Abstract
Purpose
Voltage-gated potassium ion channels have long been implicated in gastrointestinal motility. Recent studies have highlighted the role of voltage-gated channel subfamily G member 3 (KCNG3) and 4 (KCNG4) genes in the electrical functioning of interstitial cells of Cajal and PDGFRα+ cells of the mouse colon. We designed this study to investigate KCNG3 and KCNG4 expression in the normal human colon and in Hirschsprung’s disease (HSCR).
Methods
HSCR tissue specimens (n = 6) were collected at the time of pull-through surgery, while control samples were obtained at the time of colostomy closure in patients with imperforate anus (n = 6). qRT-PCR analysis was undertaken to quantify KCNG3 and KCNG4 gene expression, and immunolabelling of KCNG3 and KCNG4 proteins was visualized using confocal microscopy.
Results
qRT-PCR analysis revealed significant downregulation of the KCNG3 and KCNG4 genes in both aganglionic and ganglionic HSCR specimens compared to controls (p < 0.05). Confocal microscopy revealed KCNG3 and KCNG4 expression within neurons, ICC and PDGFRα+ cells of the myenteric plexus and smooth muscle layers, with a reduction in both proteins in aganglionic and ganglionic HSCR colon compared to controls.
Conclusion
KCNG3 and KCNG4 gene expression is significantly downregulated in HSCR colon, suggesting a role for these genes in colonic motility. KCNG3 and KCNG4 downregulation within ganglionic specimens highlights the physiologically abnormal nature of this segment in HSCR patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Electrically silent voltage-gated potassium channels (i.e. Kv5-Kv6 and Kv8-Kv9) have two subunits, membrane-integrated α- and peripheral β-subunits. α-Subunits may assemble as tetramers and form functional K-channels. β-Subunits cannot form channels by themselves [1, 2]. These channels contribute significantly to the excitability of several cell types, including neurons and cardiac myocytes. They regulate the resting membrane potential, the membrane repolarization and the action potential shape and firing frequency. KV channels perform these roles by opening, closing and inactivating upon changes in membrane potential [3].
Coordinated gut motility and function is not solely dependent on the form of innervation it receives but also on the cells that regulate neurotransmission along with the enteric nervous system (ENS). Smooth muscle cells (SMC), interstitial cells of Cajal (ICC) and platelet-derived growth factor receptor α-positive (PDGFRα+) cells make up the ‘SIP syncytium’, communicating with each other and the ENS to regulate peristalsis in the gastrointestinal tract. Recent studies have highlighted the role of voltage-gated channel subfamily G members 3 (KCNG3) and 4 (KCNG4) genes in the electrical functioning of ICC and PDGFRα+ cells of the mouse colon [4, 5].
Hirschsprung’s disease (HSCR) is a congenital condition, affecting 1:5,000 live births, which is characterised by the absence of ganglia in the distal colon. The extent of the aganglionic segment varies between patients from involving only a short segment of rectosigmoid to total colonic aganglionosis. We designed this study to investigate the expression of KCNG3 and KCNG4 in the normal human colon and in HSCR.
Materials and methods
Tissue samples
This study was approved by the Ethics Medical Research Committee, Our Lady’s Children’s Hospital, Dublin, Ireland (Ref. GEN/292/12) and tissue samples were obtained with informed parental consent. HSCR specimens from six patients who underwent pull-through surgery were studied. These specimens were divided into aganglionic and ganglionic specimens. We compared the most distal aganglionic segments with the most proximal ganglionic segments. HSCR patients were aged 6 ± 3 months old. No additional health issues existed in these patients. Colonic control samples included six specimens from patients who underwent colostomy closure following surgical correction of imperforate anus. Control samples were taken from patients who were 11 ± 4 months old. None of the imperforate anus patients had HSCR. Tissue specimens were either snap-frozen in liquid nitrogen and stored at − 80 °C for protein extraction or embedded in OCT Mounting Compound (VWR International, Leuven, Belgium) for immunofluorescence and stored at − 80 °C until use.
