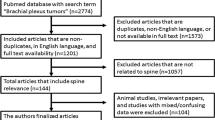
Abstract
Purpose
Peripheral nerve sheath tumors (PNSTs) are rare in pediatric patients, especially in the brachial plexus. Research on PNSTs is lacking. This article presents a retrospective cohort study of pediatric patients diagnosed and treated with PNSTs, specifically brachial plexus tumors.
Methods
All pediatric patients intervened in a single center between 2007 and 2023 with brachial plexus tumors were systemically analyzed.
Results
Eleven pediatric patients with 14 brachial plexus PNSTs were studied. The gender distribution was 64% female and 36% male, with an average age of 10.7 years. Ninety-one percent had a previous NF-1 diagnosis. Right brachial plexus presented a higher prevalence (64%). Pain, Tinel’s sign, and stiffness masses were common during diagnosis. Motor deficits were noted in 43% of the patients. Surgery was indicated for symptoms, particularly pain and rapid growth, increasing malignancy risk. Due to suspected malignancy, an en bloc resection with safety margins was performed. Among the patients, 57% received a histopathological diagnosis of MPNST (malignant peripheral nerve sheath tumor). Treatment included radiotherapy and chemotherapy. Clinical follow-up was conducted for all cases, involving clinical and oncological evaluations for all MPNSTs.
Conclusions
This article present a series of pediatric brachial plexus tumors, especially in NF-1, and emphasizes the importance of thorough evaluation for this group. Swift diagnosis is crucial in pediatrics, enabling successful surgery for small lesions with limited neurological symptoms, improving long-term outcomes. Prompt referral to specialized services is urged for suspected masses, irrespective of neurological symptoms. Benign tumor postsurgical progression shows better outcomes than MPNSTs, with complete resection as the primary goal. Needle-guided biopsy is not recommended.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Peripheral nerve sheath tumors (PNSTs) are exceedingly rare, especially in the pediatric population (PP), often associated with specific genetic syndromes like neurofibromatosis types 1 (NF-1) and 2 (NF-2). Limited literature exists on this topic, with few studies focusing on uncommon brachial plexus tumors (BPTs) in the PP [1]. Although various syndromes, such as Gorlin, Li-Fraumeni, Cowden, and Carney syndromes, are linked to these tumors, comprehensive research on BPTs in the PP is lacking [2,3,4,5,6,7,8,9].
PNSTs are classified as benign or malignant, neurogenic being the most common type. Common types include neurofibromas, schwannomas, and malignant nerve sheath tumors (MPNSTs) [4]. Neurofibromas (NFs), the most prevalent benign PNSTs, constitute a significant portion of neurogenic genetic tumors, with NF-1 being the dominant hereditary syndrome. Approximately 30% of NF cases are associated with NF-1, which has a prevalence of 0.0003% in the PP [1, 10,11,12,13]. NFs exhibit variations as cutaneous, subcutaneous, spinal, or plexiform types. Plexiform NFs, often linked to NF-1, present complexities due to intraneural NF conglomerate patterns, increasing the risk of sarcomatous degeneration and increasing the relative risk for MPNSTs [1, 5, 14, 15]. Schwannomas constitute 5% of pediatric neurogenic tumors. Confirmed schwannoma cases necessitate assessment for syndromes like NF-2 mutation, schwannomatosis, and Carney complex. Originating from a nerve root or fascicle, schwannomas exhibit an eccentric growth pattern displacing unaffected fascicles [1, 10, 16].
The incidence of MPNSTs is extremely low in the general population (0.001%), but the risk increases to 2–29% in patients with a history of NF-1, with an estimated lifetime risk of 10% [17, 18]. The greatest impact occurs in the postpubertal phase, typically involving proximal extremities, torso, and the cervical region. Although most occurrences are localized, there are instances of metastases to regional lymph nodes and distant metastases [19].
Considering the association between PNSTs and genetic conditions, this study retrospectively examined a cohort of pediatric patients exclusively diagnosed with brachial plexus PNSTs. Remarkably, 91% of patients in this cohort already had NF-1 at PNST diagnosis. Investigating the correlation between this pathology and genetic syndromes aims to optimize treatment strategies [1].
Methods
The study focused on pediatric cases with BPTs treated at the Neurosurgery Division of Gaffrée and Guinle University Hospital of Federal University of Rio de Janeiro State (UNIRIO), Brazil, from 2007 to 2023. A total of 11 patients were identified, 14 of whom were surgically treated.
The clinical data were obtained from medical records and included gender, age, family history, neurologic symptoms, physical examination, location of the tumor, MRI findings, preoperative biopsy, surgical data, postoperative results, secondary treatments (radiotherapy or chemotherapy), complications, tumor growth, histopathologic results, and clinical outcomes (Table 1).
