Abstract
Objective
The effectiveness of decompressive craniectomy (DC) in the context of neurocritical care in adult patients has been recently under debate. The aim of our study was to evaluate the impact of decompressive craniectomy in severe traumatic brain injury (TBI) in children, focusing on short and long-term neurological and neuropsychological outcomes.
Methods
Retrospective review of the medical records of children admitted at a level I trauma center, between January 2012 and December 2015, submitted to DC due to severe TBI. Additionally, an extensive review of literature on this subject was carried out.
Results
Sixteen patients underwent DC for TBI at our institution during the evaluated period. 62.5% were males and the mean age was 12 years. Road traffic accident (RTA) was the main mechanism of trauma (62.5%). Average Glasgow Coma Scale (GCS) at admission was 5.2, whereas 75% of the patients presented with pathological pupillary reaction. Initial computed tomography (CT) showed skull fractures in 62.5% and acute subdural hemorrhage (ASH) in 56.3% of the patients. The mean intracranial pressure (ICP) was 27.2 mmHg prior to surgery, and the mean time window between admission and DC was 36.3 h. Unilateral DC was performed in 68.8% of the cases. The average Glasgow Outcome Scale (GOS) at 6-month follow-up was 3.7, whereas 70% of the survivors presented good recovery (GOS 4–5). Abnormal pupillary reaction at hospital admission increased 3-fold the risk of long-term neuropsychological disturbances. Follow-up evaluation revealed cognitive abnormality in 55.6% of the patients. The overall mortality at 6-month follow-up was 37.5%.
Conclusion
The present study indicates towards a potential benefit of DC in children with severe TBI; nevertheless, our data demonstrated a high incidence of neuropsychological impairment in the long-term follow-up. Psychological and cognitive assessment should be computed in prognosis evaluation in future prospective studies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Traumatic brain injury (TBI) is a major global health concern and remains the first cause of death and long-term disability among children worldwide [1]. Data from the Centers for Disease Control and Prevention (CDC) indicate that 2.5 million Americans sustained a TBI ranging from mild to severe in 2014, whereas 37,200 children suffered severe TBI, with up to 1.3 million life-years potentially adversely affected [2, 3].
The presence of brain swelling and uncontrolled elevated intracranial pressure (ICP) strongly correlate with outcome and are well-known causes of death and neurologic disability after severe TBI [4,5,6]. Recently, randomized controlled clinical trials (RCTs) have evaluated the effectiveness of decompressive craniectomy (DC) as a salvage treatment for refractory intracranial hypertension in the setting of severe TBI in adults [6, 7].
Regarding the pediatric population, over half of the patients develop intracranial hypertension following severe TBI. Although DC has been proven to be highly effective in controlling ICP both in adults and children, most studies addressing short- and long-term clinical outcomes in children consist of case series or retrospective data analysis [8]. Therefore, there is still no strong evidence advocating in favor or against DC in severe TBI in the pediatric population, so that current guidelines have been adapted from studies in adults [4, 9] and recommend DC not as a stand-alone procedure, but associated with surgery for evacuation of intracranial hematomas or in case of neurological deterioration due to intracranial hypertension refractory to medical treatment [4, 8, 9]. Furthermore, to the best of our knowledge, there is a lack of information on the long-term cognitive and psychological function of these patients following severe TBI and DC.
In the current study, we aim to report on a single-center experience with DC for the management of severe TBI in children, addressing standard epidemiological, clinical, and surgical aspects, but also emphasizing long-term cognitive impairment, neurological, and psychological outcomes under the light of the current literature.
Methods
A retrospective study was performed on medical records of a total of 16 pediatric patients under 18 years old, submitted to DC following severe TBI, between January 2012 and December 2015, at a level I trauma center. Data regarding age at hospital admission, gender, mechanism of injury, presenting GCS score, initial radiological imaging, time window from hospital admission to surgical treatment, neurological outcome, and mortality were obtained from patient charts. Additionally, long-term cognitive and psychological performances were evaluated. Glasgow Outcome Scale (GOS) was applied as outcome measure, whereas GOS 4 and 5 were classified as “good outcome” and GOS 1 to 3, “bad outcome” for statistical analysis.