Immunofluorescence staining and confocal microscopy
Frozen blocks of HSCR colon and normal control samples were sectioned transversely at a thickness of 10 µm, mounted on SuperFrost® Plus slides (VWR International, Leuven, Belgium) and fixed with 10% buffered formalin for 5 min. Sections underwent cell membrane permeabilization with 1% TritonX-100 for 20 min at room temperature. After blocking with 10% normal goat serum (Sigma-Aldrich Ltd, Arklow, Ireland) for 30 min to avoid non-specific absorption, sections were incubated with primary antibodies; rabbit anti-KCNG3 (Abcam, UK), rabbit anti-KCNG4 (Abcam, UK), rabbit anti-PGP9.5 (Sigma-Aldrich, Ireland), rabbit anti-PDGFRα (Abcam, UK), mouse anti-PDGFRα (Abcam, UK), mouse anti-smooth muscle actin (Sigma-Aldrich, Ireland), mouse anti-huC/D (Santa Cruz, USA), rabbit anti-smooth muscle actin (Abcam, UK), rabbit anti-c-kit (Abcam, UK) and mouse anti-c-kit (Abcam, UK), all used at a dilution of 1:100 in PBS + 0.05% TritonX-100, overnight at 4 °C. Sections were then washed in PBS + 0.05% Tween and incubated with corresponding secondary antibodies; goat anti-mouse Alexa Fluor® 488, dilution 1:200 and goat anti-rabbit Alexa Fluor® 647, dilution 1:200, Abcam, UK, for 1 h at room temperature. After washing, sections were counterstained with DAPI antibody, dilution 1:1000 (Roche Diagnostics GmbH, Mannheim, Germany) for 10 min, washed, mounted and cover-slipped with Fluorescent Mounting Medium (DAKO Ltd, Cambridgeshire, UK). All sections were independently evaluated by two investigators with a LSM 700 confocal microscope (Carl Zeiss MicroImaging GmbH, Jena, Germany).
qRT-PCR
TRIzol reagent (Invitrogen) was used for the acid guanidinium-thiocyanate–phenol–chloroform extraction method to isolate total RNA from HSCR and control tissues (n = 6 for each group) according to the manufacturer’s protocol. Spectrophotometrical quantification of total RNA was performed using a NanoDrop ND-1000 UV–Vis spectrophotometer (Thermo Scientific Fisher, Wilmington, USA). The RNA solution was stored at − 20 °C until further use. cDNA synthesis and quantitative polymerase chain reaction reverse transcription of total RNA was carried out at 85 °C for 3 min (denaturation), at 44 °C for 60 min (annealing) and at 92 °C for 10 min (reverse transcriptase inactivation) using a Transcriptor High Fidelity cDNA Synthesis Kit (Roche Diagnostics, West Sussex, UK) according to the manufacturer’s instructions. The resulting cDNA was used for quantitative real-time polymerase chain reaction (qRTPCR) using a LightCycler 480 SYBR Green I Master (Roche Diagnostics, Mannheim, Germany) in a total reaction mix of 20 µl per well. The following gene-specific primer pairs were used: human KCNG3 (Eurofins) sense primer 5′ AGT ACT TCT TCG ACC GGC AC and Human KCNG3 (Eurofins) antisense primer 5′ AGA AGG AGA GCT CGC ACA TC, as well as Human KCNG4 (Eurofins) sense primer 5′ GGT CTT CAG CAA TGC CCA TG and Human KCNG4 (Eurofins) antisense primer 5′ CGG TAG TAA AGG CCC TTG ATG. For normalization purposes, real-time RT-PCR was performed for glyceraldehyde 3-phosphate dehydrogenase (GAPDH). GAPDH sense primer 5′ACA TCG CTG AGA CAC CAT GG and GAPDH antisense primer 5′ GAC GGT GCC ATG GAA TTT GC were used. After 5 min of initial denaturation at 95 °C, 55 cycles of amplification for each primer were carried out. Each cycle included denaturation at 95 °C for 10 s, annealing at 60 °C for 15 s, and elongation at 72 °C for 10 s. Relative mRNA levels of gene expression were determined using a LightCycler 480 System (Roche Diagnostics) and the relative changes in gene expression were normalized against the level of GAPDH gene expression in each sample. Experiments were carried out in duplicate for each sample and primer.
Statistical analyses
A one-way ANOVA was conducted to determine a statistically significant difference between aganglionic, ganglionic and healthy controls (p < 0.05). Data are presented as mean ± standard error. Specimens were classified into three groups: aganglionic (n = 6), ganglionic (n = 6) and healthy controls (n = 6).