In three cases, double surgery was needed; patient #2 had a spread of 8 years between the first surgery and contralateral in the second. Patient #4 underwent a second surgery on the same side that same year later. For patient #10, 11 months later, a recurrent lesion was observed at the same surgical site, despite the completion of chemotherapy cycles.
All patients, except for patient #11, underwent gross total resection using microsurgical techniques. Patient #11 had a partial excision due to BP extension. The middle cord was excised with appropriate safety margins. Electrophysiological studies were conducted for presurgical planning in some patients, as it is a difficult exam to perform in children, and intraoperative nerve stimulation was used in all patients, aiding in the precise localization of functional and non-functional tumor regions.
Postsurgical outcomes for BPNST cases revealed no motor deficits, whereas for a few patients, mild postoperative motor deficits were observed. Clinical follow-up was carried out every month for the first 6 months and subsequently every 3 months, in BPNSTs. For MPNSTs, follow-up was monthly during the first 12 months after surgery via clinical and pediatric oncology, radiotherapy, or/and chemotherapy.
Results
A total of 11 patients with 14 PNSTs were investigated. The mean age was 10.7 years (range 2–17 years). Regarding sex, 64% of the tumors occurred in females (9/14), and 36% occurred in males (5/14). Forty-four percent (4/9) of the females presented a histological diagnosis of MPNST (patient #4 tumor recurred and evolved into a malignant lesion).
The majority of patients (91%) received a diagnosis of NF-1, except for patient #10. Upon subsequent histopathological analysis, eight out of the 14 tumors (57%) were identified as MPNSTs.
All patients experienced moderate to intense pain that affected the upper limb. Clinical assessment was conducted using the Pain Assessment Scale (PAS), with scores ranging from 5 to 9 points. The primary features exhibited included nocturnal pain, leading to disruptions in a child’s sleep due to its intensity, and persistent pain throughout the day. For specific cases where quantifying pain was challenging, the Wong-Baker Faces scale was utilized as an alternative method.
Six patients (43%) presented an associated motor deficit; patients #1 and #2 (right tumor) presented a motor deficit of M2; patient #9, with M3; and patients #4, #10, and #11, with M4 (highlighting that patients #4 and #10 underwent a second surgery at the same site). The remaining patients did not present any motor deficits at the time of diagnosis.
Presurgically, Tinel’s sign and a stiff mass were diagnosed. Among the tumors, 11 had Tinel’s sign-positive tumors (79%), while a stiff mass was observed in nine tumors (64%), as a hard lesion is difficult to mobilize, intricate to palpate, and painful.
A higher incidence was evident within the right brachial plexus, accounting for 64% (9/14) of the occurrences. Within this subset, 89% (8/9) of the patients were at the supraclavicular level, while the remaining 11% (1/9) were located infraclavicular. According to the histopathological analysis, 56% (5/9) of the tumors were classified as BPNSTs, while 44% (4/9) were diagnosed as MPNSTs. In each patient, an association with NF-1 was evident.
According to the histopathological examinations, six surgical tumor samples were diagnosed as NF without signs of atypia. Notably, patients #4 and #10 experienced recurrences on the same side as the initial occurrence, with subsequent diagnoses of MPNSTs. In patient #10, this recurrence was verified through imaging studies and manifested as a palpable painful mass. Surgical resection was performed, during which the lesion was comprehensively removed. This procedure encompassed a 1-cm distal resection of the involved nerve segment and a 2-cm proximal resection.
Among the patients, only patient #8 had a plexiform neurofibroma in histopathological study. Patients #1, #5, #9, #10, and #11 received direct histopathological diagnosis of MPNST. In Patient #10, following several pathological revisions, a final diagnosis of malignant extrarenal rhabdoid tumor was established, after second surgery. Patients with MPNST, at diagnosis, had large tumors, leading to increased nerve involvement, suggesting a poorer long-term prognosis. However, early diagnosis and treatment improved outcomes. Each MPNST patient underwent radiotherapy and chemotherapy under oncology specialists’ care. Notably, 86% of MPNST patients had concurrent lung metastases, showing a significant correlation.
Postsurgery, pain symptoms notably improved, with values consistently below 2 on PAS.
Discussion
Regarding the low incidence of PNSTs in the PP, this study included 14 operated PNSTs located at the BP in 11 patients. The current paper represents the most extensive surgical database analysis within the literature on pediatric BPTs [5, 7, 13, 20]. While Kline et al. conducted a comprehensive study on peripheral nerve tumors in adults and PPs, their focus did not extend to children or the BP region, as in this article, particularly in NF-1 patients [8]. Similarly, Constine et al. conducted a substantial investigation exclusively in children within a diverse PP cohort but did not detect BPTs [6].
