Abstract
Purpose
After conducting a nationwide survey of persistent cloaca (PC), we assessed whether or not the timing of definitive anorectoplasty affects the long-term bowel function of patients with PC.
Methods
Patient information was obtained via questionnaire, and a total of 169 PC patients who underwent posterior sagittal anorectourethrovaginoplasty (PSARUVP) were enrolled in this study. Patients were classified into 2 groups based on their operative period, which was analyzed by the area under the receiver operating characteristic curve: the early group (EG) underwent anorectoplasty at ≤ 18 months old (n = 106), and the late group (LG) underwent anorectoplasty at > 18 months old (n = 63). The bowel function was evaluated using the evacuation score of the Japan Society of Anorectal Malformation Study Group. We also examined the postoperative results of vaginoplasty.
Results
The total evacuation score was significantly higher in the EG than in the LG (5.2 ± 1.7 vs. 4.2 ± 1.8, p = 0.003). The frequency of bowel movement and the constipation scores were significantly higher in the EG than in the LG (1.4 ± 0.6 vs. 1.2 ± 0.7, p < 0.05, 2.4 ± 1.0 vs. 2.1 ± 1.0, p < 0.05, respectively). Postoperative vaginal stenosis was observed in 18 cases (10.7%), of which 16 could be reconstructed transperineally.
Conclusion
PSARUVP should be performed in early infancy and facilitate vaginal reconstruction.
Similar content being viewed by others

Avoid common mistakes on your manuscript.
Introduction
Persistent cloaca (PC) is the most severe type of anorectal and gynecological malformation. PC patients have many comorbidities, and—in addition to anorectal malformations—urinary and gynecological diseases must be evaluated, and treatment strategies should be considered. Therefore, it is often difficult to determine the appropriate operative procedure and optimal timing for PC patients.
Regarding anorectoplasty procedures, PSARP, reported by Pena in 1982, is the gold standard and the method practiced worldwide [1]. The most commonly performed operative procedure for PC is posterior sagittal anorecto-urethro-vagino-plasty (PSARUVP), in which urethroplasty and vaginoplasty are performed simultaneously with PSARP during infancy [2]. However, the need to evaluate and treat associated anomalies may lead to a delay in the timing of radical surgery for PC.
Kubota et al. conducted a nationwide survey of PC in 2014 to determine the current status of this disease in Japan [3, 4]. In that survey, there were only 19.6% of cases in which anorectoplasty was performed before 12 months old. We performed a subgroup analysis of the data from our nationwide survey. The present study clarified the influence of the timing of anorectoplasty on the bowel function when performing PSARUVP and investigated the procedures used for vaginoplasty and associated complications based on a nationwide survey.
Materials and methods
Study population
The nationwide survey, “Establishment of treatment guidelines for smooth transitional care of persistent cloaca, cloacal exstrophy and Mayer–Rokitansky–Kuster–Hauser syndrome” was conducted [5, 6]. In that study, we assessed whether or not the timing of definitive anorectoplasty affects the long-term bowel function of patients with PC. Patient information was obtained by sending a questionnaire to 244 university hospitals and children’s hospitals in our country to investigate the etiologic events and clinical outcomes in patients with PC. Responses concerning 466 cases of PC were obtained from 113 institutions (46.3%).
The flow chart of the nationwide survey for PC is shown in Fig. 1. A total of 169 PC patients (36.3%) who underwent PSARUVP were extracted and enrolled in the present study to determine the optimal timing of anorectoplasty for patients with PC.
Flowchart of the PC patients underwent PSARUVP. PSARUVP posterior sagittal anorectourethrovaginoplasty, PC persistent cloaca, PSARP posterior sagittal anorectoplasty, SP-SAP sacroperineal or sacroabdominoperineal rectoplasty, LAARP laparoscopy-assisted anorectoplasty, ASARP anterior sagittal anorectoplasty
Data collection
To clarify the optimal timing of anorectoplasty in patients with PC, we investigated their long-term bowel function, the timing of anorectoplasty and vaginoplasty procedures, and their postoperative complications. Patients were classified into two groups based on their operative period: the early group (EG) for those who underwent surgery at ≤ 18 months old and the late group (LG) for those who underwent surgery at > 18 months old, using the cut-off value of the area under the receiver operating characteristic (AUROC) curve analysis of the age at anorectoplasty.
