Abstract
Purpose
Utilization of intraoperative neurophysiology (ION) to map and assess various functions during supratentorial brain tumor and epilepsy surgery is well documented and commonplace in the adult setting. The applicability has yet to be established in the pediatric age group.
Methods
All pediatric supratentorial surgery utilizing ION of the motor system, completed over a period of 10 years, was analyzed retrospectively for the following variables: preoperative and postoperative motor deficits, extent of resection, sensory-motor mappability and monitorability, location of lesion, patient age, and monitoring alarms. Intraoperative findings were correlated with antecedent symptomatology as well as short- and long-term postoperative clinical outcome. The monitoring impact on surgical course was evaluated on a per-case basis.
Results
Data were analyzed for 57 patients (ages 3–207 months (93 ± 58)). Deep lesions (in proximity to the pyramidal fibers) constituted 15.7% of the total group, superficial lesions 47.4%, lesions with both deep and superficial components 31.5%, and ventricular 5.2%. Mapping of the motor cortex was significantly more successful using the short-train technique than Penfield’s technique (84% vs. 25% of trials, respectively), particularly in younger children. The youngest age at which motor mapping was successfully achieved was 3 vs. 93 months for each method, respectively. Preoperative motor strength was not associated with monitorability. Direct cortial motor evoked potential (dcMEP) was more sensitive than transcranial (tcMEP) in predicting postoperative motor decline. dcMEP decline was not associated with tumor grade or extent of resection (EOR); however, it was associated with lesion location and more prone to decline in deep locations. ION actively affected surgical decisions in several aspects, such as altering the corticectomy location and alarming due to a MEP decline.
Conclusion
ION is applicable in the pediatric population with certain limitations, depending mainly on age. When successful, ION has a positive impact on surgical decision-making, ultimately providing an added element of safety for these patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Intraoperative neurophysiology (ION) is widely used in various neurosurgical procedures. Mapping and monitoring techniques assist, respectively, in localizing various eloquent regions and in continuously assessing the functional integrity of specific neurological pathways [13, 16,17,18,19].
ION has been widely implemented in adult neurosurgery for resection of lesions in proximity to or within the brainstem, spinal cord, and supratentorial locations abutting the motor pathways [1, 3, 4, 7, 9, 18, 23]. In the pediatric population, most publications relate to the use of ION in brainstem and spinal cord regions [5, 17]. ION use for supratentorial surgery has been described, mainly for adult surgery [13], although ION has been used for pediatric supratentorial lesions too [2, 14, 16, 17] and with increasing rates over time [22].
Potential reasons for underuse of ION in supratentorial locations may be attributed to the assumption that the developing corticospinal tract (CST) in children may have only a limited ability to monitor these pathways, in both healthy and diseased brains, as well as a potentially limited ability to map motor-related regions with direct stimulation [2, 19].
Given the cumulative data in the pediatric population regarding the importance of maximizing resection of low-grade tumors, as well as the complete removal of epileptic foci, it is logical to expand the use of ION to supratentorial regions, while concurrently evaluating its efficacy in the different age groups.
In this study, we describe our experience using ION in pediatric supratentorial surgery for various pathologies.
Methods
We conducted this study following an institutional review board approval. Patient and parent consents were waived. All patients under the age of 18 years who underwent surgery for supratentorial pathology in which ION was utilized, between the years 2006 and 2015, were included. Data was retrospectively collected, including demographics, preoperative neurological status, lesion location and grade, surgical data, such as approach and complications, intraoperative neurophysiological data, and neurological outcome (both immediate and long-term at follow-up). Data relied on patient files and on imaging captures from the PACS system.
Lesion locations were categorized into superficial, deep, superficial-deep, and ventricular as follows. Superficial locations included cortical regions involving the primary motor cortex (M1) region, precentral or postcentral gyri. Deep locations included subcortical lesions in proximity to the CST, and lesions involving the thalamus, basal-ganglia, or cerebral peduncle. Superficial-deep locations involved both regions. Ventricular locations included those located in the lateral or third ventricle.
Clinical assessment was performed preoperatively, immediately (within 24 h) postoperatively, at time of discharge, at 1 month after surgery, and at last follow-up visit. Clinical assessment included upper and lower extremity strength and presence of bulbar signs.
A standardized anesthesia protocol was used. All children were given general anesthesia. Inhaled induction was used if intravenous (IV) access had not been secured before arrival to the operating room; otherwise, IV fentanyl 2–4 mcg/kg, propofol 2–3 mg/kg, and rocuronium 0.6 mg/kg were given for induction. Inhaled agents were discontinued following induction, as was any use of neuromuscular blockade. Maintenance of anesthesia consisted of total IV anesthesia using propofol 2% (100–250 mcg/kg/min) and remifentanil (0.2–0.4 mcg/kg/min). A nonsteroidal agent was given before emergence, and morphine up to 0.1 mg/kg was added as needed for immediate postoperative pain control. Dexamethasone 0.5 mg/kg and antiepileptic drugs were added upon surgeon request.