In NF-1-related PNSTs in the PP, females exhibited a greater occurrence rate (64%), with no conclusive genetic link to sex predisposition established. Hormonal factors, like GH receptors, related to tumor size and growth during adolescence and pregnancy have been explored but are not linked to genetic factors associated with sex [21].
Clinical evaluation is crucial in identifying PNSTs, with common signs including lumps along nerve paths, sensory deficits, muscle weakness, atrophy, and local pain. Pediatric symptoms often manifest as behavioral changes, while “silent PNSTs” present as growing palpable masses. Our patients predominantly experienced pain, with 79% showing Tinel’s sign and 64% having rigid masses. Six patients had motor deficits, assessed with the MRC scale. Painful palpable masses with a positive Tinel sign were most prevalent, mirroring adult findings with motor and sensory deficits [2, 5, 20]. In pediatric cases, intense pain is a critical symptom indicating a potential tumor. Imaging studies are essential for determining tumor type and location [20].
Preoperative electrophysiological studies like ENMG (electroneuromyography) are not routinely used due to their limited impact compared to MRI [7, 20]. They generally do not provide additional clinical or imaging insights, nor do they influence surgical decisions. ENMGs may only yield some modification in nerve distribution and denervation, mainly related to MPNSTs. In PP, ENMGs face limitations due to invasiveness and sedation, especially in neonates [22]. However, intraoperative electrophysiological monitoring is crucial during surgery to assess the extent of nerve injury and tumor-related nerve fascicle involvement, reducing the risk of additional damage. It also aids postoperative follow-up to assess potential injuries and recovery.
Preoperative imaging studies like ultrasound can only define injury type, but its usage is diminishing due to the evolution of other more precise diagnostic methods [5] (Fig. 1).
Ultrasound image corresponding to a 3-year-old patient (patient #10), who was diagnosed with a malignant extrarenal rhabdoid tumor (MPNST). Image A, hypoechoic in appearance, 1.3 × 1.1 cm (white arrow) with edema in the underlying subcutaneous tissue. Image B, performed three months later, shows posterior acoustic enhancement of similar characteristics with peripheral vascularization and an increase in the size of 2.3 × 1.5 × 1.5 cm (white arrow). Nerve fascicles can be observed at the periphery of the tumor, a feature characteristic of BPNSTs, though it appears in an MPNST
MRI is the optimal imaging method for diagnosing PNSTs and assessing their relationship with nearby structures, though it is not ideal for distinguishing benign from malignant tumors (Fig. 2). Histopathological studies are the gold standard for determining tumor nature. Short T1 inversion recovery (STIR) sequences effectively characterize tumors, differentiating them from soft tissues. Contrast-enhanced sequences distinguish BPNSTs from MPNSTs, revealing non-homogeneous contrast enhancement, irregular margins, infiltrative features, bone destruction, necrosis, and hemorrhage in the latter. On T2, MPNSTs appear hyperintense compared to muscle and lack common target signs [7, 23]. Wasa et al. emphasized key imaging factors for distinguishing BPNSTs from MPNSTs: increased mass size, enhancement patterns, peripheral edema-like features, and intratumoral cystic lesions [24]. If two or more factors suggest malignancy, a histopathological study is recommended. If only one factor is present, the risk–benefit of surgical resection should be evaluated.
Patient #10, MRI of an MPSNT. Images A, B, and C present axial slices of the left cervical and shoulder regions, captured using T1, T2, and T2 fat saturation sequences, respectively. These images reveal an expansive, solid, oval, fusiform lesion with well-defined edges. The lesion appears hyperintense on T2 (white thin arrow) and hypointense on T1(asterisk). Its size was 3.6 × 2.7 × 2.5 cm (L × T × AP). Importantly, no signs of infiltration into the surrounding tissue were observed
Ultrasound and MRI can suggest a BPNST, necessitating correlation with symptoms and histopathological confirmation to determine if the lesion is benign.
Beyond Wasa et al. criteria, this study proposes an additional consideration: tumor size. BPNSTs typically range from 3.4 to 5.5 cm, while MPNSTs tend to be larger, with a mean size of 7.2 to 10 cm [1].
