Abstract
Background
Distraction-induced enterogenesis, whereby the intestine lengthens with application of linear forces, is an emerging area which may provide a unique treatment for short bowel syndrome. With an increase in overall tissue mass, there is an increase in oxygen and nutrient demand. We hypothesized that a neovascularization within the mesentery is necessary to support the growing small bowel.
Methods
A curvilinear hydraulic device was used to induce growth within the small bowel of Yorkshire pigs, and the intestine was harvested after 14 days. High-resolution gross pictures were recorded of the mesentery at implantation and at harvest, and CT imaging of the bowel and mesentery was performed at harvest after dye injection.
Results
After 2 weeks of distraction, an average of 72.5 % (8.7 cm) bowel lengthening was achieved. Gross images of the mesentery between major vessels showed a blossoming of the microvasculature and this was confirmed by CT imaging with 3D reconstruction. Mesenteric sample taken from the distracted segment had a fourfold increase in the volume of microvasculature versus controls.
Conclusion
Enterogenesis results not only in increased bowel length, but also significant increase in the mesenteric microvascularity. Presumably, this sustains the lengthened segment after application of longitudinal forces.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Background
Short bowel syndrome (SBS) is defined as insufficient surface area to absorb nutrients required for normal growth and development [1]. Typically this problem stems from surgical resection following necrotizing enterocolitis, gastroschisis, omphalocele, intestinal atresia and midgut volvulus [2]. These patients must gain much, if not all, of their nutrition and hydration through the parenteral route. Though strategies are improving in maintaining this population there remains a reported 10–30 % 5-year mortality rate [3]. This is due to the numerous complications that arise from the underlying disease process and from the administration of parenteral nutrition (PN) including systemic infections [4].
There is a period of rapid growth and adaptation in the neonatal period [5] leading to high variability in the outcome of the children affected with this disorder. The ability of a child to wean from PN is dependent on the length of bowel after resection [6] and likely the anatomical segment remaining [7]. Children with less than 10 % of their predicted small bowel length remaining are far less likely to ever wean from PN compared to those with greater lengths [8].
For those unable to wean from PN, current treatment strategies include administration of growth hormones [9], surgical lengthening of the small bowel [10, 11], and small bowel transplantation [12] with widely variable results. Mechanotransduction is an emerging area of research which may provide a novel treatment for SBS. With mechanotransduction, growth is induced by stress through the conversion of mechanical force to chemical signals that stimulate hypertrophy or hyperplasia [13]. This principle has been described for centuries in long bone osteogenesis [14] and has also since been applied to soft tissue for reconstruction [15]. Distraction-induced enterogenesis is a process whereby the intestine lengthens with application of intraluminal linear forces.
Functional lengthening of small bowel has been shown in several animal models [16–19]. This function of the newly grown bowel is supported by diverse morphologic evaluation, epithelial cell proliferation, mucosal permeability, and disaccharidase activity [17]. Our lab has produced ~2-fold increases in small bowel length over a period of 2 weeks. We hypothesized that a neovascularization within the mesentery is necessary to support the growing small bowel, and that this new vascularity further supports a mechanism for the production of enterogenesis.
Methods
We conducted an investigation of vessel growth using our well-established swine model for distraction enterogenesis. In collaboration with the University of Michigan Mechanical Engineering department, a specially designed and machined curvilinear hydraulic device was produced (Fig. 1). We elected to perform experiments on a series of Yorkshire pigs weighing 30–50 kg due to our previous experience with the species. The surgical procedure proceeded through a midline laparotomy incision. A Roux limb was created approximately 90 cm from the ligament of Treitz. The proximal end of the distal bowel was brought out in the left lower abdomen and matured. This allowed for drainage of mucus production as well as access and manipulation of the device as the hydraulic line exited the bowel through this stoma. The remaining bowel was placed back into continuity with a side-to-side functional end-to-end anastomosis creating a typical Roux-En-Y configuration. The mesenteric defect was closed to prevent internal hernia. The device was secured into the Roux limb with a series of buttressed silk sutures. The antimesenteric border was marked with stitches to aid in the identification of growth. High-resolution, gross photographs were recorded of the mesentery at implantation. The abdomen was then closed in standard fashion.