ION methodology
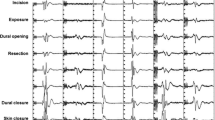
All neurophysiological recordings were performed using a Medtronic NIM Eclipse Neurological Workstation (Medtronic, Minneapolis, MN). Data collection and interpretation was performed by trained intraoperative neurophysiologists employed by Tel Aviv Medical Center. Multiple ION techniques were utilized, and a general outline is described in Table 1.
Motor threshold was calculated as the minimal stimulation intensity required to evoke a robust motor response (> 50 μV) from at least one muscle.
Techniques
-
1.
Mapping
-
a)
Motor cortex mapping
-
i)
Monopolar (fast) short-train technique: cortical stimulation was carried out using a ball-tip monopolar probe (Rhythmlink Columbia, SC) that delivered a train of 5–7 anodal pulses, 0.5 msec pulse width each, at a frequency of 250 Hz, which is equivalent to an interstimulus interval of 3.5 msec. Stimulation intensity ranged between 2.0 and 25.0 mA, and stimulation repetition rate was 1.2 Hz. Simultaneous 100 msec time-locked sweeps of motor evoked potentials (MEPs) were collected from paired subdermal needle electrodes placed in several muscles of interest: contralateral orbicularis oris, trapezius, deltoid, biceps, extensor carpi radialis, abductor pollicis brevis, quadricep, tibialis anterior, and ipsilateral abductor pollicis brevis. Collected data were displayed on both live and stack formats. MEPs were reported to correlate to M1 location if were above 50 μV amplitude, appeared on any channel or number of channels and replicated at least twice. M1 was identified if responses were proven specific, with adjacent areas of negative findings. To enhance specificity, when motor responses were established, repeat stimulation was undertaken at lower intensities to rule out electrical-spreading effects.
-
ii)
Bipolar long-train (Penfield) technique: the exposed cortex was stimulated using a 0.5-cm spaced bipolar probe and Ojemann cortical stimulation generator (Integra Life Sciences Plainsboro, NJ), delivering alternating pulses at 50 Hz for 2–3 s. Evoked EMG activity was displayed on the monitoring unit from the same muscle array mentioned above. In cases where the exposed cortex did involve the primary cortex, and in which direct cortical MEP monitoring was to be performed (see below), two neighboring contacts from a cortical strip electrode were stimulated in an identical fashion.
-
b)
Somatosensory evoked potential phase reversal: subdermal needle electrodes were placed along the medial aspect of the contralateral wrist for bipolar stimulation of the median nerve (200 μsec pulse duration; 10–20 mA intensity; 4.7 Hz stimulation rate). Somatosensory evoked potentials (SSEP) were recorded over the exposed temporoparietal cortex using an 8-contact silastic-embedded subdural strip electrode (PMT Corp., Chanhassen, MN) placed in proximity to the primary motor cortex and referenced to the FpZ position on the forehead. Eight channel simultaneous recordings were analyzed for phase reversal of the primary SSEP, approximating the sensory-motor border. If classic phase reversal was not identified, the electrode showing the maximal negativity of N20 waveform was identified and associated with the location of the primary sensory cortex.
-
c)
Subcortical pyramidal tract mapping: a ball-tip monopolar probe (Rhythmlink Columbia, SC) was used for direct high-frequency multipulse cathodal stimulation of subcortical regions of interest. MEP recordings were collected from the identical muscles as cortical MEP described above. Stimulating intensities started at 1 mA and were increased by 0.5 mA to motor threshold or to a maximum of 25 mA.
-
i)
-
2.
Monitoring
-
a)
Transcranial electric motor evoked potentials (tcMEP): tcMEPs were recorded from the bilateral abductor hallucis brevis and tibialis anterior muscles using paired subdermal needle electrodes. Corkscrew-style or straight subdermal needle-stimulating electrodes were placed approximately 2 cm anterior to the C3 and C4 scalp positions according to the 10–20 international system. When the exposure site interfered with these positions, electrodes were allocated as close as possible above the M1. Transcranial stimulation was carried out using a train of 5–7 anodal pulses (interpulse interval 3 msec, train rate 1 per sec) and the minimal intensity to consistently evoke MEP in the desired limbs.