Evaluating the bowel function
The bowel function was evaluated based on the clinical data of the latest visit at the outpatient clinic using the evacuation score of the Japan Society of Anorectal Malformation Study Group [7]. In this study, we evaluated four parameters: frequency of bowel movement, soiling, constipation, and incontinence.
The total scores were analyzed based on the sum of the frequency of the bowel movement score and soiling score. For constipation and incontinence, a lower score is preferred. The maximum score is 8 points, which indicates an excellent bowel function. The clinical stratification was evaluated according to the total evacuation score (ES), as follows: 0 to 4, poor; 5 to 6, good; and 7 to 8, excellent.
Statistical analyses
To evaluate the effect of operative timing on the bowel function, the total ES (poor vs. good and excellent) was assessed by AUROC curve analyses. The cut-off value was determined based on the sensitivity and specificity. Mann‒Whitney U test and Fisher’s exact test were used for comparisons between two groups, and p values of < 0.05 were considered to indicate statistical significance. Data are expressed as the mean ± standard deviation.
All statistical analyses were performed with EZR (Saitama Medical Center, Jichi Medical University, Saitama, Japan), which is a graphical user interface for R (The R Foundation for Statistical Computing, Vienna, Austria). More precisely, it is a modified version of R commander designed to add statistical functions frequently used in biostatistics [8].
Ethical approval
This research project, “Establishment of treatment guidelines for smooth transitional care of persistent cloaca, cloacal exstrophy and Mayer–Rokitansky–Küster–Häuser syndrome,” was supported by the Health and Labour Sciences Research Grants for Research on Rare and Intractable Diseases from 2014 to 2016 in Japan (registration number: general-068). This study was performed in accordance with the Ethical Guidelines for Medical and Health Research Involving Human Subjects by the Ministry of Health, Labour, and Welfare of Japan in 2014 and was in compliance with the 1964 Declaration of Helsinki (revised in 2013). This study was approved by the Research Ethics Committee of Kagoshima University Hospital (registration number: 170347).
Results
Dividing method for the operative period of PC patients using the AUROC curve
We analyzed the postoperative bowel function using the AUROC curve of the age at anorectoplasty (Fig. 2). In the AUROC curve analysis, the cut-off value of the age at anorectoplasty was 18 months old, with an area under the AUROC curve of 0.636 (95% confidence interval: 0.543–0.729). This result indicated that those who had undergone anorectoplasty after 18 months acquired a poor bowel function (p < 0.01). Based on this cut-off value, as described above, patients were classified into either the EG (n = 106) or the LG (n = 63).
Mean age at anorectoplasty and at the latest evaluation of the bowel function
The mean age at anorectoplasty of PC patients in the EG was 11.4 ± 5.4 months old, while that in the LG was 32.9 ± 21.3 months old (p < 0.001). The mean age at the latest evaluation of the bowel function in the EG was 11.5 ± 6.5 years old, while that in the LG was 12.3 ± 7.2 years old (p = 0.50).
The comparison of the postoperative bowel function between the EG and LG
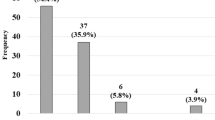
The bowel functions at the latest evaluation of the PC patients are shown in Table 1. The total evacuation score in the EG was significantly better than that in the LG (5.2 ± 1.7 vs. 4.2 ± 1.8, p = 0.003), as were the frequency of bowel movement score and constipation score (1.4 ± 0.6 vs. 1.2 ± 0.7, p < 0.05; 2.4 ± 1.0 vs. 2.1 ± 1.0, p < 0.05, respectively). There were no significant differences in the incontinence or soiling scores between the two groups. The proportion of total ES and the details of the four scores according to the age at anorectoplasty are shown in Fig. 3.