After 24 h of recovery, the device was progressively expanded with 0.55-cc injections of water twice daily. The injection schedule was calculated based on an expected rate of distraction to allow a twofold increase over a 13-day period per our laboratory’s previously established protocols. Tissue harvest was performed on post-operative day 13 upon completion of expansion. Harvest was completed through a repeat laparotomy. The lengthened segment of bowel and a more distal control segment of bowel and their corresponding mesentery were visualized and photographed with high-resolution photography and were then harvested for analysis. Although the photographed areas showed increases in vascularity, they were predominately a qualitative inference of vascularization.
Multiple avenues of vascular quantification were attempted in separate pigs. Many techniques were considered and three different approaches were explored. The first method attempted was immunofluorescence (IF). Sections were embedded in an optimum cutting temperature compound (Tissue-Tek; Sakura Finetechnical, Tokyo, Japan) and snap frozen. Immunofluorescent staining was performed as previously described [20] with monoclonal anti-smooth muscle actin antibodies (F3777 Sigma, Sigma-aldrich; working dilution, 1:1,000). Confocal imaging and quantification involved visualization of multiple stained slides and included focusing on six random high-powered (20×) images of each slide. Then manual isolation of vessel area using software ImageJ provided a total vessel area. The vessel data was tabulated and compared between groups.
A second method attempted to quantify vascular differences was the use of laser Doppler. Blood flow to the mesentery was measured using a Lisca PIM II Laser Doppler Perfusion Imager (Perimed AB, Stockholm, Sweden), placed 11 cm above the bowel segment of interest. Blood flow patterns were analyzed using LDPIwin 2.3 software (Perimed AB) as previously described [21].
In our latest experiment, prior to explanting the device and small bowel, Microfil® Injection compound (Flow Tech, Inc., Carver, MA, USA) was injected to aid in vascular analysis. In short, a 24-gauge angiocatheter was introduced into large branches of the superior mesenteric artery (SMA) to allow for investigation of the selected vascular bed. Associated large draining veins were isolated and encircled with 2-0 silk suture but not tied. These veins were cut to allow drainage of blood. Heparinized normal saline (300 cc) was injected into the angiocatheter to flush the mesentery of interest clear of blood. The product was prepared according to the manufacturer’s instructions. A 5:4 ratio of MV-diluent to MV-122 yellow compound was mixed on the back table. The mixture was catalyzed with 5 % (by volume) of MV Curing Agent. Selective injection into the SMA branches was performed until the Microfil® compound was seen leaking from the previously cut mesenteric veins. Both the cannulated artery and the draining veins were ligated with previously placed silk ties. Samples of mesentery were taken from the lengthened portion, as well as more distal non-surgically manipulated segment of small bowel (control). After a 24 h incubation (4 °C) period the tissue was taken for CT scanning. Images were then analyzed with GE Healthcare’s Microviewer software.
The tissue was taken to the University of Michigan’s Center for Molecular Imaging for microCT analysis. GE Healthcare’s Microviewer software was utilized to identify and isolate microvasculature in each two-dimensional CT plane. The area of interest was outlined by hand in each 2D image separating large mesenteric vessels from microvasculature. The compilation of these images was used to construct a three-dimensional (3D) polygonal isosurface (48.05 μm/cubic voxel) on which vessel density analysis could be computed. Volumetry was based on the difference in Hounsfield units.
While vascular growth was our primary aim, microscopic evaluation for histomorphology of the bowel was performed. Transepithelial resistance (TER) was also investigated as a measure of mucosal viability via Ussing chamber analysis for epithelial barrier function (EBF).
Results are expressed as the mean ± SD, and paired t tests were used for statistical comparison.
Results
All animals survived without complication to the study endpoint. Weight was unchanged from implantation to harvest date (37.2 ± 2.9 vs. 38.7 ± 2.9, P = 0.73). Bowel lengthening was achieved as the distracted segment was 72.5 % (8.7 cm) larger at the time of explant. Morphologic evaluation was notable for a statistically significant increase in villus height in the stretched portion of the bowel (438.8 ± 12.96 vs. 501.2 ± 10.36, P < 0.05). Although crypt depth decreased, the differences were not significant (329.0 ± 14.92 vs. 371.0 ± 23.90, P > 0.17). Ussing chamber analysis was similar to our previously published [17] data showing a decrease in TER in the distracted segment (112.5 ± 0.037 vs. 84.72 ± 0.03, P < 0.05).