-
b)
Direct cortical motor evoked potentials (dcMEP): following the phase reversal technique, the silastic-embedded subdural strip was maintained over the primary motor cortex, for intermittent stimulation (0.2 Hz) and MEP recording from the identical muscles as cortical MEP described above. Stimulation was carried out using two neighboring contacts immediately anterior to the location of the phase reversal waveform, as described above. Stimulation intensities were restricted to a maximum of 25 mA, and cathodal return was placed at the Fz scalp position using a subdermal needle electrode. Baseline dcMEPs were established prior to any surgical manipulation and were compared with all subsequent recordings to identify potential deterioration.
-
c)
Electrocorticography (ECOG): during cortical and subcortical stimulation, ECOG data were recorded through the strip electrode using contacts not utilized by dcMEP and scanned for after-discharge potentials. Once after-discharge potentials were detected, stimulation was halted to prevent the emergence of epileptic seizure.
ION methodologies were applied during surgery in a tailored approach relative to the surgical stages. A general outline is described in Table 1.
Monitorability was defined as the ability to elicit a motor response following electrical stimulation (regardless of the technique—cortical/subcortical mapping or MEP monitoring—used).
Clinical assessment was performed preoperatively, immediately (within 24 h) postoperatively, at time of discharge, at 1 month after surgery, and at last follow-up visit. Clinical assessment included upper and lower extremity strength and presence of bulbar signs.
Effect of ION on surgery
The effect of ION on surgery was classified as follows:
Low subcortical threshold alarm—this suggests proximity to the CST and could lead to alteration in surgical plan (such as performing a more limited resection at a certain location).
Cortical mapping alarms—when cortical mapping elicited a positive motor response, altering corticectomy location.
MEP alarm—when either tcMEP or dcMEP attenuation or change in stability was observed, and this change correlated with a surgical event or invasive maneuver, and the extent of said attenuation deviated from the standard variability evaluated until that point, this was considered a noteworthy change and the surgical team was informed for further investigation.
No impact due to unreliable ION—baseline data were not available due to technical (such as impossible placement of electrodes) or nontechnical reasons.
No impact despite reliable ION—robust baseline data were collected but maintained stable throughout monitoring and thus did not alter the course of surgery at any point. This can be evident, for example, in lesion cases that do not involve motor areas.
Statistical analysis
Data was tabulated in an Excel spreadsheet. SPSS software was used for all statistical analyses (IBM SPSS statistics, version 25, IBM Corp., 2017, Armonk, NY, USA). Categorical variables were reported as number and percentage. Continuous variables were evaluated for normal distribution using histogram and reported as median and interquartile range (IQR). Correlations between continuous variables were evaluated using Spearman’s correlation coefficient. Association between categorical variables was evaluated using Fisher’s exact test. Association between categorical and continuous variables was performed using Mann-Whitney test or Kruskal-Wallis test. All statistical tests were two tailed, and a p value less than 0.05 was considered significant.
Results
Over a period of 10 years, 58 children underwent supratentorial lesion resections under electrophysiological guidance. One patient underwent an awake craniotomy with linguistic mapping and was not included in the current study. Age range of the included subjects was 3–207 months (93 ± 58) at day of surgery.
Indications for surgery included tumor-related surgery in 40, epilepsy-related resections in 13, vascular lesions in 3, and removal of a brain abscess in 1.
Pathology locations were unilateral in 52 cases and midline in 5. Twenty-seven lesions were categorized as superficial, 9 deep, 18 superficial-deep, and 3 ventricular. Forty-eight lesions were low-grade, and 9 were high-grade. Of the tumoral lesions and lesional epilepsy, 33 underwent gross total resection (GTR), and 11 a subtotal resection (STR).
Of the entire patient population, 11 had an immediate postoperative motor decline. At last follow-up (2 weeks–8 months), 6 had significantly improved or returned to baseline condition, one had a permanent motor decline, and 4 were not available for long-term follow-up.
Mapping
Cortical mapping
Fifty-one children had a mapping attempt using a short-train technique; 43 (84%) of whom had a positive motor response. All positive responses to Penfield mapping were positive to short-train mapping, but not vice versa. Thirty-nine underwent both short-train stimulation and Penfield stimulation; 10 (25%) had a positive motor response for both techniques. As a whole, monopolar short-train stimulation elicited a motor response in 34/39, as opposed to 10/39 responding to the Penfield technique (Table 2).
MEP monitorability and age
The median age for successful motor response following short-train stimulation was 107 (46–150) months, with the youngest age 3 months, as opposed to the failure group, which displayed a median age of 45 (18–86) months (p = 0.04). The median age for successful motor response following Penfield stimulation was 151 (128–191) months, with the youngest age 93 months, as opposed to the failure group, with a median age of 53 (24–115) months (p < 0.001). Amongst infants (<24 months), 6/8 short-train attempts elicited a motor response, as opposed to 0 of 7 in the Penfield group. Thus, in this subgroup, the short-train technique stimulation was more effective at eliciting a motor response.