PET studies are restricted to high-risk MRI-identified patients, due to cost and complexity, as whole-body evaluation of metastases, often near the lungs due to BP, are available (Fig. 3) [2, 6, 17, 19]. Its advantage lies in determining the SUV, gauging malignancy, disease progression, and prognosis more specifically than histopathology. An SUV over 3 predicts 94% accurate premature death within 36 months [25, 26]. Studies explored combined FDG PET and MRI, with Derlin et al. finding both differentiate PNSTs, though PET/CT is more sensitive. Higher SUV, intratumoral FDG activity, and poorly defined margins on MRI associated with MPNST [27]. Spinner et al. suggested managing PNSTs with an SUVmax under 4.3 as BPNSTs, reserving surgery for symptomatic lesions. SUVmax 4.3–8.1 lesions should undergo MRI due to few false-positive MRI results. Due to SUV measurement variability, some opt for a 3.5 SUV max threshold. Lesions with SUVmax above 8.1 need biopsy, focusing on the most FDG-avid mass area. For high SUVmax or symptomatic BPNSTs, MRI defines nerve involvement and tumor relationship for safe biopsy/resection, usually advised [28]. It is pertinent to underscore that while this information holds significance, the majority of studies have primarily centered on adults, resulting in limited findings for the PP.
[18F] FDG-PET/CT study corresponding to a patient #10, postoperative control and staging. Surgical manipulation in the left axillary region was indicated by the presence of a seroma/collection 25 × 17 × 17 mm (inflammatory/postsurgical etiology). No signs of tumor remnants were observed in the region of the left brachial plexus. Glycolytic hypermetabolism in right lung opacities, with suspicion of secondary implants. Similarly, cervical hypermetabolic nodal findings (levels IV and V), as well as findings in the left axillary/retropectoral and mediastinal hilars findings, can be considered indicative of susceptibility to secondary implants
Given suspected malignancy and potential vascular involvement, presurgical evaluation may require a CT angiography with vascular reconstruction. In cases of extensive tumor extension toward adjacent structures, our team suggested broad resection of involved nerve structures, followed by posthistopathological radiotherapy or chemotherapy.
In NF1 cases, an interdisciplinary assessment precedes surgery, especially for rapidly growing and painful cases; targeting to minimize malignancy risk through complete resection, proven to increase life expectancy and reduce recurrence [5, 18]. Selective pharmacological treatments are crucial for symptomatic NF-1 patients with inoperable plexiform neurofibromas. Selumetinib, an FDA-approved oral medication for inviduals aged 2 and older, inhibits MAPK kinases (MEK) 1 and 2, which is pivotal in NF-1 development. Evidence indicates Selumetinib’s benefits: pain relief, improved appearance, and enhanced function. This NF-1 pharmacotherapy, in early stages, holds potential positive outcomes [29, 30].
Zipfel et al. underscore the significance of early surgical resection in the PP according to our criteria: notable growth, pain, motor issues, or suspected malignancy based on clinical/imagining data [5]. This finding prompted a surgical approach for treating PNSTs. In BPNST patients, the approach closely mirrors adult protocols. Schuhmann et al. performed intracapsular tumor resection, using a nerve stimulator and magnification (microscope/loops) to ensure nerve fascicle–free capsule segments (Figs. 4 and 5) [1, 5, 31]. Large tumors or specific NF cases may leave residual tumors to avoid a major deficit. In these cases, a nerve graft is not necessary if good microsurgical criteria are applied.
Surgical case of an 11-year-old female with a history of NF-1 affecting the right brachial plexus. The patient had been experiencing a palpable mass for the past 5 years, which led to her being referred to the hospital due to intense pain (patient #3). A Patient in the dorsal decubitus surgical position, with a slight inclination of the cervical region toward the contralateral side, supported by the interscapular area. Notably, multiple café-au-lait spots were visible in the supra and infraclavicular regions, as well as on the right upper limb. B, C Visualization of the supraclavicular surgical approach, where the tumor lesion can be observed (white arrow). D The results of the histopathological study confirming the compatibility with a typical neurofibroma
Surgical images of a 16-year-old female patient with a history of neurofibromatosis type 1 (NF-1) presenting with a painful palpable mass in the right infraclavicular brachial plexus. (patient #6). A Surgical positioning and preoperative markings at the right supraclavicular and infraclavicular levels. B Exposure of the tumor mass (asterisk) revealing a well-defined, lobulated mass integrated with the nervous structures of the infraclavicular brachial plexus. C, D Complete excision of the lesion with preservation of the adjacent nervous structures
MPNSTs require a major surgical approach: wide incisions, which allow for larger resections, en bloc resection of the nerve, and resection of the tumor lesion with tumor-free margins (3 cm if possible). In these cases, the risk/benefit of resection should be agreed upon with the patient’s tutors due to the risk of a major motor and sensory injury. Unlike for BPNSTs, intracapsular resection is not usually performed due to the risk of dissemination and contamination with malignant cells in the surgical field and the risk of worsening clinical and oncological status [5, 7, 17, 20, 32].
