Abstract
Purpose
Cell therapy is a promising approach to treat enteric neuropathies such as Hirschsprung disease (HD). Recent studies have reported that enteric neurons derived from stem cells (ENCCs) can be grafted into the HD colon. Thus, we investigated the migration and generation of enteric neurospheres from SOX10-VENUS+ mice after transplantation into control or Ednrb KO mice, which are a model of HD.
Methods
Single-cell suspensions were isolated from the fetal guts of SOX10-VENUS+ mice E13.5 and dissociated. These cells were cultured for 7 days under non-adherent conditions to generate neurospheres, which were co-cultured with dissociated control or SOX10-VENUS− Ednrb KO mouse gut on E13.5. 4 days later, these cells were fixed and the expression of the neuronal marker, Tuj1, was evaluated.
Results
Transplanted neurospheres had undergone abundant neuronal migration and differentiation of ENCCs in the control gut compared with the HD gut. The average length and intersections were significantly decreased in HD colon compared with controls (p < 0.05), and a similar pattern was observed in the HD small intestine (p < 0.05).
Conclusions
We demonstrated that transplanted ENCCs did not differentiate properly in HD gut. These results highlight the importance of the neuronal environment in the recipient gut for enteric nervous system development.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The enteric nervous system (ENS) is a network of neurons and glia in the wall of the gastrointestinal tract that controls intestinal functions including motility, secretion, absorption, sensation, and immunity [1]. Enteric neural crest-derived cells (ENCCs) enter the foregut mesenchyme and migrate rostral caudally along the entire gut during the development of the ENS [2]. Hirschsprung disease (HD) results from the failure of ENCCs to complete the colonization of the distal intestine during fetal development [3]. Moreover, gut microenvironment has also been implicated in previous studies of the pathogenesis of HD [4].
Stem cell transplantation might be a future alternative to surgical interventions for HD. Recent studies have demonstrated that enteric neurons derived from various types of stem cells can be engrafted onto the HD colon. Transplanted cells require an appropriate microenvironment to survive and differentiate. However, whether transplanted ENCCs migrate and generate enteric neurons, as occurs in the normal gut, remains clear.
Sox10, which belongs to the SOX family of transcription factors, is essential for ENS development and is expressed throughout the early neural crest [5]; therefore, it is a well-known marker of ENCCs. Previously, we developed genetically engineered transgenic (SOX10-Venus Tg) mice to visualize the behavior of migrating ENCCs expressing green fluorescent protein, known as the Venus mouse [6]. Using this model, we reported the successful isolation of Sox10 positive cells and that ENCCs from HD mice favored glial differentiation compared with controls [7].
In this paper, we investigated the migration and generation of enteric neurospheres from SOX10-VENUS+ mice when transplanted into control or Endothelin-B receptor knockout (Ednrb KO) mice, which are a model of HD.
Materials and methods
Animal model
We generated and raised Sox10-Venus Transgenic mice in accordance with a protocol described elsewhere to visualize neural crest lineage cells [6]. Endothelin receptor-B null Ednrbtm1Ywa mice were obtained from the Jackson Laboratory (Bar Harbor, USA). Heterozygote littermates (referred to as Ednrb± and homozygotes referred to as Ednrb−/− in this article) were used as breeding pairs. Sox10-Venus+/Ednrb−/− mice were raised at the Juntendo University School of Medicine (Tokyo, Japan) by crossing Ednrb± mice with Sox10-Venus Tg mice and repeat crossing with Sox10-Venus+/Ednrb± mice. The genotypes of mice and embryos were determined by polymerase chain reaction. The day when a vaginal plug was detected was classified as embryonic day 0.5 (E0.5). Sox10-Venus−/Ednrb+/+ mice, Sox10-Venus−/Ednrb−/− mice, and controls (Sox10-Venus+/Ednrb+/+) were sacrificed on E13.5 by cervical dislocation. Each embryo was harvested and the gut was dissected under a stereoscopic microscope (M165FC FSM, Leica, Germany). All animal procedures were reviewed and approved by the Animal Care and Use Committee (Institutional review board No. 280082).
Generation and culture neurospheres
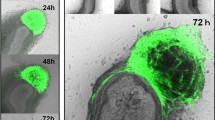
ENCCs for cell transplantation were isolated from the whole gut of Sox10-Venus+/Ednrb+/+ mice at E13.5. Dissected specimens were washed in calcium and magnesium-free DPBS (Gibco, USA) containing 1% penicillin/streptomycin (P/S, Gibco). Gut tissue was digested in an enzyme mixture containing collagenase 1 (Gibco) in DPBS at 37 °C for 40 min by mechanical trituration. Cells were then plated at 2 × 106 cells/ml in a 25-cm2 flask in NSM medium (DMEM/F12 (Sigma–Aldrich, UK)) containing 20 ng/ml bFGF (PeproTech, UK), 20 ng/ml EGF (PeproTech, UK), N2 supplement (Invitrogen, UK), B27 supplement (Invitrogen, UK), and 1% P/S (Gibco, USA). After 7 days, primary cell aggregates (neurospheres) were obtained. Neurospheres from mice were used for cell transplantation.