Age was negatively correlated with the stimulation threshold for motor responses in both the Penfield and the short-train techniques (Fig. 1).
Ability to evoke MEP, lesion location, and pathological grade
Lesion location did not significantly correlate with the ability to evoke MEP data (p = 0.57). Tumor grade was not correlated with the ability to evoke MEP either (p = 1).
Ability to evoke MEP and preoperative neurological status
Preoperative motor strength did not significantly correlate with the monitorability of MEPs (p = 0.54 upper extremity, p = 0.17 lower extremity). We classified patients according to their preoperative status: weak (motor strength 0–3/5) and strong (motor strength 4–5/5). We found no significant association between the two subgroups and the ability to evoke MEP data (p = 0.21 upper extremity, p = 0.11 lower extremity).
Sensory-motor mapping (phase reversal)
Phase reversal technique was utilized in 45 cases. A classic phase reversal of the N20 component and a maximal negativity waveform were achieved in 17 and 13 patients, respectively. Both were regarded as indicators for the location of the central sulcus. Attempts to achieve any form of sensory-motor mapping failed in 15 patients. The median age of children for which classical phase reversal was obtained was significantly older than the median age of the maximal negativity and failure group (123.7 ± 56 months vs. 86 ± 58 months and 64 ± 64 months, respectively). Children with preoperative motor strength of 4–5/5 had a significantly higher chance of having a classic phase reversal pattern (12/15), compared with those with weaker preoperative strength (3/15) (p = 0.01 upper extremity, p = 0.04 lower extremity). Lesion location was not correlated with the ability to obtain a phase reversal pattern (p = 0.74).
Subcortical mapping
Subcortical stimulation was utilized in 30 patients. Successful mapping was established in 24 patients (motor threshold range = 0.9–23.0 mA) and was not correlated with age (p = 0.5).
Correlation between subcortical motor threshold and postoperative motor strength was calculated amongst 23 patients. Two patients displayed a transient postoperative motor decline, associated with supplementary motor area (SMA) syndrome, and were excluded from this analysis. For the remaining 21 patients, there was a significant correlation between subcortical motor threshold and any motor decline immediately after surgery. Of the 10 patients with threshold of 6 mA and lower, 4 had a motor decline following surgery. None of the 11 patients with a subcortical motor threshold higher than 6.0 mA showed a motor decline (p = 0.02).
Monitoring
Monitoring technique
tcMEP and dcMEP techniques were utilized in 26 and 37 cases, respectively. Both tcMEP and dcMEP were utilized in 16 cases.
Immediate postoperative neurological data was fully available for 18/26 and 26/37 patients with tcMEP and dcMEP data, respectively. Two patients with SMA syndrome were excluded from the analysis. We found a significant negative correlation between intraoperative dcMEP stability and clinical motor decline (p < 0.001). The sensitivity, specificity, and positive and negative predictive values of significant dcMEP attenuation for new postoperative deficit were 100%, 78%, 60%, and 100%, respectively (Table 3).
Six of the 10 patients with attenuated dcMEP data showed motor decline following surgery; 3 recovered at long-term follow-up and one remained compromised. Two patients were lost to long-term follow-up.
Similar to dcMEP stability, tcMEP stability also correlated with motor decline (p = 0.005). The sensitivity, specificity, positive predictive value, and negative predictive value were 66%, 100%, 100%, and 86%, respectively (Table 4).
Effect of lesion and surgical factors on dcMEP stability
dcMEP stability was not affected by lesion grade (low vs. high) (p = 1). Deep tumor location was significantly associated with dcMEP instability compared with superficial and superficial-deep locations (75%, 21%, and 37%, respectively, p = 0.05). dcMEP stability was not correlated with the surgical approach (p = 0.328) or extent of resection (gross total vs. subtotal) (p = 0.173).
Effect of ION on surgical decisions
ION affected surgical decisions in several manners, as outlined in Table 5. dcMEP instability affected mainly deep lesions (p = 0.048). In all other aspects of ION effect on surgery, there was no significant difference between the various locations.