When dealing with tumors in isolated nerves or those unrelated to the BP, their removal may require excising the nerve itself. However, addressing an MPNST associated with the BP requires meticulous evaluation for potential same-surgery resection due to risks associated with both tumor removal and compromised surrounding nerves and structures, as highlighted by Kline et al. [5]. In such cases, the prospect of significant postoperative morbidity looms, impacting both pediatric and adult populations.
This emphasizes the need for a reliable diagnosis before surgically removing a PNST to prevent unnecessary upper limb motor function loss. It suggests that MPNSTs from a plexus could initially be treated with en bloc resection of their nerve of origin, sparing not entirely encompassed nerves. This approach, as evidenced by recent studies, has the potential to obviate the necessity for amputation, which has been observed in certain cases. These findings suggest promising prospects for children to not only survive but also thrive well beyond the conventional 5- to 10-year time frame [32, 33].
Specialized histopathologist expertise is vital. From a histopathological perspective, MPNSTs may resemble various tumor types, particularly epithelioid, synovial sarcoma, and rhabdoid tumors. Shared features include lobular arrangement, epithelioid morphology, prominent nucleoli, myxoid stroma, and rhabdoid cells. Crucially, assessing S100 and/or GFAP expression is vital; notably, S100 is the most reliable MPNST marker, with a 50% positivity rate [34].
Epithelioid MPNSTs often show nerve origins and focal spindle cells, aligning with typical malignant patterns. Immunohistochemistry usually detects widespread S100 positivity and occasional epithelial. Conversely, malignant extrarenal rhabdoid tumors, often aggressive and high grade, may mimic MPNSTs, especially in children. They can occur in renal or extrarenal sites and are distinguished by INI1 (SMARCB1) gene loss. Patient #10’s case underscores diagnostic challenges, initially diagnosed with epithelioid MPNST and later with a malignant extrarenal rhabdoid tumor. This emphasizes the need for thorough histopathological evaluation and molecular marker consideration for accurate diagnosis and management [34,35,36].
In patients with uncertain histopathological diagnoses, like patient #10, FoundationOne CDx provides advanced genomic profiling. This technique analyses tumoral tissue for genetic mutations, enhancing the understanding of tumor growth and progression, offering tailored therapies and personalized clinical trial options. FoundationOne CDx is reserved for specific cases guiding the most suitable treatment [37, 38].
According to our research, resection surpasses open biopsy for overall survival, consistent with findings in the adult literature [33]. Needle biopsies should be avoided due to the risk of damaging fascicles, causing pain or motor/sensory deficits, and potential inadequate sampling for accurate diagnosis [39]. In terms of prognosis and treatment, patients undergoing surgery had better long-term outcomes than those with only needle biopsies [33]. Notably, BPNSTs had better clinical outcomes than MPNSTs, who required adjuvant postsurgical treatments but still had lower survival rates.
A systemized approach to diagnosing and treating brachial plexus PNSTs in children is crucial for positive outcomes (i.e., flowchart in Fig. 6).
The postoperative outcomes for BPNSTs are generally favorable, with complete surgical resection leading to good recovery; minor motor or sensory deficits countered with rehabilitation. MPNSTs pose a higher risk of neurological deficits postresection.
Postoperative imaging is selectively necessary for BPNSTs, primarily in cases of partial resection. Especially, plexiform NFs may require follow-up MRI 3 months postsurgery to detect residual tumors that could lead to recurrence or malignant transformation.
For MPNSTs, postoperative radiotherapy (RT) or chemotherapy (QT) is recommended. RT improves local control but does not significantly impact overall survival. The radiation dosage adheres to adult soft tissue sarcoma guidelines, with preoperative RT in adults aiming to reduce fibrosis and enhance limb function, albeit with higher postoperative complication rates. Children receive lower irradiation doses to minimize growth-related side effects [32, 40,41,42]. Balancing the benefits and potential side effects of RT is crucial in MPNST treatment.
One rare postoperative complication is the occurrence of a lymphatic fistula, as observed in isolated cases, often related to axillary or thoracic surgeries [43].
Pediatric survival rates are generally more favorable. However, those associated with syndromic conditions tend to have worse prognoses. Surgical procedures involving major nerves, plexuses, or nerve roots carry a higher risk of morbidity [42, 44].
Conclusions
Prompt referral is vital for PNSTs patients with pain, growth, significant size, activity limitations, or NF-1 history. Early diagnosis is crucial, where even asymptomatic lesions benefit from surgery. Defining clinical symptoms, especially pain, and using imaging studies, though not always definitive, guide diagnosis in most cases.
BPNSTs typically have favorable postsurgical outcomes, while MPNSTs require close monitoring and additional treatments. Needle-guided biopsy is discouraged due to low efficacy and risk; image-guided biopsy may be considered. Complete surgical resection is paramount, particularly in high-risk scenarios. Involving an experienced pathologist in PNSTs and sarcomas is essential.