Isolation and culture of Venus-negative cells as recipient cells
The entire gut from Sox10-Venus−/Ednrb−/− mice and control mice (Sox10-Venus−/Ednrb+/+) were dissected after cervical dislocation at E13.5. Dissected specimens were washed in calcium and magnesium-free DPBS (Gibco, USA) containing 1% P/S (Gibco) and divided into the small intestine and colon. Each gut tissue was digested in an enzyme mixture containing collagenase 1 (Gibco, USA) in DPBS at 37 °C for 40 min by mechanical trituration. Cells were plated on fibronectin-coated glass coverslips (neuVitro Corporation, USA) in 35-mm dishes with NSM medium for 7 days.
Transplantation of neurospheres into Venus-negative cells
Venus-positive neurospheres were co-cultured with E13.5 either control gut or HD gut.
Immunofluorescence staining
After 4 days, cultures were fixed in 4% paraformaldehyde (PFA). After washing twice in PBS + 0.5% Triton X-100 (PBT), they were incubated with blocking solution (PBT + 1% BSA) at RT for 2 h. Primary antibodies Tuj1 (rabbit; BioLegend, #801202; a neuronal marker) were diluted 1:500 in blocking solution and cultured with cells at RT for 1 h. After several washes with PBS, secondary antibodies (anti-rabbit Alexa Fluor; Invitrogen, UK; 1:300) were added in blocking solution for 1 h at RT. Finally, samples were washed with distilled water, and mounted on microscopic slides using Vectashield containing DAPI (Vector Laboratories, USA). Cells were then examined and counted. Images were acquired on a confocal laser-scanning microscope (TCS-SP5, Leica, Germany). Five neurospheres (n = 5) were counted for each group.
Analysis of neuronal morphology
All neurite outgrowth processes and associated branches were traced and analyzed using NeuronJ plugin, ImageJ software, and Sholl analysis. The mean lengths of neurites were measured. Then, the numbers of neurite intersections were recorded. For Sholl analysis, concentric circles with a radius of 200 μm were drawn around the cell body, and the numbers of neurites crossing at each circle were automatically counted.
Statistical analysis
Differences between the two groups were tested using an unpaired t test. Statistical significance was defined when p < 0.05.
Results
Immunofluorescence staining
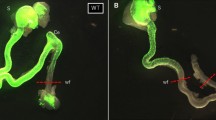
After 4 days, transplanted enteric neurospheres had undergone abundant neuronal migration and ENCCs had differentiated in the control gut compared with the HD gut. The neurospheres expressed ENCCs markers such as SOX10 (green). There were many Tuj1-positive neurites in the control and HD groups except in the colons of the HD group. Transplanted neurospheres were connected to not only other neurospheres but also recipient cells (DAPI stained cells). In the HD group, the shapes of neurospheres in the colon were not complete circles compared with the controls (Fig. 1).
The average of neurite length
The average of neurite length in the controls was no significantly differences between small intestine and colon in controls. Whereas, in HD group, colon was significantly decreased in HD compared to small intestine (p < 0.05). It was also significantly decreased compared to control colon (p < 0.001). Interestingly, the average length of small intestine in HD was significantly decreased compared with controls (p < 0.05), indicating that even ganglionic area in HD is not same as controls (Fig. 2).
Neurite complexity
We performed Sholl analysis, a standard assay to assess neurite complexity, to quantify the patterns of neurite trees. The number of neurites crossing circles at various radial distances from the cell soma was measured.
As shown in Fig. 3, in control group, small intestine there was significantly decreased the number of intersection at 0 um from soma compared with colon (p < 0.05). On the other hand, co-culture with control small intestine, there were significantly increased number of neurite intersections from 160 to 200 um compared with both control and HD small intestine (p < 0.05). Moreover, we observed a significantly decreased the number of intersection of neurites in HD colon from 0 to 60 um compared to both control colon and HD small intestine (p < 0.05).
Discussion
HD results from the failure of ENCCs to correctly colonize the entire gut. Current treatment involves the surgical removal of the aganglionic segment; however, functional outcomes are variable and many patients suffer life-long complications such as defecation problems and enterocolitis [8]. Cell-based therapy is considered a promising therapeutic approach for HD, especially because several studies have shown that ENCC-based transplantation successfully restored the ENS [9,10,11]. However, this approach has limitations, which is poor survival, differentiation, and migration of the grafted cells [12] and the precise mechanism by which donor-derived cells integrate within recipient tissue remains poorly understood.