Results
Immunofluorescence staining
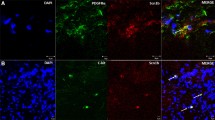
Immunohistochemistry in conjunction with confocal microscopy revealed that the distribution of KCNG3 and KCNG4 was decreased in both the aganglionic and ganglionic HSCR colon compared to normal controls. KCNG3 was found to be co-localised with ICC and neurons (Fig. 1), while KCNG4 was found to be co-localised with neurons and PDGFRα+ cells (Fig. 2), within both the myenteric and submucosal plexuses, as well as within the smooth muscle layer.
qRT-PCR
The relative mRNA expression levels of KCNG3 and KCNG4 were significantly decreased in both aganglionic and ganglionic HSCR specimens compared to normal controls (p < 0.05) (Fig. 3).
Discussion
Although the most striking histological feature in HSCR is the absence of ganglion cells, it is unlikely that this is the only cause of functional intestinal obstruction. There are a number of other histopathological findings, both in the aganglionic segment and in the proximal ganglionic segment in HSCR, which may account for the frequent discrepancy encountered between the length of the non-functional bowel and the degree of obstruction; and for the persistent obstructive symptoms after a pull-through operation for HSCR. The mechanisms underlying persistent bowel symptoms in HSCR patients who have had a properly performed pull-through operation are poorly understood and understudied. As mentioned earlier, gastrointestinal motility is controlled by four groups of cells, the ENS along with SMC, ICC and PDGFRα+ cells; the ‘SIP syncytium’. Together, these four networks of cells regulate secretory activities and peristalsis of the bowel.
K+ channels play important roles in excitable cells, in which they are involved in several physiological processes, such as action potential firing, neurotransmitter release and smooth muscle contractility [6]. The cell membrane potential hyperpolarizes whenever K+ channels open, as K+ flows out of the cell due to the large transmembrane electrochemical gradient cell excitability decreases. In the smooth muscle, this results in a reduction of contractility, that is mainly triggered by Ca2+ influx through voltage-gated Ca2+ channels following membrane potential depolarization in the different cell types within the SIP syncytium. In contrast, the inhibition of K+ channels that are open at the resting membrane potential in the SIP syncytium determines membrane depolarization, with an increase in voltage-gated Ca2+ channels opening and thus smooth muscle contraction. Furthermore, K+ channels also play a role in setting the resting membrane potential resulting in different levels of basal muscle tone which vary depending on their location within the gastrointestinal tract [6].
SMC are the effector cells of the bowel, and as a result of communication from the ENS, ICC and PDGFRα + cells, govern intestinal peristalsis. ICC are mesenchyme-derived cells that play a key role as pacemaker cells in gut, regulating the propagation of electrical slow waves in gastrointestinal muscle. They also modulate inhibitory and excitatory neurotransmission and have been shown to have stretch receptor activity in GI muscle [7]. The distribution of ICC varies depending on location within the bowel layers. Our research group has previously reported an absence of ICC in the aganglionic bowel in patients with HSCR, and a marked reduction in ICC in both the ganglionic and transition zone regions [8,9,10]. PDGFRα+ cells play a role in inhibitory neurotransmission as they contain purinergic receptors and have been found to display gap junctions with SMC [11]. Our group has previously reported a decreased expression of PDGFRα+ cells in HSCR colon [12].
Voltage-gated K+ channels contribute significantly to the excitability of several cell types, including neurons and cardiac myocytes. Based on sequence homology, the Shaker-related KV channel subunits are divided into eight subfamilies: KV1–KV6 and KV8–KV9. Members of the KV5, KV6, KV8 and KV9 subfamilies are collectively called “silent” subunits because they do not form functional homotetramer channels at the plasma membrane, but they assemble with KV2 subunits to form functional heterotetramers [3]. A study by Lee et al. identified several ion channels and transporters characteristic of ICC cellular identity and function in the murine colon, naming KCNG3 among them [4]. They reported, however, that prominent expression of the KCNG3 gene was surprisingly not accompanied by expression of other subunits. KCNG3 expression was found to be higher in colonic ICC versus jejunal ICC in the mice studied [4]. In another recent study by the same research group, Ha et al. reported high expression of the KCNG4 gene in murine colonic PDGFR+ cells [5].