This paper offers a comprehensive analysis of pediatric BPTs, outlining an efficient diagnostic and treatment flowchart.
Data availability
No datasets were generated or analysed during the current study.
Abbreviations
- PNST:
-
Peripheral nerve sheath tumors
- BP:
-
Brachial plexus
- BPTs:
-
Brachial plexus tumors
- PAS:
-
Pain assessment scale
- BPNSTs:
-
Benignant tumors of the nerve sheath
- MPNSTs:
-
Malignant tumors of the nerve sheath
- PP:
-
Pediatric population
- NF-1:
-
Neurofibromatosis type 1
- NF-2:
-
Neurofibromatosis type 2
- NRSTS:
-
Nonrhabdomyosarcoma soft tissue sarcoma
- GH:
-
Growth hormone
- MAPK:
-
Mitogen-activated protein kinase
- MRI:
-
Magnetic resonance imaging
- STIR:
-
Short T1 inversion recovery
- STS:
-
Soft tissue sarcomas
- SUV:
-
Standardized uptake value
References
Guedes F, Socolovsky M, Rasulic L, Garozzo D, Zager EL (2022) Diagnostic assessment and treatment of peripheral nerve tumors. Springer, Cham
DeCou JM, Rao BN, Parham DM et al (1995) Malignant peripheral nerve sheath tumors: the St. Jude children’s research hospital experience. Ann Surg Oncol 2(6):524–529. https://doi.org/10.1007/BF02307086
Yaworski A, Koujok K, Cheung K, Ying Y, McMillan HJ (2021) Intrinsic peripheral nerve and root tumor and pseudotumoral lesions at a tertiary care pediatric hospital. Childs Nerv Syst 37(4):1229–1236. https://doi.org/10.1007/s00381-020-04995-8
Costales JR, Socolovsky M, Sánchez Lázaro JA, ÁlvarezGarcía R, Costales DR (2019) Peripheral nerve injuries in the pediatric population: a review of the literature. Part III: peripheral nerve tumors in children. Childs Nerv Syst 35(1):47–52. https://doi.org/10.1007/s00381-018-3976-6
Zipfel J, Al-Hariri M, Gugel I et al (2020) Surgical management of peripheral nerve sheath tumors in children, with special consideration of neurofibromatoses [published correction appears in Childs Nerv Syst. 2021 Sep;37(9):2953]. Childs Nerv Syst 36(10):2433-2442https://doi.org/10.1007/s00381-020-04703-6
Bates JE, Peterson CR, Dhakal S, Giampoli EJ, Constine LS (2014) Malignant peripheral nerve sheath tumors (MPNST): a SEER analysis of incidence across the age spectrum and therapeutic interventions in the pediatric population. Pediatr Blood Cancer 61(11):1955–1960. https://doi.org/10.1002/pbc.25149
Siqueira MG, Martins RS, Teixeira MJ (2009) Management of brachial plexus region tumours and tumour-like conditions: relevant diagnostic and surgical features in a consecutive series of eighteen patients. Acta Neurochir (Wien) 151(9):1089–1098. https://doi.org/10.1007/s00701-009-0380-8
Kim DH, Murovic JA, Tiel RL, Kline DG (2004) Operative outcomes of 546 Louisiana State University Health Sciences Center peripheral nerve tumors. Neurosurg Clin N Am 15(2):177–192. https://doi.org/10.1016/j.nec.2004.02.006
Gosk J, Rutowski R, Zimmer K, Rabczyński J (2004) Brachial plexus tumours–own experience in diagnostics and surgical treatment. Folia Neuropathol 42(3):171–175
Coffin CM, Dehner LP (1989) Peripheral neurogenic tumors of the soft tissues in children and adolescents: a clinicopathologic study of 139 cases. Pediatr Pathol 9(4):387–407. https://doi.org/10.3109/15513818909022361
Donner TR, Voorhies RM, Kline DG (1994) Neural sheath tumors of major nerves. J Neurosurg 81(3):362–373. https://doi.org/10.3171/jns.1994.81.3.0362
Mackinnon SE (2015) Nerve Surgery. Thieme, New York (Chapter 18)
Dunning-Davies BM, Parker AP (2016) Annual review of children with neurofibromatosis type 1. Arch Dis Child Educ Pract Ed 101(2):102–111. https://doi.org/10.1136/archdischild-2014-308084
Korf BR (1999) Plexiform neurofibromas. Am J Med Genet 89(1):31–37. https://doi.org/10.1002/(sici)1096-8628(19990326)89:1%3c31::aid-ajmg7%3e3.0.co;2-w
Gabhane SK, Kotwal MN, Bobhate SK (2009) Morphological spectrum of peripheral nerve sheath tumors: a series of 126 cases. Indian J Pathol Microbiol 52(1):29–33. https://doi.org/10.4103/0377-4929.44958
