Abstract
Purpose
To present a new technique of retroperitoneoscopic Anderson–Hynes dismembered pyeloplasty (AHDP) in infants and children with ureteropelvic junction obstruction (UPJO) based on our clinical experience.
Methods
From March 2003 to February 2007, retroperitoneoscopic AHDP was performed in 60 (44 boys and 16 girls) UPJO infants and children with a three-port lateral retroperitoneal approach. The retroperitoneal space was entered via a 1-cm longitudinal incision beneath the 12th rib and further developed by a glove balloon. Video-retroperitoneoscopy was undertaken with a 5-mm laparoscope between the mid axillary line and 1 cm away from the superior border of iliac crest. Dismembered pyeloplasty was carried out with the Anderson–Hynes anastomosis where 5-0 or 6-0 vicryl sutures were over a double-J ureteric stent. Anastomosis was completed with freehand intracorporeal suture techniques. Follow-up studies were conducted by intravenous urography and renal ultrasonography.
Results
Among the 60 patients (62 sides) with retroperitoneoscopic AHDP, only the first two cases were converted to open surgery due to difficulties in developing the retroperitoneal space, and the remaining cases succeeded. The average operative time was 70 ± 12.6 min (ranging from 55 to 130 min), the average estimated blood loss was 10 ± 2.2 ml (ranging from 5 to 20 ml), and the average postoperative hospital stay was 7 ± 1.3 days (ranging from 3 to 15 days). Aberrant artery vessel was intraoperatively observed in seven patients. Postoperative urinary leakage occurred in two patients, but spontaneously disappeared on the 6th and 11th days after the surgery, respectively; and one of them underwent open surgery for recurrent UPJO 8 months later. During an average follow-up period of 24 months, we performed radiographic assessment by intravenous urography and found that all the cases showed good results except the patient who underwent open surgery later.
Conclusions
Our experience with retroperitoneoscopic AHDP demonstrates that this technique is safe, effective and time saving for treating UPJO in infants and children.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The surgical correction of ureteropelvic junction obstruction (UPJO) has been a urological challenge for more than one century. Open Anderson–Hynes dismembered pyeloplasty (AHDP) via a retroperitoneal approach remains the gold standard for correcting UPJO in children and adults, with a success rate of >90% [1, 2]. Schuessler et al. [3] first introduced laparoscopic pyeloplasty in 1993, which has become a popular option since it is minimally invasive and a success rate comparable to that of open pyeloplasty. In addition, this method has advantages of low morbidity, short hospital stay and convalescence. Laparoscopic pyeloplasty can be performed through the transperitoneal or retroperitoneal approach. Retroperitoneoscopic AHDP has the virtues of open pyeloplasty and without the morbidity of a large flank incision. The application of retroperitoneoscopic AHDP in children was not as popular as in adults [4, 5]. With technical improvements, we found that retroperitoneoscopic AHDP is feasible and effective, even in infants and children. In this study, we summarized our experience of retroperitoneoscopic dismembered pyeloplasty in infants and children.
Patients and methods
Patients
From March 2003 to February 2007, we performed retroperitoneoscopic AHDP in 60 patients (62 sides) including 44 boys and 16 girls, with an average age of 36 months (ranging from 4 to 168 months, 7 cases under 1 year) at two institutions (Tongji Hospital and Wuhan Children’s Hospital). Among them, 39 had UPJO on the left side, 19 on the right side, and two had bilateral obstruction. The two sides of the bilateral cases were corrected with a 6- and 8-week interval, respectively.