Mesenteric vascular imaging with immunofluorescence is shown in Fig. 2. This demonstrated a challenge in the interpretation and quantification of vascularization due to the variability of vessel size in any given sampled area (Fig. 2a). Attempts at quantifying the degree of vascularity with ImageJ is shown in Fig. 3. This showed wide variability among independent researchers. Variability within individual slides made reproducibility challenging (Figs. 2, 3). The first researcher reported a significant decrease in vessels in the distracted group (11,650 ± 1,582 vs. 4,969 ± 1,336, P < 0.05). The second researcher found no difference (5,224 ± 1,709 vs. 6,920 ± 1,868, P = 0.57). A third researcher found a significant increase in vessels in the distracted group (4,180 ± 1,066 vs. 16,080 ± 360, P < 0.05). To compensate for this variability we then imaged larger 1 cm2 portions of mesentery to reduce variability (Fig. 2b). Multiple high-power confocal images were merged with a tiling technique. This alternative immunofluorescence technique also had poor reproducibility and high degree of variation between multiple investigators and was thus abandoned.
Immunofluorescence imaging using anti-smooth muscle antibody. a Three representative images are shown for each study group, control and distracted. This image demonstrates the intra-sample variability in amount of immunofluorescence staining of vasculature in the mesenteric samples. b Confocal, tiled representative image of mesentery. In this case the mesentery was scanned to an area of 1 cm2. However, this further broadening of the scanned image failed to obviate the problems seen with single image analysis
Laser Doppler was imaged on the anti-mesenteric surface of the small bowel serosa and the mesentery (Fig. 4). These results showed a significant increase in mesenteric flow at the time of the harvest when compared to the time of implant (0.89 ± 1.18 vs. 0.57 ± 0.9, P < 0.05). Despite the increase in vascularity by Doppler studies, two flaws in this comparison were noted. This includes problems with the precise placement of the defined region of interest which could markedly change the results, as well as the overall sensitivity of the Doppler’s ability to quantify microvessels. Because of this, we elected to explore more robust measurements.
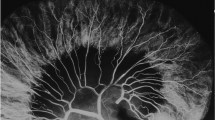
We finally approached our mesenteric vessel analysis in a different manner. First, we inspected and took high-resolution gross photographs of the mesentery. This showed a blossoming of the microvasculature between major vessels. Figure 5 gives visual representation of our findings. Two-dimensional imaging was again difficult to quantify. The GE Healthcare’s Microviewer software was next utilized to identify and isolate microvasculature in each two-dimensional CT plane, and this is shown in the selected image by the dotted area. A 3D polygonal isosurface (3D structure) was generated. The percentage of vessels within the generated 3D polygon was reported based on volumetry using the difference in Hounsfield units. The mesenteric sample taken from the distracted segment had a vessel volume of 8.3 % of the total volume isolated compared to 2.0 % in the mesenteric sample taken from the control segment. This was a fourfold increase in the volume of microvasculature versus controls as measured by dye density. Although not quantitative, a raw 3D reconstruction of the mesentery including the large vessels is shown in Fig. 6. This displays a clear visual evidence of this increase in vascularization with distraction-induced growth.
Photo-insets: gross photograph insets depict an increase in branching from major mesenteric vessels in the distracted sample. Upper panel representative two dimensional CT images used to isolate the microvasculature (outlined in orange dotted lines). Lower panel multiple images were then used to generate a 3D reconstruction of the mesentery excluding the major vessels (yellow structures). This was analyzed for differences in microvascular density by way of CT volumetry. Two control segments are shown from same sample to aid in interpretation of data
Discussion
There is growing evidence in the literature that the application of mechanotransduction to the small bowel results in a lengthening of the intestine. The results of this study showed reproducibility in our swine model for distraction-induced enterogenesis with a hydraulic driven device. Morphology was notable for an increase in villus height, increase in crypt depth (non-significant) and decline in the TER, similar to our previous reports [17, 18]. In this present work, we utilized a modification of the hydraulic device by engineering it in a curvilinear configuration. This allowed for the potential of a near threefold increase in lengthening, and the curvilinear shape allowed it to expand more easily within the confines of the abdominal cavity. We hypothesized that this increase in tissue volume would require an increase in blood flow to compensate for the increase in nutrient and oxygen demand. Our study showed that a striking volume of neovascularization was seen after a twofold expansion of the small intestine.