Rodriguez FJ, Stratakis CA, Evans DG (2012) Genetic predisposition to peripheral nerve neoplasia: diagnostic criteria and pathogenesis of neurofibromatoses, Carney complex, and related syndromes. Acta Neuropathol 123(3):349–367. https://doi.org/10.1007/s00401-011-0935-7
Farid M, Demicco EG, Garcia R et al (2014) Malignant peripheral nerve sheath tumors. Oncologist 19(2):193–201. https://doi.org/10.1634/theoncologist.2013-0328
Reilly KM, Kim A, Blakely J et al (2017) Neurofibromatosis type 1-associated MPNST state of the science: outlining a research agenda for the future. J Natl Cancer Inst 109(8):djx124. https://doi.org/10.1093/jnci/djx124
Carli M, Ferrari A, Mattke A et al (2005) Pediatric malignant peripheral nerve sheath tumor: the Italian and German soft tissue sarcoma cooperative group [published correction appears in J Clin Oncol. 2006 Feb 1;24(4):724. Koscielniak, Eura [corrected to Koscielniak, Ewa]]. J Clin Oncol 23(33):8422–8430. https://doi.org/10.1200/JCO.2005.01.4886
Guedes F, Brown RS, Torrão-Junior FJL, Barbosa DAN, Ravanini GAG, Amorim RMP (2019) Pediatric peripheral nerve tumors: clinical and surgical aspects. Childs Nerv Syst 35(12):2289–2297. https://doi.org/10.1007/s00381-019-04306w
Cunha KS, Barboza EP, Fonseca EC (2008) Identification of growth hormone receptor in plexiform neurofibromas of patients with neurofibromatosis type 1. Clinics (Sao Paulo) 63(1):39–42. https://doi.org/10.1590/s1807-59322008000100008
Mazal AT, Faramarzalian A, Samet JD, Gill K, Cheng J, Chhabra A (2020) MR neurography of the brachial plexus in adult and pediatric age groups: evolution, recent advances, and future directions. Expert Rev Med Devices 17(2):111–122. https://doi.org/10.1080/17434440.2020.1719830
Stull MA, Moser RP Jr, Kransdorf MJ, Bogumill GP, Nelson MC (1991) Magnetic resonance appearance of peripheral nerve sheath tumors. Skeletal Radiol 20(1):9–14. https://doi.org/10.1007/BF00243714
Wasa J, Nishida Y, Tsukushi S et al (2010) MRI features in the differentiation of malignant peripheral nerve sheath tumors and neurofibromas. AJR Am J Roentgenol 194(6):1568–1574. https://doi.org/10.2214/AJR.09.2724
Brenner W, Friedrich RE, Gawad KA et al (2006) Prognostic relevance of FDG PET in patients with neurofibromatosis type-1 and malignant peripheral nerve sheath tumours. Eur J Nucl Med Mol Imaging 33(4):428–432. https://doi.org/10.1007/s00259-005-0030-1
Azizi AA, Slavc I, Theisen BE et al (2018) Monitoring of plexiform neurofibroma in children and adolescents with neurofibromatosis type 1 by [18 F]FDG-PET imaging. Is it of value in asymptomatic patients? Pediatr Blood Cancer 65(1). https://doi.org/10.1002/pbc.26733. https://doi.org/10.1002/pbc.26733
Derlin T, Tornquist K, Münster S et al (2013) Comparative effectiveness of 18F-FDG PET/CT versus whole-body MRI for detection of malignant peripheral nerve sheath tumors in neurofibromatosis type 1. Clin Nucl Med 38(1):e19–e25. https://doi.org/10.1097/RLU.0b013e318266ce84
Broski SM, Johnson GB, Howe BM et al (2016) Evaluation of (18)F-FDG PET and MRI in differentiating benign and malignant peripheral nerve sheath tumors. Skeletal Radiol 45(8):1097–1105. https://doi.org/10.1007/s00256-016-2394-7
Dombi E, Baldwin A, Marcus LJ et al (2016) Activity of selumetinib in neurofibromatosis type 1-related plexiform neurofibromas. N Engl J Med 375(26):2550–2560. https://doi.org/10.1056/NEJMoa1605943
Gross AM, Wolters PL, Dombi E, et al (2020) Selumetinib in children with inoperable plexiform neurofibromas [published correction appears in N Engl J Med. 2020 Sep 24;383(13):1290]. N Engl J Med 382(15):1430–1442. https://doi.org/10.1056/NEJMoa1912735
Das S, Ganju A, Tiel RL, Kline DG (2007) Tumors of the brachial plexus. Neurosurg Focus 22(6):E26. Published 2007 Jun 15. https://doi.org/10.3171/foc.2007.22.6.27
Martin E, Flucke UE, Coert JH, van Noesel MM (2020) Treatment of malignant peripheral nerve sheath tumors in pediatric NF1 disease. Childs Nerv Syst 36(10):2453–2462. https://doi.org/10.1007/s00381-020-04687-3