The diagnosis was established by renal ultrasonography, intravenous urography (IVU) with high-volume contrast medium, magnetic resonance urography, retrograde pyelography or/and diuretic renal scans. There were 41 patients with a symptom: 14 showed mild to moderate flank or abdomen pain; 12 presented urinary tract infection; and 15 had abdominal masses. Nineteen patients were asymptomatic, 11 of them showed prenatal diagnosed hydronephrosis, 6 of them were discovered incidentally by renal ultrasonography for health examination, and 2 were discovered incidentally by computed tomography for abdomen injury. No patients had undergone renal surgery previously. Before the operation, all the patients had hydronephrosis with a dilated extrarenal renal pelvis, and among them, 18 were moderate and 42 were severe. Patients with urinary tract infection received antibiotic therapy until the urine was sterile. Preoperative preparations were performed according to the standard procedure.
Intravenous urography and renal ultrasonography were performed 3 and 6 months after the operation, respectively, and then follow-up examinations were performed annually.
Operative techniques
The patients were subjected to general endotracheal anesthesia and fixed on the operating table at the lateral decubitus position. The involved side was over-extended by raising the lumbar bridge of the table and straightening the ipsilateral limb, so that the space between the costal margin and the iliac crest was maximally exposed. A three-port balloon-dissecting retroperitoneal laparoscopic approach was employed, as previously described [6]. Briefly, a 1-cm skin incision was made below the 12th rib at the posterior axillary line, and the peritoneum was pushed forward by the index finger. The balloon expansion was carried out to make a retroperitoneal working space through two sessions of inflation, and 200–300 ml (500 ml for older children) of air was injected to maintain the balloon dilation for 3–5 min. Under the guidance of the index finger, a 3- or 5-mm trocar was introduced under the subcostal margin along the anterior axillary line, and another 5-mm trocar was put above the iliac crest in the mid axillary line for the laparoscopy. Then, a third 5-mm trocar was inserted into the 1-cm incision. CO2 was bubbled to maintain a pressure of 8–12 mmHg.
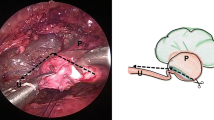
Gerota’s fascia was cut longitudinally, and the peri-renal fat was removed to reveal the posterior surface of the kidney. The lower pole of the kidney was identified. Then the dilated renal pelvis and the upper ureter were fully freed by blunt and sharp dissection, and the status of UPJO was assessed (Fig. 1a). The renal pelvis was partially separated from the most dependent part, cephalad toward the renal pelvis, and the most dilated renal pelvis remained undismembered. Then, the ureter was spatulated laterally and extended inferiorly through its narrow portion for about 2 cm (Fig. 1b). A 3# catheter was placed to avoid suturing the anterior wall to the posterior walls of the ureter. The most dependent part of the renal pelvis was sutured to the apex of the spatulated ureter with 5-0 or 6-0 vicryl suture (6-0 for children under three) (Fig. 1c). After anchoring with the suture, the renal pelvis and the ureter were further trimmed, the catheter was removed, and the stenotic segment of UPJ and the redundant renal pelvis were dissected. The posterior ureteropelvic anastomosis was achieved with a running suture, where every two sutures were coupled with a lock-stitch suture. The rest of the renal pelvis was closed with a running suture or an interrupted suture. A double-J stent (4.7F or 5F for children, 3F for infants) was antegradely inserted (Fig. 1d), with its proximal end put in the renal pelvis. For the doubtful cases, the fluoroscopy analysis was performed to verify the stent position in the urinary tract. The anterior wall of the ureteropelvic anastomosis was closed with an interrupted suture (Fig. 1e). The sutures were placed and tied intracorporeally, and all the knots were outside the urinary tract. If there was a posterior crossing vessel, the vessel was transposed ventrally to the UPJ for anastomosis. A closed suction drain was placed through the port incision in the mid axillary line into the perinephric space near the repaired site. Bleeding was monitored carefully after the pneumoretroperitoneum pressure was lowered. Then, CO2 was evacuated and the port sites were closed. Prophylactic antibiotics (a third-generation cephalosporin) were routinely used. The Foley catheter was removed 2 or 3 days after the operation. The closed suction drain was removed if the drainage output had not increased and there was less than 10-ml output in 24 h after the Foley catheter was removed. The double-J stent was removed 4–6 weeks after the surgery.