Postoperative complications associated with anorectoplasty
Postoperative complications associated with anorectoplasty are shown in Table 2. Postoperative complications associated with PSARUVP were recognized in 26 (15.4%) of 169 patients. Postoperative complications were recognized in 19 (17.9%) patients in the EG and in 7 (11.1%) patients in the LG. Recanalization procedures for the neo-anus were required after PSARUVP in 9 (8.5%) patients in the EG, while prolapse of rectal mucosa was recognized in 8 (7.5%) patients, and anal stenosis was recognized in 2 (1.9%) patients. Conversely, reconstruction procedures for the neo-anus were required in 5 (7.9%) patients in the LG, while prolapse of rectal mucosa was recognized in 1 (1.6%) patient, and anal stenosis was recognized in 1 (1.6%) patient. These complications showed no significant differences between the EG and LG.
Vaginoplasty procedure and postoperative complications according to the performed procedure
The vaginoplasty procedure and the postoperative complications according to the performed procedure are shown in Table 3. Of the 168 PC patients who underwent PSARVUP, 28 (16.6%) required additional surgery after initial vaginoplasty. Total urogenital mobilization (TUM) was performed in 33 (19.8%) patients, and 5 (15.2%) patients required additional procedures. Skin flaps were performed in 28 (16.8%) patients, and 3 (10.7%) patients required additional procedures. Bowel interposition was performed in 23 (13.8%) patients, and 4 (17.4%) patients required additional surgery. Vaginal switching was performed in 4 (2.4%) patients, and no additional surgery was needed. Tubularized penile flap was performed in 1 (0.6%) patient, and the patient required vaginal orifice-plasty later. Fifteen patients who underwent unknown vaginoplasty (18.8%) required additional procedures (vaginal orificeplasty: 7, vaginal dilation: 3, vaginourethral fistula closure: 2, vaginal septum incision: 2, unknown: 1).
Vaginal reconstructions after PSARUVP were required in 18 (62.3%) of 28 patients. Of these patients, trans-perineal surgery was performed in 16 patients (13 patients: vaginoplasty, 3 patients: vaginal dilatation), and bowel interposition was performed in 2 patients.
Discussion
We clarified the influence of the timing of anorectoplasty on the bowel function when performing PSARUVP and investigated the operative procedures of vaginoplasty and its associated complications in patients with PC who were identified in a nationwide survey in Japan. The major findings of this study were as follows: (1) PSARUVP was performed in 169 out of 466 PC cases (36.3%); (2) with 18 months old as the cut-off value for the timing of anorectoplasty, the total ES was significantly lower in those who underwent anorectoplasty later than in those who underwent it earlier; (3) the scores of frequency of bowel movement and constipation were significantly better in the EG than in the LG; (4) while various vaginoplasty procedures were performed, postoperative vaginal stenosis was still recognized in 18 cases (10.7%), of which 16 were able to be reconstructed transperineally.
In the nationwide survey regarding PC that was used in this study, anorectoplasty was able to be performed before 12 months old in only 19.6% of cases [3, 4]. This is because PC patients have many associated anomalies, such as cardiac anomalies, and—in addition to anorectal malformations—urinary and gynecological diseases should be evaluated, and a treatment strategy must be considered. In general, patients with PC usually undergo anorectoplasty at a weight of 8 kg in Japan, and this timing of anorectoplasty seemed to be delayed compared with other reports. Therefore, it is important for pediatric surgeons to recognize the optimal timing for surgery to achieve a good postoperative defecation function in PC patients.
Performing anorectoplasty for anorectal malformation at an early age has some advantages with respect to the postoperative bowel function [9, 10]. Performing anorectoplasty at an early age allows for the passage of stool earlier, with early establishment of the brain-defecation reflex [11, 12]. The present study showed a significant difference in the frequency of bowel movement scores. This may be due to differences in the timing for anorectoplasty. The present study also showed a significant difference in the constipation score, and the incontinence and soiling scores in the EG tended to be better than those in the LG. These results suggest that PC patients should undergo anorectoplasty in the early period of infancy.