Additionally, we searched PubMed/Medline for entries with terms “decompressive craniectomy” and “traumatic brain injury” until March 2019, limited to papers written in the English and Spanish languages. The following inclusion criteria were applied: reports of a total of 5 or more patients, under 18 years old, decompressive craniectomy (unilateral, bilateral, or bifrontal), and minimum follow-up of 1 month after surgery. For the data analysis, only complete reports were considered.
Descriptive and statistical analyses were performed using SPSS 22 (SPSS Inc., Chicago, Illinois, USA). Chi-square test was performed to compare the distribution of categorical variables, and a non-parametric Mann-Whitney U test was employed to evaluate intergroup comparisons of continuous variables. Kaplan-Meier log rank (Mantel-Cox) was applied for survival analysis. p < 0.05 was considered as statistically significant.
Results
Patient population and neurological evaluation
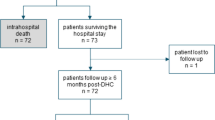
Sixteen children underwent DC for the treatment of severe TBI in the studied period. The gender distribution consisted of ten males (62.5%) and six (37.5%) females. The mean age at hospital admission was 12 years (range (R) 0.42–17.0, SD = 4.7). Common mechanisms of traumatic injury were road traffic accident (RTA) in 10 out of 16 patients (62.5%), physical aggression in three (18.8%), fall in one case (6.3%), gunshot in one patient (6.3%), and blunt head trauma in one child (6.3%). The mean time from trauma to admission at the emergency department was 2.45 h (R 0.7–14, SD = 43.1). Mean GCS at admission was 5.2 (R 3–13, SD = 2.7). Regarding pupil reaction to light at admission, we found bilateral non-reactive mydriatic pupils in five (31.3%), unremarkable reaction in four (25%), slow reactive pupils in four (25%), and anisocoria in three (18.8%) patients. Abnormal pupillary reaction to light at hospital admission increased 5-fold the risk of death and was significantly associated with unfavorable outcome (p = 0.04) (Fig. 1).
Neuroimaging characteristics
Initial CT imaging at admission was classified according to Marshall et al. [10] as follows: Marshal II in 12.5% of the cases, Marshall III in 56.3%, and Marshall IV in 31.3% of the patients. Ten out of 16 (62.5%) children presented skull fracture; nine (56.3%) patients, acute subdural hematoma; five (31.3%), brain contusion; and two (12.5%), intraventricular hemorrhage. Mean ICP prior to DC was 27.2 mmHg (R 4–60 mmHg, SD = 17.5).
Decompressive craniectomy and complications
The mean time window from hospital admission to surgical DC was 36.3 h (R 2–144 h, SD = 38.7). Primary DC (as soon as admitted) was carried out in six children (37.5%). Eleven (68.8%) out of 16 patients underwent unilateral DC, whereas bifrontal craniectomy was performed in five children (31.3%). No patient in this series underwent bilateral fronto-temporo-parietal DC.
Nearly all patients developed clinical complications at some point of the follow-up. Nosocomial pneumonia was diagnosed in 10 out of 16 patients (62.5%), trauma-related hydrocephalus in five children (31.3%), meningitis or ventriculitis in four (25%), and surgical wound infection in three patients (18.8%).
Outcome
Mean hospitalization time was 55.9 days (3–373, SD = 98.2). Among patients who overcame the first 6 months after hospital discharge, the mean GOS was 3.7 (SD = 1.25) and 4.1 (SD = 1.29) at 6-month and at two-year follow-up, respectively, whereas 7 out of 10 patients (70%) presented good recovery (GOS 4–5). The mortality rate at 1- and 6-month follow-up was 31.2% and 37.5%, respectively.
Concerning the long-term psychological and cognitive evaluations, four out of 10 children presented unremarkable assessments, whereas two showed aggressive behavior, two had mild cognitive impairment, one remained restricted to bed in vegetative state, and one child with persistent severe cognitive impairment. Interestingly, long-term neuropsychological outcome was strongly correlated with the severity of neurological presentation at admission. Abnormal pupillary reaction to light at admission increased 3-fold the risk of long-term psychological and cognitive disturbances at 6-month follow-up (p = 0.03).