Retroperitoneoscopic Anderson–Hynes dismembered pyeloplasty. a The lower and middle poles of the kidney are separated and freed to fully expose the upper segment of the renal pelvis and ureter. The causes of the obstruction were evaluated. b The renal pelvis is cut along a curve parallel to the kidney edge to partially divide the renal pelvis; and the ureter is longitudinally incised with the cut beyond the obstruction site by 1–2 cm. c A 5-0 vicryl suture is used to combine the lower corner and the lowest end of the cut ureter. d A double-J stent is placed in an antegrade manner. e The anterior wall of anastomosis is sutured with an interrupted suture. f After the pyeloplasty, the ureteropelvic part assumes a funnel shape
Results
In this study, we successfully performed 60 retroperitoneoscopic AHDP in total. Only two patients (3%) were converted to open surgery due to dense perinephric adhesions and profuse oozing during the process of developing the retroperitoneal space. These two cases occurred at the very beginning of our practice. An aberrant artery vessel crossing on the dorsal side of UPJ was observed in seven patients, and all the vessels were transposed ventrally to UPJ. No major intraoperative complications took place, such as injury to adjacent organs and major vessels.
The average operative time was 70 ± 12.6 min (ranging from 55 to 130 min), and the average estimated blood loss was 10 ± 2.2 ml (ranging from 5 to 20 ml). None of the patients required blood transfusion. Oral nutrition administration was resumed 1–2 days after the surgery. Hospital stay was between 3 and 15 days, with an average postoperative stay of 7 days. Most patients preferred to be discharged when the sutures were removed. The average time of returning to normal activity was 16.5 ± 3.5 days (ranging from 11 to 27 days). Postoperative urinary leakage occurred in two patients, but spontaneously disappeared on the 6th and 11th day after the surgery, respectively. Other than these two cases, all the patients made a rapid and smooth recovery, and the average follow-up time was 24 months. IVU and renal ultrasonography demonstrated a fluent UPJ anastomosis and resolution of hydronephrosis, except one of the two leakage cases later underwent open surgery for recurrent UPJO 8 months after the operation. This recurrent obstruction was due to a fibrotic scar around UPJ, which was caused by the earlier urine extravasations. The other 59 patients showed good results with an average follow-up period of 24 months.
Discussion
Open AHDP remains the reference standard for treating PUJO for all kinds of pathological origins, including a crossing vessel in children. The limitations of open surgery are obvious: massive surgical trauma, incision pain and protracted recovery. With the development of techniques and equipments of minimally invasive procedure, antegrade and retrograde endopyelotomies have been improved for the UPJO treatment. However, the lower success rates and the risk of bleeding of these surgeries significantly hamper the extensive application of endopyelotomies in clinical practice [7, 8].
Recently, laparoscopic techniques have been developing quickly in the field of urological surgery. Laparoscopic pyeloplasty achieves a success rate that is close to and sometimes even higher than that of open surgery [6]. Bauer et al. [9] found that the success rate of laparoscopic AHDP is as high as 98% compared with the success rate of 94% in open surgery; and no significant difference is observed in hydronephrosis. But 3 months after the operation, the patients in the open surgery group still have pain of lumbar region, while those in the laparoscopic operation group all resume normal activities, suggesting that laparoscopic operation has the advantages of small incision and quick postoperative recovery. Chen et al. [10] compared the long-term effects of the three procedures for the UPJO treatment and found that the curative rate of endopyelotomy is lower than that of the open surgery especially in the presence of distorted ureter, crossing vessels and other factors. Compared with endopyelotomy, laparoscopic pyeloplasty has a similar time of hospital stay and postoperative recovery, but it has the results comparable to that of open surgery.