Discussion
This is the first study describing systematic use of ION in pediatric supratentorial surgery. From the onset of our study, we were faced with the reality that interpretation of neurophysiologic recordings in children was different from those in adults. For example, absent motor responses to cortical stimulation, especially in the younger patients, did not necessarily correlate with nonprimary motor cortex location, perhaps due to lack of excitability because of immaturity of the motor pathways (potential false negative mapping). Conversely, evoking positive motor responses at times necessitated high stimulation intensities, causing current spread to distant cortical or subcortical regions, potentially leading to false positive mapping. Likewise, variations in general motor excitability made the interpretation of subcortical mapping difficult. Nevertheless, in this study, we have demonstrated that ION is a valid, and useful, surgical adjunct, which may be applied even in infants. Utilization of ION in children may have an impact on the ability to maximize and safely resect supratentorial tumors, as well as epileptic foci, even in the young population.
Our key take home messages are as follows:
Short-train monopolar stimulation technique elicited motor responses at younger ages compared with the long-train bipolar Penfield technique.
The current needed to elicit a motor response following short-train stimulation decreased with age.
The ability to evoke a motor response was independent of lesion location or grade.
Preoperative motor strength was not associated with the ability to evoke MEP.
There was no correlation between lesion location and likelihood of successful phase reversal.
Preoperative strength of 4/5 or higher was correlated with a classic phase reversal pattern, as opposed to a weaker motor status which did not.
Subcortical mapping threshold for a motor response of less than 6 mA was associated with a higher rate of postoperative motor decline compared with a higher threshold.
dcMEP was more sensitive than tcMEP in predicting postoperative motor decline.
dcMEP decline was not associated with tumor grade, or EOR; however, it was associated with lesion location and more prone to decline in deep locations.
ION actively affected surgical decisions in several aspects, such as altering the corticectomy location and alarming due to a MEP decline.
Several of these ION impacts are not unique to the pediatric population; however, we have shown that they are just as valid in the pediatric population as in the adult one.
Stimulation techniques in children
Perhaps one of the most significant findings of this paper relates to comparisons between the success of monopolar fast short-train and “classic” long-train bipolar Penfield techniques. Our results support the ability to elicit motor responses at younger ages using the short-train technique even in infants, our youngest being 3 months old. Children younger than this were not included in the study. These results echo those presented by Jain et al., which showed the superiority of short-train monopolar stimulation over traditional Penfield stimulation, both in accuracy and ability to elicit a motor response and in lower epileptogenesis amongst children undergoing epilepsy surgery [6]. In a recent editorial by Sala, both techniques were compared, and the advantages of the short-train over Penfield techniques were described with a clear advocacy for the monopolar short-train technique as an ideal in most situations [15].
Implementation of ION in tumor and epilepsy surgery
Overall survival and progression-free survival have been shown to correlate with EOR in pediatric LGG [24]. However, proximity to primary motor cortex (M1) and the CST, such as for thalamic tumors, may hamper complete resection. In the adult population, fMRI may assist in defining functional cortical regions preoperatively, and awake craniotomy with intraoperative motor stimulation and evaluation, as well as ION, is used to improve EOR while reducing motor-related risks. Since there are limitations in children for the use of fMRI, as well as the inability to perform awake surgery, other techniques are used preoperatively (such as DTI of the motor pathways in thalamic lesions) or intraoperatively (such as ION) [10, 19].
In extratemporal refractory epilepsy, which is more prevalent in children than in adults, maximal resection of the epileptic zone (EZ) is correlated with seizure freedom, improved quality of life, and reduction of epilepsy associated morbidity and mortality. Removal of the EZ in proximity to the motor pathways, or performing a frontal lobectomy with motor sparing, or a TPO resection or disconnection (with motor-sensory sparing), necessitates motor mapping and monitoring to verify motor pathway integrity during surgery [25].
Implementing ION in pediatric supratentorial surgery relates to these pathologies in several ways:
Delineating the primary motor cortex (M1) for motor preservation in epilepsy and tumor resections (by using phase reversal and M1 mapping)
Monitoring CST integrity with dcMEP and tcMEP in order to identify impending injury and altering the surgical course
Identifying the location of the subcortical CST by means of subcortical mapping
We have shown that ION has a role even in infants; however, ION techniques must be tailored to the patients’ age [2]. Even then, ION is of limited sensitivity to predict postoperative motor decline and is affected by several factors, such as lesion location. Nevertheless, the fact that ION has a role in pediatric supratentorial surgery is of utmost importance, as it may facilitate “maximal safe resection” of various pathologies. In a recent paper, we have described the use in pediatric supratentorial surgery of an “electrified CUSA” that combines subcortical stimulation for subcortical motor mapping during CUSA aspiration [14]. This technique, previously described in adults [12, 21], was shown to be successfully applied in 11 children ages 1–18 years.