In this study, we used Ednrb KO mice, which develop distal colorectal aganglionosis, as a model for HD. In this model, it was shown that by E14.5, the control ENCCs wavefront had reached the terminal hindgut, whereas the mutant wavefront had only reached the proximal colon. Then, the mutant ENCC advanced through the proximal colon, and their network became denser but with more narrow strands compared with those in the control gut [13]. Thus, at E13.5 ENCCs migrate small intestine in HD as ganglionic segment.
The findings of our study demonstrate, the neurite branches in control colon was significantly increased compared with control small intestine nearby soma, whereas when the distance from soma the number of intersection of neurite was decreased. Accordingly, our results suggested that resulting in different effect of neuronal development which depending in their location within the gastrointestinal tract.
Furthermore, we have shown that neurite outgrowth and branches in both ganglionic and aganglionic segment of HD are significantly decrease compared with controls. These results are supported by published data showing that gut microenvironment has support enteric nervous growth [14]. Our results suggest that the ganglionic regions in HD and controls have different features for enteric neuronal development. In recent year, our team has published the colonic extracellular matrix (ECM) is altered in disease states, which suggests the integrative ability of ENCC-derived donor cells is likely to be altered by the recipient microenvironment ENCC integration.
Using our in vitro transplantation approach, we successfully demonstrated that transplanted neurospheres have shown to migrate extensively with controls. It would be a useful tool for advanced cell-based therapy and drug discovery research for HD. However, it should be noted that the dissociated bowel condition a markedly different environment from that found gut itself.
Further research into the molecular mechanisms underlying these neuronal processes might lead to the development of successful cell therapies for this complex condition.
References
Furness JB (2012) The enteric nervous system and nerurogastroenterology. Nat Rev Gastroenterol Hepatol 9(5):286–294
Obermay F, Hotta R, Enomoto H et al (2013) Development and developmental disorders of the enteric nervous system. Nat Rev Gastroenterol Hepatol 10(1):43–57
Burns AJ, Hofstra RMW (2016) The enteric nervous system. From embryology to therapy. Dev Biol 15(41782):127–128
Parikh DH, Tam PK, Lloyd DA et al (1992) Quantitative and qualitative analysis of the extracellular matrix protein, laminin, in Hirschsprung`s disease. J Pediatr Surg 27(8):995–996
Kawaguchi J, Nichols J, Gierl MS et al (2010) Isolation and propagation of enteric neural crest progenitor cells from mouse embryonic stem cells and embryos. Development 137:693–704
Shibata S, Yasuda A, Renault-Mihara F et al (2010) Sox10-Venus mice: a new tool for real-time labeling of neural crest lineage cells and oligodendrocytes. Mol Brain 3:31
Fujiwara N, Miyahara K, Nakazawa-Tanaka N et al (2016) Altered differentiation of enteric neural crest-derived cells from endothelin receptor-B null mouse model of Hirschsprung’s disease. Pediatr Surg Int 32(12):1095–1101
Heuckeroth RO (2018) Hirschsprung disease—integrating basic science and clinical medicine to improve outcomes. Nat Rev Gastroenterol Hepatol 15(3):152–167
Pan WK, Zheng BJ, Gao Y et al (2011) Transplantation of neonatal gut neural crest progenitors reconstructs ganglionic function in benzalkonium chloride-treated homogenic rat colon. J Surg Res 167(2):e221–e230
Lindley RM, Hawcutt DB, Connell MG et al (2008) Human and mouse enteric nervous system neurosphere transplants regulate the function of aganglionic embryonic distal colon. Gastroenterology 135(1):205-216.e6
Hotta R, Stamp LA, Foong JPP et al (2013) Transplanted progenitors generate functional enteric neurons in the postnatal colon. J Clin Invest 123(3):1182–1191
Mucci MA, Pasricha F (2007) Neural stem cells for the treatment of disorders of the enteric nervous system: strategies and challenges. Dev Dyn 236(1):33–34
Druckenbrod NR, Epstein ML (2009) Age-dependent changes in the gut environment restrict the invasion of the hindgut by enteric neural progenitors. Development 136(18):3195–3203
Ci H, Rauch U, Klotz M et al (2012) The microenvironment in the Hirschsprung’s disease gut supports myenteric plexus growth. Int J Colorectal Dis 27(6):817–829
Acknowledgements
The authors thank Ms Mirei Takahashi for excellent technical support.
Funding
This work was supported by JSPS KAKENHI Grant no. 19K09103.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Fujiwara, N., Miyahara, K., Nakazawa-Tanaka, N. et al. In vitro investigation of the differentiation of enteric neural crest-derived cells following transplantation of aganglionic gut in a mouse model. Pediatr Surg Int 38, 755–759 (2022). https://doi.org/10.1007/s00383-022-05105-2
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00383-022-05105-2