The frequency of vaginal stenosis after vaginoplasty performed during infancy is reported to be 36–41% [13, 14]. Couchman et al. reported that 9 (56.2%) of 19 patients who underwent vaginoplasty before 1 year old required at least 1 additional procedure because of obstruction to the menstrual flow or sexual intercourse [15]. Even if vaginoplasty is performed in infancy, many cases require additional surgery for vaginal stenosis after puberty. Therefore, there are some negative opinions concerning the performance of early vaginoplasty. In the present study, 18 patients required vaginal reconstruction due to vaginal stenosis after PSARUVP during puberty, and 16 patients (88.9%) were treated by transperineal procedures. In other words, PC patients with vaginal stenosis who underwent PSARUVP were able to receive additional vaginal treatment without the need for laparotomy. However, if initial vaginoplasty is planned in puberty, patients with a history of multiple prior laparotomies may require additional laparotomy for vaginoplasty. Although additional surgical treatment for stenotic vagina may be required after puberty, performing vaginoplasty in infancy may allow for less-invasive vaginal reconstruction surgery in future.
The frequency of complications after PSARUVP varies depending on the vaginoplasty procedure used. Vaginal stenosis occurs frequently and is a postoperative complication that must be resolved. Stenosis after vaginoplasty is more likely to occur after surgery using the gastrointestinal tract than other procedures [16], especially when using the small intestine for vaginal replacement [17]. Although the vaginal switch procedure is not always associated with fewer complications than other approaches, Bischoff et al. reported that the original vaginal tissue was eventually maintained as the actual vagina in 75% of patients [18]. The original vaginal tissue can be expected to grow due to the response to hormones as vaginal tissue [19], and mucus can be avoided due to the use of the intestine. Therefore, the vaginal switch procedure is superior to vaginoplasty using other organs in that it uses the original vaginal tissue [18].
In the present study, vaginal stenosis occurred in 3 (13.0%) of 23 patients in whom the intestine was used for vaginal replacement but did not occur in the patients who underwent the vaginal switch procedure. In this nationwide survey, the vaginal switch procedure was used in only 2.4% of vaginoplasties. The vaginal switch procedure is applicable in specific cases of cloaca and urogenital sinus when the patients have two large hemivaginas, located very high in the pelvis, and the transverse distance between both hemiuteri is longer than the longitudinal length of the vaginas [18]. The reason for few patients undergoing the vaginal switch procedure in Japan is that the indications for this procedure are limited, and most pediatric surgeons are not proficient in this procedure. However, the vaginal switch procedure has been reported superior approach, as it does not reduce blood flow to the organ [18]. Therefore, the vaginal switch procedure should be considered more frequently if the vaginal shape is suitable for the procedure.
Conclusion
In patients with PC, there are various disease types and many associated anomalies, and in some cases, the timing of radical surgery is delayed. The results of this study indicate that performing PSARUVP as early as possible leads to an improved postoperative bowel function and facilitates treatment of vaginal stenosis, which frequently occurs after vaginoplasty. The early assessment of the disease type and planning of radical surgery as early as possible can result in improved outcomes regarding the bowel function and overall vaginoplasty operation in patients with PC.