Child’s age
We defined evocability as the ability to elicit a motor response following either direct or indirect stimulation of the brain, with the underlying maturation of the motor system as the main predictor of success [2]. Thus, in principle, infants and young children would be less suitable for utilizing the full battery of ION techniques. However, similar to others, our results suggest a higher success rate by using the monopolar short-train stimulation technique (as opposed to the Penfield technique) in infants under the age of 2 years and even as young as 3 months [19]. Also, as stated by others, our data supports that monopolar short-train stimulation threshold values inversely correlated with age, with a higher threshold needed in younger ages [8, 19]. This was also reflected by using stimulation currents of up to 25 mA (as opposed to up to 20 mA—typically used in adults) for eliciting a response. It is notable though that use of the Penfield technique has been shown to be relatively epileptogenic, thus providing an additional benefit for the monopolar short-train technique both in the adult context but also in young children [17]. Similar to prior publications, our data suggests that successful recordings of either classic or nonclassic phase reversal are also dependent upon age [17].
Preoperative neurological status
It is reasonable to assume that the baseline clinical motor status would have a negative effect on mapping, due to antecedent weakness and possible compromised integrity of the motor system. In our study, however, we saw no significant correlation between poor motor strength and the monitorability of MEPs. This may be due to the lack of statistical power because of a relatively small number of patients in whom no motor baseline was available (6), as well as to the difficult ability to accurately assess clinical motor strength in this young population. Another reason for this unexpected lack of correlation may be due to the fact that we considered only two groups of motor strength (0–3 vs. 4–5). Usually, unless the paresis is severe, muscle MEPs can still be elicited, and therefore, some children with motor strength of 2–3 could retain muscle MEPs.
Lesion-related variables
Interestingly, several lesion characteristics had no correlation to ION. Location was not associated with evocability; however, deep lesions were associated with dcMEP instability. This is an important point that strengthens the case for using ION even in young patients and especially with deep located lesions. Lesion grade was not associated with evocability, ability to achieve a phase reversal pattern, and dcMEP stability. These points support ION use in low- as well as high-grade lesions.
Impact on surgical strategy
In our approach to tumor and epilepsy surgery under neurophysiological guidance, the location of the lesion had an impact on the planned surgical strategy, as well as affecting surgical decisions. For instance, in superficial lesions, direct cortical stimulation was utilized with an emphasis on determining a safe corticectomy region avoiding primary motor cortex, rather than the subsequent dcMEP monitoring. By comparison, for deep-seated lesions, dcMEP and tcMEP were emphasized to assess pyramidal tract integrity during the surgical approach and stages of resection, alongside subcortical mapping to assess proximity to the CST. For ventricular tumors, ION assisted during the approach (with lateral retraction on the brain during an interhemispheric approach or posterior retraction during a frontal transcortical approach), and also during tumor resection, when in proximity to the internal capsule.
In slightly less than half of the patients, the ION contribution was categorized as “no effect despite reliable monitoring.” This is not to say that the ION contribution was irrelevant or nonexistent. With close, tight monitoring, including reliable reassurance that no injury is indicated, the aggressiveness of surgical resection could be maximized, with the surgeon possessing a greater degree of confidence regarding the neurological well-being.
Types of corticospinal tract monitoring
In general, the role of tcMEP in pediatric supratentorial surgery could not be determined in our work due to the small number of patients monitored by this method. It is noteworthy however that the sensitivity of the tcMEP method in predicting a postoperative motor deficit was shown to be only 66%. It has been suggested that the nature of transcranial stimulation may bypass the lesion and excite the tracts beneath the tumor location, possibly contributing to a false negative in the supratentorial settings, even more prominently in children where small cranial size and thinness are evident [2, 19].
The sensitivity of dcMEP recordings in predicting an immediate postoperative motor decline was found to be 75%; however, once excluding the 2 patients with SMA syndrome, sensitivity was 100%. There could be several reasons leading to limited sensitivity: first, some of the lesions were located frontally near the SMA. Because dcMEPs are mediated by the pyramidal tracts and primary motor cortex only, they are not expected to be sensitive to SMA region lesions, which tend to result in transient weakness. This may explain the two patients who emerged with significant motor deficit yet displayed stable dcMEP monitoring and subcortical mapping thresholds of 16 and 17.5 mA. Since in principle MEPs are not expected to be sensitive to prefrontal lesions, we excluded these patients from the dcMEP analysis, and the sensitivity of dcMEP to true corticospinal injury was shown to be 100%.
Second, in our series, the vast majority of dcMEP recordings were made from the contralateral upper extremity (APB, Thenar), and this muscle group was used as a general representation of complete pyramidal tract integrity. The spatial limitation of the placement and orientation of the strip electrode along the cortex makes stimulation of the complete primary motor cortex impossible. As a result, MEP data representing the face, arm, or leg may not be available, potentially decreasing the dcMEP sensitivity.