In this study, we revealed a downregulation of the KCNG3 and KCNG4 genes in both ganglionic and aganglionic HSCR specimens compared to normal control colon. We also observed decreased KCNG3 and KCNG4 protein expression within the ganglionic and aganglionic HSCR specimens compared to normal control colon. The expression of KCNG3 and KCNG4 on neurons, ICC and PDGFRα+ cells of the human colon suggests a role for these proteins in colonic neurotransmission. The downregulation of both KCNG3 and KCNG4 in the ganglionic colon HSCR patients highlights the physiologically abnormal nature of the ganglionic segment in some HSCR patients. The presence of ganglion cells alone can no longer serve as a standard for HSCR patients who continue to have persistent bowel symptoms despite a properly performed pull-through operation. We need extensive studies on the proximal resected ganglionic segment in HSCR patients to search for subtle changes in the ENS, SIP syncytium and voltage-gated potassium channels to fully understand the etiology of the disease and better success in its treatment.
References
Heinemann S, Rettig J, Scott V, Parcej DN, Lorra C, Dolly J, Pongs O (1994) The inactivation behaviour of voltage-gated K-channels may be determined by association of alpha- and beta-subunits. J Physiol Paris 88(3):173–180
Regnier G, Bocksteins E, Marei WF, Pintelon I, Timmermans JP, Leroy J, Snyders DJ (2017) Targeted deletion of the Kv6.4 subunit causes male sterility due to disturbed spermiogenesis. Reprod Fertil Dev 29(8):1567–1575. https://doi.org/10.1071/RD16075
Bocksteins E, Snyders DJ, Holmgren M (2017) Independent movement of the voltage sensors in KV2.1/KV6.4 heterotetramers. Sci Rep 7:41646. https://doi.org/10.1038/srep41646
Lee MY, Ha SE, Park C, Park PJ, Fuchs R, Wei L, Jorgensen BG, Redelman D, Ward SM, Sanders KM, Ro S (2017) Transcriptome of interstitial cells of Cajal reveals unique and selective gene signatures. PLoS One 12(4):e0176031. https://doi.org/10.1371/journal.pone.0176031
Ha SE, Lee MY, Kurahashi M, Wei L, Jorgensen BG, Park C, Park PJ, Redelman D, Sasse KC, Becker LS, Sanders KM, Ro S (2017) Transcriptome analysis of PDGFRalpha + cells identifies T-type Ca2 + channel CACNA1G as a new pathological marker for PDGFRalpha + cell hyperplasia. PLoS One 12(8):e0182265. https://doi.org/10.1371/journal.pone.0182265
Curro D (2014) K(+) channels as potential targets for the treatment of gastrointestinal motor disorders. Eur J Pharmacol 733:97–101. https://doi.org/10.1016/j.ejphar.2014.03.049
Ward SM, Sanders KM (2006) Involvement of intramuscular interstitial cells of Cajal in neuroeffector transmission in the gastrointestinal tract. J Physiol 576(Pt 3):675–678
Rolle U, Piotrowska AP, Nemeth L, Puri P (2002) Altered distribution of interstitial cells of Cajal in Hirschsprung disease. Arch Pathol Lab Med 126 (8):928–933. https://doi.org/10.1043/0003-9985(2002)126%3C0928:ADOICO%3E2.0.CO;2
Piotrowska A, Solari V, Puri P (2003) Distribution of interstitial cells of Cajal in the internal anal sphincter of patients with internal anal sphincter achalasia and Hirschsprung’s disease. Arch Pathol Lab Med 127(9):1192–1195
Nemeth L, Puri P (2001) Three-dimensional morphology of c-Kit-positive cellular network and nitrergic innervation in the human gut. Arch Pathol Lab Med 125(7):899–904
Kurahashi M, Nakano Y, Hennig GW, Ward SM, Sanders KM (2012) Platelet-derived growth factor receptor alpha-positive cells in the tunica muscularis of human colon. J Cell Mol Med 16(7):1397–1404. https://doi.org/10.1111/j.1582-4934.2011.01510.x
O’Donnell A, Coyle D, Puri P (2016) Deficiency of platelet-derived growth factor receptor-α-positive cells in Hirschsprung’s disease colon. World J Gastroenterol 22(12):3335–3340
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
O’Donnell, A.M., Nakamura, H., Tomuschat, C. et al. Altered expression of KCNG3 and KCNG4 in Hirschsprung’s disease. Pediatr Surg Int 35, 193–197 (2019). https://doi.org/10.1007/s00383-018-4394-2
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00383-018-4394-2