While Microfil® has been used for decades, this combination of Microfil® contrast agent with GE Healthcare’s Microviewer software is a new method of vessel analysis that has great promise to quantify changes in vascularity [21, 22]. Our lab had struggled to quantify neovascularization in the past relying on methods that have proven unreliable. As well, demonstration of increases in vascularization with tissue engineering has been quite challenging [15].
In an attempt to quantify mesenteric vasculature, we had considered many techniques prior to microCT volumetry. While the process of IF staining is straightforward, quantification was challenging. The vascularization within mesentery varies greatly. Whether from a distracted or control segment, a dramatically different amount of vessel staining within that sample can occur even if only separated by a few millimeters (Figs. 2, 3). This is due to a few simple phenomena.
Sections in one area can sample a region of high vessel density while a shift in only a few millimeters can sample a region of relative vascular paucity. Secondly, vessels are three-dimensional structures; the 2D slide can vary greatly depending on the plane of transection of that vessel. Lastly, sampling of a section with large vessels and microvasculature can make the microvascular measurement obsolete as the large volume vessels will predominate in the calculation. Random sampling of the mesentery becomes a game of chance rather than that of empiric evidence. In this study, two researchers found statistical significance. However, their data was conflicting as one showed an increase and the other a decrease in vascularity in distracted mesentery versus control. Our attempt to reduce variability (Fig. 2b) with multiple high-power microscopic images merged in a tiling technique proved to not sufficiently overcome these aforementioned issues.
A second method attempted to quantify vascular differences was the use of laser Doppler. This tool gives real-time interpretation of blood flow in a defined region. While this technique could allude to vessel volume, it was also flawed. Flow is calculated over a defined area selected by the user which results in quantification of flow in that region (Fig. 4). In this study, the same area of mesentery was measured at implant and harvest. At first the images seem reliably accurate. However, the region of interest is small and even a few millimeter adjustments could skew the data greatly. The inclusion or exclusion of a large mesenteric vessel could change the output drastically. Additionally, this method is only sensitive enough to quantify large vessels and the branching microvasculature visualized by the naked eye was not tabulated in the final vessel volume by the software.
The technique described in this study using Microfil® and GE Healthcare’s Microviewer software provided the most optimal answers to the problems with the previous vascular analytical tools. While the Microfil® data in this study includes only one animal, the potential for continued research with this technique is vast, and this technologic method needs to be repeated multiple times for validation. Nevertheless, our data shows an extensive expansion of the microvasculature in the mesentery with distraction enterogenesis and this new technique may prove a more reliable method of vessel quantification than previous measures.
One could call this imaging technique into question as the user must select the area of mesentery between the major vessels. This does leave room for operator error and biased sampling. However, this is an imperative step to this technique as a difference in the number of large feeding vessels will drastically skew the volume computed by the software. It is the authors’ contention that if 3D imaging is paired with the representative slices used to construct the polygonal isosurface used for data computation, much of the ambiguity will be eliminated. Secondly, this approach does not account for increases in diameter or length in the large mesenteric vessels as they are excluded from analysis. While not performed in this study, a similar method of isolation could be performed on single vessels should the researcher desire.
In addition to repeating these visual data, our future goal is to correlate these findings to possible mechanisms of action. An evaluation of growth factors (e.g., vascular epithelial growth factor, fibroblast growth factor, and platelet derived growth factor) and their corresponding receptors may add insight to the underlying pathophysiology of what is shown in this study.
Concluding remarks
Enterogenesis results in increased bowel length. This study shows a significant increase in the mesenteric microvascularity as well. Presumably, this sustains the lengthened segment after application of longitudinal forces by providing nutritional support and oxygen delivery to the newly grown tissue. This adds additional evidence that distraction enterogenesis produces functional, lengthened small bowel and may lead to a future treatment of SBS.