Data availability
The data sets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Pena A, Devries PA (1982) Posterior sagittal anorectoplasty: important technical considerations and new applications. J Pediatr Surg 17:796–811
Pena A (1989) The surgical management of persistent cloaca: results in 54 patients treated with a posterior sagittal approach. J Pediatr Surg 24:590–598
Kubota M (2017) The current profile of persistent cloaca and cloacal exstrophy in Japan: the results of a nationwide survey in 2014 and a review of the literature. Pediatr Surg Int 33:505–512
Harumatsu T, Muto M, Kawano T, Sugita K, Yano K, Onishi S et al (2023) Analysis of the potential risk factors for defecation problems and their bowel management based on the long-term bowel function in patients with persistent cloaca: results of a nationwide survey in Japan. Pediatr Surg Int 39:96
Kyrklund K, Pakarinen MP, Koivusalo A, Rintala RJ (2014) Long-term bowel functional outcomes in rectourethral fistula treated with PSARP: controlled results after 4–29 years of follow-up: a single-institution, cross-sectional study. J Pediatr Surg 49:1635–1642
Rintala RJ, Lindahl HG (2001) Fecal continence in patients having undergone posterior sagittal anorectoplasty procedure for a high anorectal malformation improves at adolescence, as constipation disappears. J Pediatr Surg 36:1218–1221
Onishi S, Nakame K, Yamada K, Yamada W, Kawano T, Mukai M et al (2016) Long-term outcome of the bowel function for 110 consecutive cases of Hirschsprung’s disease: comparison of the abdominal approach with transanal approach more than 30years in a single institution—is the transanal approach truly beneficial for bowel function? J Pediatr Surg 51:2010–2014
Kanda Y (2013) Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant 48:452–458
Harumatsu T, Kaji T, Nagano A, Matsui M, Yano K, Onishi S et al (2021) Early definitive operation for patients with anorectal malformation was associated with a better long-term postoperative bowel function. Pediatr Surg Int 37:445–450
Pena A (1993) Management of anorectal malformations during the newborn period. World J Surg 17:385–392
Albanese CT, Jennings RW, Lopoo JB, Bratton BJ, Harrison MR (1999) One-stage correction of high imperforate anus in the male neonate. J Pediatr Surg 34:834–836
Liu G, Yuan J, Geng J, Wang C, Li T (2004) The treatment of high and intermediate anorectal malformations: one stage or three procedures? J Pediatr Surg 39:1466–1471
Levitt MA, Stein DM, Pena A (1998) Gynecologic concerns in the treatment of teenagers with cloaca. J Pediatr Surg 33:188–193
Hall R, Fleming S, Gysler M, McLorie G (1985) The genital tract in female children with imperforate anus. Am J Obstet Gynecol 151:169–171
Couchman A, Creighton SM, Wood D (2015) Adolescent and adult outcomes in women following childhood vaginal reconstruction for cloacal anomaly. J Urol 193(5 Suppl):1819–1822
Burgu B, Duffy PG, Cuckow P, Ransley P, Wilcox DT (2007) Long-term outcome of vaginal reconstruction: comparing techniques and timing. J Pediatr Urol 3:316–320
Levitt MA, Pena A (2010) Cloacal malformations: lessons learned from 490 cases. Semin Pediatr Surg 19:128–138
Bischoff A, Levitt MA, Breech L, Hall J, Pena A (2013) Vaginal switch–a useful technical alternative to vaginal replacement for select cases of cloaca and urogenital sinus. J Pediatr Surg 48:363–366
Breech L (2016) Gynecologic concerns in patients with cloacal anomaly. Semin Pediatr Surg 25:90–95
Acknowledgements
We thank the members who participated in the nationwide survey in Japan and the researchers who are referenced in the manuscript [3]. We also thank Mr. Brian Quinn for his comments and help with the manuscript. This study was supported by the Research Project for Rare and Intractable Diseases of the Ministry of Health, Labour and Welfare (MHLW), a research grant from The Mother and Child Health Foundation, and a research grant from the Kawano Masanori Memorial Public Interest Incorporated Foundation for Promotion of Pediatrics.
Author information
Authors and Affiliations
Contributions
HT and SK wrote the main manuscript text. YK, M, and KT prepared the tables and figures. NA, OS and MM researched the literature. I and KM gave conceptual advice. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest in association with the present study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Harumatsu, T., Sugita, K., Onishi, S. et al. Posterior sagittal anorecto-urethro-vagino-plasty in the late period was associated with the long-term bowel function in patients with persistent cloaca: results of a nationwide survey in Japan. Pediatr Surg Int 39, 244 (2023). https://doi.org/10.1007/s00383-023-05526-7
Accepted:
Published:
DOI: https://doi.org/10.1007/s00383-023-05526-7