Epidemiological features of the cohort extracted from the medical files are summarized in Table 1.
Pearson’s chi-squared test (X2) or Mann-Whitney U test was employed, accordingly, for statistical analysis. There was no statistically significant correlation between the following variables and outcome: gender (p = 0.6), age (< 10 or ≥ 10 years) (p = 0.7), time window from trauma to hospital admission (< 2 h or ≥ 2 h) (p = 0.5), GCS at admission (p = 0.2), presence of brain shift on initial CT scan (< 5 mm or ≥ 5 mm) (p = 0.6), ICP values prior to DC (< 20 mmHg or ≥ 20 mmHg) (p = 0.4), Marshall classification (p = 0.8), surgical technique for DC (unilateral or bifrontal (p = 0.8), side of DC (p = 0.9), primary or secondary DC (p = 0.6), and occurrence of post-operative meningitis (p = 0.3). However, length of hospitalization (< 30 days or ≥ 30 days) correlated with outcome (good or bad), (p = 0.04), whereas the mean hospitalization time was 79.7 (R 9–373) and 16.1 (3–55) days, among survivors and deceased patients, respectively. To compare continuous variables, Mann-Whitney U test was performed; however, no correlation with outcome the following variables could be established: age (p = 0.5), length of time from trauma to hospital admission (p = 0.6), brain shift (mm) (p = 0.5), length of time from trauma to DC (p = 1.0), and ICP (mmHg) prior to DC (p = 0.4).
Literature review
Systematic review of the literature is summarized in Table 2.
A total of 183 peer-reviewed papers were found. Out of them, only 14 publications (236 patients) matched our inclusion criteria and were finally selected.
Mean age among the 236 children submitted to DC across the selected studies was 6.3 years (R 1.18–14.5), whereas 138 patients (58.5%) were male. The mean time from hospital admission to surgery was 41.8 h (R 1.0–97.6). One hundred eighty-five out of 236 (78.5%) children presented at admission with GCS < 8, whereas the mean value at admission across studies was 5.6 (R 4.28–8.87). One hundred eighteen out of 236 patients (50%) were admitted with anisocoria, while 14.5% presented bilateral non-reactive mydriatic pupils.
Concerning initial radiological imaging, 48.8% presented with acute subdural hematoma, skull fracture in 43.3%, midline brain shift in 14.9%, diffuse brain edema in 34.3%, brain contusion in 33.3%, epidural hematoma in 12.9%, and intraventricular hemorrhage in 4.5%.
Unilateral decompressive craniectomy was performed in 70.7% of the patients, bilateral DC in 11.5%, bifrontal DC in 14.9%, and posterior fossa decompression in 2.9%. Primary DC was carried out in 97 out of 236 (41.2%) children. The reported overall mortality across studies at 1- and 6-month follow-up was 21.1% (47/223) and 20.9% (49/236), respectively. Mean GOS was 4.0, whereas 81.8% of the survivors had good recovery (GOS 4–5) and 18.2% unfavorable outcome (GOS 2–3).
Illustrative case
A 3 year-old male involved in a RTA was admitted at the emergency department 40 min after TBI. At admission he presented a GCS of 7, isocoric and reactive pupils, and unremarkable vital parameters. The multidisciplinary trauma evaluation revealed polytraumatic injuries with multiple bone fractures and splenic lesion. The CT scan of the head showed no evidence of intracranial bleeding or severe edema. Decision was taken to monitor the ICP, which was initially within the normal range. Subsequent ICP monitoring showed 36 h after TBI a harsh and refractory to treatment increase of ICP (up to 32 mmHg). Radiological imaging showed diffuse severe brain edema (Fig. 2). Thereafter, a bifrontal DC was performed. The patient recovered well and could be discharged on the 37th day following TBI with a GOS of 5 (Fig. 2). Cranioplasty was performed uneventfully 55 days after DC, and the patient followed-up regularly in a clinic. Five years after TBI, although areas of left-sided temporal and cerebellar encephalomalacia could still be identified on MRI scan (Fig. 3), the patient presented good performance at school and unremarkable cognitive and psychological development.
