Abstract
Purpose
The management of subglottic stenosis (SGS) remains challenging. Although laryngotracheal reconstruction with a costal cartilage graft (LTR) has been widely performed, restenosis with cicatricial tissue may require long-term stenting, especially in patients with severe SGS. An anterior cricoid split (ACS) with long-term stenting has been shown to be useful for patients with mild SGS. Thus, we evaluated the clinical outcomes of patients, including severe SGS, who underwent ACS compared to those with LTR.
Methods
A retrospective chart review was conducted in 25 patients with severe SGS (Grades III and IV) who underwent initial laryngoplasty (ACS or LTR) in our hospital from January 2009 to April 2018.
Results
17 patients (8 with Grade III and 9 with Grade IV) underwent ACS, and 8 (6 with Grade III and 2 with Grade IV) underwent LTR. The median duration of stenting was 11 months (range: 0.8–50) in the ACS group and 12 months (range: 0.4–29) in the LTR group. Thirteen of 17 patients (76.5%) in the ACS group were decannulated, whereas 4 of 8 patients (50%) in the LTR group were decannulated (p = 0.2).
Conclusion
ACS might be useful even for children with severe SGS. The optimal duration of stenting should be investigated further.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Subglottic stenosis (SGS) is a relatively rare condition, and improvement of neonatal care has further decreased the occurrence [1]. However, the management of SGS, especially severe SGS, remains a challenge. An anterior cricoid split (ACS) has been reported to be successful for neonates and small infants [2,3,4,5]. Laryngotracheal reconstruction with a costal cartilage graft (LTR) was commonly advocated in cases of failed ACS [6]. Recently, LTR has been widely used for management of subglottic stenosis [7]. However, dislodgement of the graft or an excessive inflammatory response can cause granulation or cicatricial tissue resulting in restenosis. Long-term T-tube stenting was suggested as the optimal treatment along with either LTR or ACS [8]. ACS with a posterior split and long-term T-tube stenting was reported to be effective for mild SGS even in older children [9]. Thus, we evaluated ACS with long-term T-tube stenting for children with severe SGS.
Methods
Following approval by our Institutional Review Board (No.R30-20), a retrospective analysis was performed on 25 patients with severe (Myer-Cotton Grades III and IV) SGS who underwent either ASC or LTR as initial laryngotracheoplasy in our hospital between January 2009 and April 2018.
Myer–Cotton grades were assessed by pediatric surgeons who performed rigid bronchoscopy. Surgical procedures for ACS and LTR were performed as described elsewhere. Briefly, under general anesthesia, ventilation was established through a tracheostomy. With rigid bronchoscopy and direct vision, we made a split in the anterior wall of the cricoid cartilage that extended to one-third of the thyroid cartilage to keep the midline and prevent injury to the vocal cord. The posterior wall of the subglottis was additionally split just down to the submucosal layer not to break the frame of the posterior plate but to widen the lumen and place the silastic T-tube (or the endotracheal tube in patients who subsequently required repair of congenital heart disease). For ACS, we placed the sutures roughly on the stent and closed the wound. For LTR, a costal cartilage graft was used to cover the gap over the stent. The surgical procedures were chosen mainly according to surgeons’ preferences. However, in the case of the hypoplastic cricoid cartilage, in which anterior wall graft would have compressed silastic T tube, ACS was performed.
Demographic data such as gender, gestational weeks, birth weight, age at operation, operative procedures, and duration of stenting were obtained. Severity of SGS was determined by rigid bronchoscopy.
We compared the decannulation rates between patients who underwent ACS and those who underwent LTR.
We undertook a pathological examination of the anterior wall of cricoid cartilage from a patient who underwent partial cricoid tracheal resection (PCTR) after failed ACS.
Statistical analysis was performed using JMP®. Continuous values were expressed as median and range. The Mann–Whitney test was used for comparison of patients with ACS and LTR and of successful and failed ACS. Fisher’s exact test was performed to compare the decannulation rates between the two groups. A value of p < 0.05 was considered significant.
Results
A total of 25 patients with severe SGS were included. All had tracheostomy before laryngotracheoplasty. Seventeen (ten males and seven females) patients underwent ACS, whereas eight (four males and four females) patients underwent LTR. The median gestational weeks and birth weight were 37 weeks (range: 23–41) and 2400 g (range: 518–3740) in the ACS group and 25 weeks (range: 23–40) and 886 g (range: 524–2794) in the LTR group (p = 0.06, 0.1). Ten patients (59%) in the ACS group and seven patients (87.5%) in the LTR group had congenital SGS (p = 0.2). Eight patients had Grade III, and nine patients had Grade IV in the ACS group; six patients had Grade III and two patients had Grade IV in the LTR group (p = 0.2). Age at operation and duration of stenting were 3 years old (range: 0.75–14) and 11 months (range: 0.8–50) in the ACS group and 4 years old (range: 2–9) and 12 months (range: 0.4–29) in the LTR group (p = 0.8, 0.7). The decannulation rate was 76.5% in the ACS group and 50% in the LTR group (p = 0.2) (Table 1). No patients who were successfully decannulated encountered restenosis, and needed tracheostomy tube any more during the study period.
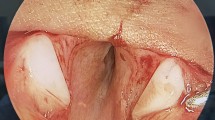
A comparison between successful decannulation and failure in the ACS group is shown in Table 2. All Grade III patients were successfully free from tracheostomy, whereas 44.4% of Grade IV patients required further intervention (p = 0.03). Figure 1 shows a Grade IV patient who was successfully decannulated after ACS and silastic T-tube stenting. We performed PCTR on one patient who failed ACS. The resected specimen showed that osteosis had filled the gap in the anterior wall of the cricoid cartilage (Fig. 2).