Third, extrasurgical (postsurgical) processes like inflammation, edema, or vascular insult can result in motor weakness and by their temporal nature of appearance be undetected by intraoperative dcMEP monitoring.
Limitations of the study
The retrospective nature of this study contributes to the lack of real-time neurological evaluation and loss of long-term follow-up. Younger age patients are also difficult to accurately examine, limiting neurological evaluation. Additionally, the heterogeneity of the study group, by age, pathology, lesion location, and baseline neurological status, limits the reliability of extrapolating conclusions.
The definitions of lesion location into “superficial, deep, etc.” are arbitrary and inaccurate. Different superficial locations may have a different impact on motor monitoring and the same for different deep locations. As stated above, breaking the data into superselective groups was not practical due to small numbers; however, this should be looked into in future studies on this topic.
Not all patients had all monitoring modalities, such as tcMEP, dcMEP, and scrtMEP. Complete data sets, including full clinical evaluations, were available only in 11 cases. Reasons for incomplete MEP evaluation included the preclusion of strip electrode placement due to prior craniotomies and adhesions, nonexposed M1, bulging brain, etc.
Our patients did not routinely have preoperative and postoperative DTI, and we could not routinely asses the distance between cortical and subcortical stimulation locations, motor responses, and distance on navigation from M1 or the CST, as demonstrated on DTI. Thus, we could not correlate the threshold of motor response to the distance from these functional structures. In the adult population, it has been shown that the ratio between scrtMEP threshold and distance from the CST is roughly 1:1 [11, 20]. We could not evaluate this ratio in the study group and could not state how it was affected by age or any other factors. Thus, when implementing scrtMEP in children, it is possible that the distance to the CST may be closer than expected. Additionally, a threshold of 6 mA may be inaccurate, as the number of patients below this threshold was limited. Further investigations incorporating low-threshold scrtMEP are recommended.
Conclusion
ION has a role in pediatric supratentorial surgery. Depending on lesion location and proximity to the CST and M1, mapping (including subcortical) and monitoring may be applied. Technical nuances should take into account the patient’s age. There is a need for a prospective, large cohort, implementing DTI, navigation, and a thorough early and late neurological evaluation, to refine the limitations and interpretation of ION in this unique population.
References
Barzilai O, Lidar Z, Constantini S, Salame K, Bitan-Talmor Y, Korn A (2017) Continuous mapping of the corticospinal tracts in intramedullary spinal cord tumor surgery using an electrified ultrasonic aspirator. J Neurosurg Spine 27:161–168
Coppola A, Tramontano V, Basaldella F, Arcaro C, Squintani G, Sala F (2016) Intra-operative neurophysiological mapping and monitoring during brain tumour surgery in children: an update. Childs Nerv Syst 32:1849–1859
Deletis V, Fernandez-Conejero I (2016) Intraoperative monitoring and mapping of the functional integrity of the brainstem. J Clin Neurol 12:262–273
Duffau H, Capelle L, Denvil D, Sichez N, Gatignol P, Taillandier L, Lopes M, Mitchell MC, Roche S, Muller JC, Bitar A, Sichez JP, van Effenterre R (2003) Usefulness of intraoperative electrical subcortical mapping during surgery for low-grade gliomas located within eloquent brain regions: functional results in a consecutive series of 103 patients. J Neurosurg 98:764–778
Fulkerson DH, Satyan KB, Wilder LM, Riviello JJ, Stayer SA, Whitehead WE, Curry DJ, Dauser RC, Luerssen TG, Jea A (2011) Intraoperative monitoring of motor evoked potentials in very young children. J Neurosurg Pediatr 7:331–337
Jain P, Whitney R, Strantzas S, McCoy B, Ochi A, Otsubo H, Snead OC 3rd, Weiss S, Donner E, Pang E, Sharma R, Viljoen A, Keller A, Drake JM, Rutka JT, Go C (2018) Intra-operative cortical motor mapping using subdural grid electrodes in children undergoing epilepsy surgery evaluation and comparison with the conventional extra-operative motor mapping. Clin Neurophysiol 129:2642–2649