The laparoscopic Anderson–Hynes procedure can be performed in two different ways: transperitoneal and retroperitoneal. In the transperitoneal approach, the operative space is large, the operative marking is distinct and the operative field is better lighted due to the peritoneum reflection. On the other side, this approach may disturb other intraperitoneal organs, such as stomach and intestines, which can result in complications. In particular, urine leakage caused by the operation may lead to some serious consequences. For the retroperitoneal approach, although the operative field is less clear, it involves fewer intraperitoneal organs, and urine leakage is easy to handle. Thus, the retroperitoneal approach is more suitable for urological surgery [6] since it makes a direct access to the target lesion and causes less injury. The advantages of this approach would be even more obvious when the operator is skillful in establishing the retroperitoneal space and familiar with its topographical features.
It is usually assumed that the small size of the retroperitoneal space in children restricts the manipulation of laparoscope, and this notion has hampered the laparoscope application. Moreover, difficulties in intraperitoneal suture with reconstructive surgery limits the introduction of retroperitoneal laparoscopic Anderson–Hynes operation.
In the past 4 years, we performed the laparoscopic Anderson–Hynes operation via the retroperitoneal approach in 60 infants and children and obtained very good results. Our topographic study under laparoscope showed that the retroperitoneal space contained less fat tissue and was anatomically less complicated, and vessels could be clearly seen under laparoscope. Moreover, there was less local inflammatory adhesion and loose tissues were easy to be separate under aeroperitoneum. All these conditions facilitate the laparoscopic Anderson–Hynes operation in children. Thus, despite the relatively small size of the retroperitoneal space, the operations of separation and anastomosis were not substantially affected in practice. In fact, according to our experience, target lesions were well exposed, and the operations went smoothly without major complications. The lumbodorsal fascia of children was thin and weak so that there was less sensation of rupture when penetrated. Since the peritoneum of children was thin, during the process of preparing the retroperitoneal space, 200–300 ml air was injected in two sessions of gas insufflations to avoid the peritoneum rupture caused by over-inflation. In addition, the air injected into the peritoneum might suppress the retroperitoneal space and thus hamper the operation. For the first two patients in our study, the laparoscopic operation was converted to open surgery because unskillful manipulation caused peritoneal rupture at the early stage. The children were made to take a lateral decubitus position, and the first trocar incision was made below the 12th rib along the posterior axillary line, and the trocar was placed at the footward end before suturing. Thus, after the laparoscope was introduced into the peritoneal cavity, the erector muscle of spine could be easily identified once the trocar was found. This procedure not only facilitated the opening of Gerota’s fascia without causing injures to peritoneum, but also prevented the trocar from getting too close to the rib, allowing the movement of the trocar. We suggest that the retroperitoneal space should be developed outside the Gerota’s fascia to avoid the formation of dangling fat patches, which blurs the operative field. The retroperitoneal space in children was small and postoperative gas leakage can make the space even smaller, which would affect operative manipulation. Furthermore, gas flow should be performed at the maximal level to supplement the gas. Finally, before the operation, the catheter should be closed by a clamp to facilitate the insertion of double-J stent.
Our experience about the freeing of kidney and pyeloplasty can be summarized as follows. First, it is not necessary to free the kidney completely, and the partial separation of the middle-lower part is enough, which helps minimize the operative trauma and complications. The upper part of the ureter should be minimally separated as long as the tension-free anastomosis can be achieved. Second, before cutting the renal pelvis, the pelvis should be spatulated first. The cutting of redundant renal pelvis and severing of stenotic segment of ureter should be performed after the anastomosis of lower corner of pelvis with the lowest part of the cutting end of ureter. This can prevent the twisting of ureter and maintain traction during anastomosis. The manipulation on the retroperitoneal space in children is very important. Third, for the suturing during pyeloplasty, the posterior wall should be sutured before the anterior wall. We used both continuous and over-and-over whip sutures for the posterior wall: with every two stitches, an over-and-over whip suture was made. This cannot only take advantage of the fast speed of continuous suture technique, but also prevent anastomotic stenosis (due to over-traction) and urine leakage (due to loose traction). Fourth, the double-J stent should be placed antegradely via the anterior wall of the stoma, when the guide wire is inserted all the way to the bladder and the other end of the stent is advanced to the renal pelvis. The double-J stent should be chosen carefully based on the age and height of children (i.e., 4.7F or 5F for children and 3F for infants).