References
Cole CR et al (2008) Very low birth weight preterm infants with surgical short bowel syndrome: incidence, morbidity and mortality, and growth outcomes at 18 to 22 months. Pediatrics 122(3):e573–e582
Sigalet DL (2001) Short bowel syndrome in infants and children: an overview. Semin Pediatr Surg 10(2):49–55
Hess RA et al (2011) Survival outcomes of pediatric intestinal failure patients: analysis of factors contributing to improved survival over the past two decades. J Surg Res 170(1):27–31
Torres C et al (2007) Role of an intestinal rehabilitation program in the treatment of advanced intestinal failure. J Pediatr Gastroenterol Nutr 45(2):204–212
Struijs MC et al (2009) Establishing norms for intestinal length in children. J Pediatr Surg 44(5):933–938
Messing B et al (1999) Long-term survival and parenteral nutrition dependence in adult patients with the short bowel syndrome. Gastroenterology 117(5):1043–1050
Turner JM et al (2011) Novel neonatal piglet models of surgical short bowel syndrome with intestinal failure. J Pediatr Gastroenterol Nutr 52(1):9–16
Spencer AU et al (2005) Pediatric short bowel syndrome: redefining predictors of success. Ann Surg 242(3):403–409 (discussion 409–412)
Peretti N et al (2011) Growth hormone to improve short bowel syndrome intestinal autonomy: a pediatric randomized open-label clinical trial. J Parenter Enteral Nutr 35(6):723–731
Kim HB et al (2003) Serial transverse enteroplasty (STEP): a novel bowel lengthening procedure. J Pediatr Surg 38(3):425–429
Bianchi A (1997) Longitudinal intestinal lengthening and tailoring: results in 20 children. J R Soc Med 90(8):429–432
O’Keefe SJ et al (2006) Short bowel syndrome and intestinal failure: consensus definitions and overview. Clin Gastroenterol Hepatol 4(1):6–10
Hoffman BD, Grashoff C, Schwartz MA (2011) Dynamic molecular processes mediate cellular mechanotransduction. Nature 475(7356):316–323
Codivilla A (1904) On the means of lengthening, in the lower limbs, the muscles and tissues which are shortened through deformity. Clin Orthop Relat Res 1994(301):4–9
Nishisho T, Yukata K, Matsui Y, Matsuura T, Higashino K, Suganuma K, Nikawa T, Yasui N (2012) Angiogenesis and myogenesis in mouse tibialis anterior muscles during distraction osteogenesis: VEGF, its receptors, and myogenin genes expression. J Orthop Res 30(11):1767–1773. doi:10.1002/jor.22136
Park J et al (2004) Enterogenesis by mechanical lengthening: morphology and function of the lengthened small intestine. J Pediatr Surg 39(12):1823–1827
Koga H et al (2012) Distraction-induced intestinal enterogenesis: preservation of intestinal function and lengthening after reimplantation into normal jejunum. Ann Surg 255(2):302–310
Okawada M, Maria HM, Teitelbaum DH (2011) Distraction induced enterogenesis: a unique mouse model using polyethylene glycol. J Surg Res 170(1):41–47
Stark R et al (2011) Restoration of mechanically lengthened jejunum into intestinal continuity in rats. J Pediatr Surg 46(12):2321–2326
Feng Y, McDunn JE, Teitelbaum DH (2010) Decreased phospho-Akt signaling in a mouse model of total parenteral nutrition: a potential mechanism for the development of intestinal mucosal atrophy. Am J Physiol Gastrointest Liver Physiol 298(6):G833–G841
Johnston M et al (2005) Subarachnoid injection of Microfil reveals connections between cerebrospinal fluid and nasal lymphatics in the non-human primate. Neuropathol Appl Neurobiol 31(6):632–640
Lin G et al (1984) Microvasculature architecture of small liver metastases in man. A correlation between microfil vascular preparations and histologic sections. Invest Radiol 19(4):296–302
Acknowledgments
The study was supported by Hartwell Biomedical Research Award, FDA P50 Pediatric Device Consortia Grant 2-P50-FD-003787-03; and NIH 2R44DK085765-02.
Author information
Authors and Affiliations
Corresponding author
Additional information
Matthew W. Ralls and Ryo Sueyoshi contributed equally to this study.
Rights and permissions
About this article
Cite this article
Ralls, M.W., Sueyoshi, R., Herman, R.S. et al. Mesenteric neovascularization with distraction-induced intestinal growth: enterogenesis. Pediatr Surg Int 29, 33–39 (2013). https://doi.org/10.1007/s00383-012-3204-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00383-012-3204-5