Keles GE, Lundin DA, Lamborn KR, Chang EF, Ojemann G, Berger MS (2004) Intraoperative subcortical stimulation mapping for hemispherical perirolandic gliomas located within or adjacent to the descending motor pathways: evaluation of morbidity and assessment of functional outcome in 294 patients. J Neurosurg 100:369–375
Lieberman JA, Lyon R, Feiner J, Diab M, Gregory GA (2006) The effect of age on motor evoked potentials in children under propofol/isoflurane anesthesia. Anesth Analg 103:316–321 table of contents
Morota N, Ihara S, Deletis V (2010) Intraoperative neurophysiology for surgery in and around the brainstem: role of brainstem mapping and corticobulbar tract motor-evoked potential monitoring. Childs Nerv Syst 26:513–521
Moshel YA, Elliott RE, Monoky DJ, Wisoff JH (2009) Role of diffusion tensor imaging in resection of thalamic juvenile pilocytic astrocytoma. J Neurosurg Pediatr 4:495–505
Nossek E, Korn A, Shahar T, Kanner AA, Yaffe H, Marcovici D, Ben-Harosh C, Ben Ami H, Weinstein M, Shapira-Lichter I, Constantini S, Hendler T, Ram Z (2011) Intraoperative mapping and monitoring of the corticospinal tracts with neurophysiological assessment and 3-dimensional ultrasonography-based navigation. Clinical article. J Neurosurg 114:738–746
Raabe A, Beck J, Schucht P, Seidel K (2014) Continuous dynamic mapping of the corticospinal tract during surgery of motor eloquent brain tumors: evaluation of a new method. J Neurosurg 120:1015–1024
Ringel F, Sala F (2015) Intraoperative mapping and monitoring in supratentorial tumor surgery. J Neurosurg Sci 59:129–139
Roth J, Korn A, Bitan-Talmor Y, Kaufman R, Ekstein M, Constantini S (2017) Subcortical mapping using an electrified Cavitron ultrasonic aspirator in pediatric supratentorial surgery. World Neurosurg 101:357–364
Sala F (2018) Penfield’s stimulation for direct cortical motor mapping: an outdated technique? Clin Neurophysiol 129(12):2635–2637. https://doi.org/10.1016/j.clinph.2018.09.021
Sala F, Coppola A, Tramontano V, Babini M, Pinna G (2015) Intraoperative neurophysiological monitoring for the resection of brain tumors in pediatric patients. J Neurosurg Sci 59:373–382
Sala F, Krzan MJ, Deletis V (2002) Intraoperative neurophysiological monitoring in pediatric neurosurgery: why, when, how? Childs Nerv Syst 18:264–287
Sala F, Lanteri P (2003) Brain surgery in motor areas: the invaluable assistance of intraoperative neurophysiological monitoring. J Neurosurg Sci 47:79–88
Sala F, Manganotti P, Grossauer S, Tramontanto V, Mazza C, Gerosa M (2010) Intraoperative neurophysiology of the motor system in children: a tailored approach. Childs Nerv Syst 26:473–490
Shiban E, Krieg SM, Haller B, Buchmann N, Obermueller T, Boeckh-Behrens T, Wostrack M, Meyer B, Ringel F (2015) Intraoperative subcortical motor evoked potential stimulation: how close is the corticospinal tract? J Neurosurg 123:711–720
Shiban E, Krieg SM, Obermueller T, Wostrack M, Meyer B, Ringel F (2015) Continuous subcortical motor evoked potential stimulation using the tip of an ultrasonic aspirator for the resection of motor eloquent lesions. J Neurosurg 123:301–306
Vadivelu S, Sivaganesan A, Patel AJ, Agadi S, Schmidt RJ, Mani P, Jea A (2014) Practice trends in the utilization of intraoperative neurophysiological monitoring in pediatric neurosurgery as a function of complication rate, and patient-, surgeon-, and procedure-related factors. World Neurosurg 81:617–623
Verla T, Fridley JS, Khan AB, Mayer RR, Omeis I (2016) Neuromonitoring for intramedullary spinal cord tumor surgery. World Neurosurg 95:108–116
Wisoff JH, Sanford RA, Heier LA, Sposto R, Burger PC, Yates AJ, Holmes EJ, Kun LE (2011) Primary neurosurgery for pediatric low-grade gliomas: a prospective multi-institutional study from the Children’s Oncology Group. Neurosurgery 68:1548–1554 discussion 1554-1545
Yang TF, Chen HH, Liang ML, Chen C, Chiu JW, Wang JC, Lai CJ, Liao KK, Chan RC (2014) Intraoperative brain mapping to identify corticospinal projections during resective epilepsy surgery in children with congenital hemiparesis. Childs Nerv Syst 30:1559–1564
Acknowledgments
We thank Tomer Ziv-Baran, PhD, for the assistance with statistical analysis, and Mrs. Adina Sherer for the editorial assistance.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Roth, J., Korn, A., Sala, F. et al. Intraoperative neurophysiology in pediatric supratentorial surgery: experience with 57 cases. Childs Nerv Syst 36, 315–324 (2020). https://doi.org/10.1007/s00381-019-04356-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00381-019-04356-0