In conclusion, our study shows that retroperitoneoscopic AHDP is effective and safe and causes fewer complications. Thus, it represents a new alternative for the UPJO treatment.
References
Mikkelsen SS, Rasmussen BS, Jensen TM, Hanghøj-Petersen W, Christensen PO (1992) Long-term follow-up of patients with hydronephrosis treated by Anderson–Hynes pyeloplasty. Br J Urol 70(2):121–124. doi:10.1111/j.1464-410X.1992.tb15688.x
Nguyen DH, Aliabadi H, Ercole CJ, Gonzalez R (1989) Nonintubated Anderson–Hynes repair of ureteropelvic junction obstruction in 60 patients. J Urol 142(3):704–706
Schuessler WW, Grune MT, Tecuanhuey LV, Preminger GM (1993) Laparoscopic dismembered pyeloplasty. J Urol 150(6):1795–1799
El-Ghoneimi A, Farhat W, Bolduc S, Bagli D, McLorie G, Aigrain Y, Khoury A (2003) Laparoscopic dismembered pyeloplasty by a retroperitoneal approach in children. BJU Int 92(1):104–108. doi:10.1046/j.1464-410X.2003.04266.x
Yeung CK, Tam YH, Sihoe JD, Lee KH, Liu KW (2001) Retroperitoneoscopic dismembered pyeloplasty for pelvi-ureteric junction obstruction in infants and children. BJU Int 87(6):509–513. doi:10.1046/j.1464-410X.2001.00129.x
Zhang X, Li HZ, Wang SG, Ma X, Zheng T, Fu B, Zhang J, Ye ZQ (2005) Retroperitoneal laparoscopic dismembered pyeloplasty: experience with 50 cases. Urology 66(3):514–517. doi:10.1016/j.urology.2005.04.007
Danuser H, Ackermann DK, Bohlen D (1998) Endopyelotomy for primary ureteropelvic junction obstruction: risk factors determine the success rate. J Urol 159(1):56–61. doi:10.1016/S0022-5347(01)64011-4
Gupta M, Tuncay OL, Smith A (1997) Open surgical exploration after failed endopyelotomy: a 12-year experience. J Urol 157(5):1613–1619. doi:10.1016/S0022-5347(01)64808-0
Bauer JJ, Bishoff JT, Moore RG, Chen RN, Iverson AJ, Kavoussi LR (1999) Laparoscopic versus open pyeloplasty: assessment of objective and subjective outcome. J Urol 162(3 Pt 1):692–695. doi:10.1097/00005392-199909010-00016
Chen RN, Moore RG, Kavoussi LR (1998) Laparoscopic pyeloplasty: indication, technique, and long-term outcome. Urol Clin North Am 25(2):323–330. doi:10.1016/S0094-0143(05)70021-5
Acknowledgments
This study was approved by the ethics committees at two participating hospitals. This study was supported by a grant from key programs of the Ministry of Science and Technology, Hubei province, P. R. China; and a Hubei Health Fund of young talents, P. R. China.
Author information
Authors and Affiliations
Corresponding author
Additional information
H. Zhou and H. Li contributed equally to this work.
Rights and permissions
About this article
Cite this article
Zhou, H., Li, H., Zhang, X. et al. Retroperitoneoscopic Anderson–Hynes dismembered pyeloplasty in infants and children: a 60-case report. Pediatr Surg Int 25, 519–523 (2009). https://doi.org/10.1007/s00383-009-2369-z
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00383-009-2369-z