Discussion
ACS with long-term stenting was effective in all patients with Grade III and in more than half of patients with Grade IV SGS, which, in this study and other reports, was comparable or better than LTR using a costal cartilage graft [1, 7, 10]. ACS has been reported as an effective procedure for infants and young children [6] with mild SGS. Richardson et al. showed that LTR with graft resulted in a higher success rate in extubation than ACS without a graft [11]. Extubation was performed at 7 to 14 days following the procedure in their study. This could have been because the duration of stenting was not sufficient for the healing process after ACS without a graft. Although single-stage LTR has been shown to be effective [12], Gustafson et al. reported in their review of 200 cases that 29% of patients required reintubation, 15% needed tracheostomy, and 11% experienced a prolapsed graft [13]. We demonstrated that ACS without a graft with a sufficient duration of stenting was feasible for older children with severe SGS, for whom even LTR requires long-term stenting [14].
Although the age at operation was not different between ACS and LTR, gestational weeks and body weight at birth were lower in the LTR group than in the ACS group. The reason for this trend was uncertain. The optimal timing needs to be investigated for both procedures. We prefer to postpone intervention until the inflammation in the subglottic region has subsided.
The pathological findings from the patient who underwent PCTR after failed ACS showed osteosis in the resected anterior wall of the cricoid cartilage. It could be possible for regeneration of cartilage or osteosis to occur to fill the gap on the anterior wall of the cricoid after ACS if the duration of stenting is long enough. We assumed that this regeneration, especially in children, was the reason why ACS without a graft with stenting was relevant to LTR.
Limitations
The limitations of this study included its small sample size and single-center retrospective nature. It was difficult to determine congenital or acquired SGS precisely from the charts since it was commonly mixed (acquired on congenital) type at the time when the patients referred to our hospital. The optimal timing and duration of stenting after ACS was not investigated, and the follow-up period was insufficient to detect restenosis after decannulation.
Conclusion
Despite the limitations of this study, ACS with long-term T-tube stenting is a simple technique and could be used as an initial laryngotracheoplasty in patients with severe SGS.
References
Raol N, Rogers D, Setlur J, Hartnick CJ (2015) Comparison of hybrid laryngotracheal reconstruction to traditional single- and double-stage laryngotracheal reconstruction. Otolaryngol Head Neck Surg 152(3):524–529
Frankel LR, Anas NG, Perkin RM, Seid AB, Peterson B, Park SM (1984) Use of the anterior cricoid split operation in infants with acquired subglottic stenosis. Crit Care Med 12(4):395–398
Seid AB, Canty TG (1985) The anterior cricoid split procedure for the management of subglottic stenosis in infants and children. J Pediatr Surg 20(4):388–390
Bagwell CE, Marchildon MB, Pratt LL (1987) Anterior cricoid split for subglottic stenosis. J Pediatr Surg 22(8):740–742
Michna BA, Krummel TM, Tracy T Jr, Brooks JW, Salzberg AM (1988) Cricoid split for subglottic stenosis in infancy. Ann Thorac Surg 45(5):541–543
Massie RJ, Robertson CF, Berkowitz RG (2000) Long-term outcome of surgically treated acquired subglottic stenosis in infancy. Pediatr Pulmonol 30(2):125–130
Yamamoto K, Monnier P, Holtz F, Jaquet Y (2014) Laryngotracheal reconstruction for pediatric glotto-subglottic stenosis. Int J Pediatr Otorhinolaryngol 78(9):1476–1479
Froehlich P, Truy E, Stamm D, Floret D, Morgon A (1993) Role of long-term stenting in treatment of pediatric subglottic stenosis. Int J Pediatr Otorhinolaryngol 27(3):273–280
Bitoh Y, Okata Y, Tsugawa J, Miyauchi H, Aida Y, Tachibanaki Y, Nakai Y, Tomioka Y (2018) Anterior-posterior cricoid split combined with silastic T-tube stenting for subglottic stenosis in children: a single surgeon's experience. Pediatr Surg Int 34(10):1041–1046
Padia R, Sjogren P, Smith M, Muntz H, Stoddard G, Meier J (2018) Systematic review/meta-analysis comparing successful outcomes after single vs. double-stage laryngotracheal reconstruction. Int J Pediatr Otorhinolaryngol 108:168–174
Richardson MA, Inglis AF Jr (1991) A comparison of anterior cricoid split with and without costal cartilage graft for acquired subglottic stenosis. Int J Pediatr Otorhinolaryngol 22(2):187–193
Smith LP, Zur KB, Jacobs IN (2010) Single- vs double-stage laryngotracheal reconstruction. Arch Otolaryngol Head Neck Surg 136(1):60–65
Gustafson LM, Hartley BE, Liu JH, Link DT, Chadwell J, Koebbe C, Myer CM 3rd, Cotton RT (2000) Single-stage laryngotracheal reconstruction in children: a review of 200 cases. Otolaryngol Head Neck Surg 123(4):430–434
Choi SS, Zalzal GH (1999) Pitfalls in laryngotracheal reconstruction. Arch Otolaryngol Head Neck Surg 125(6):650–653
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Akiko Yokoi and other co-authors have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Yokoi, A., Nakao, M. & Bitoh, Y. An anterior cricoid split and long-term silastic T-tube stenting for children with severe subglottic stenosis. Pediatr Surg Int 36, 697–700 (2020). https://doi.org/10.1007/s00383-020-04657-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00383-020-04657-5