Lu VM, Wang S, Daniels DJ, Spinner RJ, Levi AD, Niazi TN (2021) The clinical course and role of surgery in pediatric malignant peripheral nerve sheath tumors: a database study. J Neurosurg Pediatr 29(1):92–99. Published 2021 Oct 8. https://doi.org/10.3171/2021.7.PEDS21263
Olsen SH, Thomas DG, Lucas DR (2006) Cluster analysis of immunohistochemical profiles in synovial sarcoma, malignant peripheral nerve sheath tumor, and Ewing sarcoma. Mod Pathol 19(5):659–668. https://doi.org/10.1038/modpathol.3800569
Klijanienko J, Caillaud JM, Lagacé R, Vielh P (2002) Cytohistologic correlations of 24 malignant peripheral nerve sheath tumor (MPNST) in 17 patients: the Institut Curie experience. Diagn Cytopathol 27(2):103–108. https://doi.org/10.1002/dc.10152
Priya D, Sadasivan B, PatilOkaly GV, MukundaPai M, Alashetty S, Kavitha BL (2021) Cytomorphological and immunohistochemical features of renal and extrarenal rhabdoid tumors. Diagn Cytopathol 49(6):711–717. https://doi.org/10.1002/dc.24727
Milbury CA, Creeden J, Yip WK et al (2022) Clinical and analytical validation of FoundationOne®CDx, a comprehensive genomic profiling assay for solid tumors. PLoS One 17(3):e0264138. Published 2022 Mar 16. https://doi.org/10.1371/journal.pone.0264138
Pinet S, Durand S, Perani A, et al (2023) Clinical management of molecular alterations identified by high throughput sequencing in patients with advanced solid tumors in treatment failure: real-world data from a French hospital. Front Oncol 13:1104659. Published 2023 Feb 27. https://doi.org/10.3389/fonc.2023.1104659
Guedes F, Henriques VM, Torrão FL et al (2024) When biopsy goes wrong: a case series of misdiagnoses and complications from biopsies of masses of unknown origin potentially originating from a peripheral nerve. J Neurosurg 140:480–488. Published online August 18, 2023. https://doi.org/10.3171/2023.5.JNS23385
Kahn J, Gillespie A, Tsokos M et al (2014) Radiation therapy in management of sporadic and neurofibromatosis type 1-associated malignant peripheral nerve sheath tumors. Front Oncol 4:324. Published 2014 Nov 17. https://doi.org/10.3389/fonc.2014.00324
van Noesel MM, Orbach D, Brennan B et al (2019) Outcome and prognostic factors in pediatric malignant peripheral nerve sheath tumors: an analysis of the European Pediatric Soft Tissue Sarcoma Group (EpSSG) NRSTS-2005 prospective study. Pediatr Blood Cancer 66(10):e27833. https://doi.org/10.1002/pbc.27833
Ferrari A, Bisogno G, Carli M (2007) Management of childhood malignant peripheral nerve sheath tumor. Paediatr Drugs 9(4):239–248. https://doi.org/10.2165/00148581-200709040-00005
Lv S, Wang Q, Zhao W et al (2017) A review of the postoperative lymphatic leakage. Oncotarget 8(40):69062–69075. Published 2017 Apr 20. https://doi.org/10.18632/oncotarget.17297
Bergqvist C, Servy A, Valeyrie-Allanore L et al (2020) Neurofibromatosis 1 French national guidelines based on an extensive literature review since 1966. Orphanet J Rare Dis 15(1):37. Published 2020 Feb 3. https://doi.org/10.1186/s13023-020-1310-3
Author information
Authors and Affiliations
Contributions
F.G made the draft. E.L.,V.H. and F.G wrote the main manuscript text. E.L, V.H and F.T data collection and data analysis. F.G, E.L, V.H bibliographic collection. F.G and F.T. edition. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Ethics approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee (Comitê de Ética em Pesquisa do Hospital Universitário Gaffrée and Guinle 5258) and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Conflict of interest
The authors declare that they have no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Guedes, F., Llorian, E., Henriques, V.M. et al. Brachial plexus peripheral nerve sheath tumors (PNSTs): clinical and surgical management in the pediatric population. Childs Nerv Syst (2024). https://doi.org/10.1007/s00381-024-06509-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00381-024-06509-2