Discussion
Primary brain injury develops immediately after trauma. Direct forces to the head generate focal insults, such as intracranial contusion and hemorrhage. Acceleration-deceleration and rotation forces can also create inertial effect, resulting in axonal shearing and widespread damage to the central nervous system [11]. Secondary brain injury is understood as an indirect result of the primary injury, occurring minutes to weeks after TBI. Cerebral edema, impaired metabolism, altered cerebral blood flow, formation of free radical, and excitotoxicity as well as elevated ICP, coagulopathy, and seizures are well-known factors that increase brain injury, potentially worsening clinical and neurological outcomes [11,12,13,14].
Sustained increased ICP is strongly associated with death and persistent disability following TBI [15]. When first-stage measures fail to control ICP, salvage second-tier strategies can be employed. The role of such strategies, such as barbiturate coma, intermittent hyperventilation, hypothermia, and decompressive craniectomy (DC), is under debated [4, 8, 11, 16].
Regarding adult population, the DECRA study suggested that patients undergoing primary bifrontal craniectomy have worse outcome than those receiving intensive medical care alone [7]. On the other hand, the RESCUEicp study randomized 408 patients (aged between 10 and 65 years) with TBI and refractory elevated ICP (> 25 mmHg) to undergo secondary DC or receive best medical therapy only. Fifty-six (13.7%) patients were under 18 years. Overall, DC resulted in lower mortality (26.9% versus 48.9%), but higher rates of vegetative state, “lower severe disability,” and “upper severe disability.” The rates of moderate disability and good recovery were similar in the two groups [17]. However, the subset of younger patients within this cohort performed significantly better at 12-month follow-up, where 45.4% of the patients in the surgical group were independent at home, as compared with 32.4% of children in the medical group (p = 0.01) [9].
When comparing the present cohort with previously published data, we found a higher prevalence of male subjects (62.5% vs 58.5) and RTA as cause of TBI (62.5% vs 31.3%) in our series. The mean age in this cohort was 12 vs 6.3 years; however, the mean GCS at hospital admission was similar (5.2 vs 5.6). Initial early CT showed showed skull fracture in 62.5% (vs 43.3%), and acute subdural hematoma was present in 56.3% (vs 48.8%) of the children. Average ICP before surgery was slightly higher in the previously published studies (mean = 27.2 mmHg vs 31.6 mmHg) and DC was performed later (mean time to DC 36.3 h vs 41.8 h). Regarding the technique, 68.8% (vs 70.7%) of the patients in our cohort had unilateral decompression, and 31.3% (vs 11.5%) had bifrontal DC. In this cohort, primary DC was conducted in 37.5% (vs 41.2%) of children.
Six-month mortality rate was 37.5% in this cohort, compared with 20.9% across reviewed studies. Furthermore, GOS at 2-year follow-up was 4.1 vs 4.0, whereas 70% (vs 81.8%) of survivors presented good long-term outcome (GOS 4–5).
The results of the present study corroborate with recent publications showing that DC is effective in controlling ICP and directly impacts on survival and neurological outcomes of severe TBI in the pediatric population. The slightly higher mortality rate in our series was directly related to a more dramatic neurological presentation at hospital admission. In our cohort, 75% of patients presented abnormal pupillary reaction, whereas 31.3% had non-reactive pupils and 18.8% anisocoric pupils at admission. The incidence of bilateral non-reactive pupils in our cohort was 2.1-fold higher than the average reported in the literature (Table 2), and abnormal pupillary reaction was associated with a 5-fold increased risk of bad outcome.
Pathologic pupillary reflex has been shown to be a prognostic factor of unfavorable outcome following TBI in adults and children, whereas isolated evaluation of GCS at admission has limited value [18, 19]. Fulkerson et al. evaluated 67 children with severe TBI and showed pupillary response to be the factor most predictive of survival and outcome. If pupillary reaction was not normal, the chance of survival dropped from 87 to 23% in that cohort [20].
Taylor et al. randomized 27 children with raised ICP to clinical treatment (n = 14) or decompressive bitemporal craniectomy (n = 13) showing better outcome at 6-month follow-up in patients submitted to DC, with an overall mortality rate of 23.1% [21]. Authors described 70% of good outcomes, while DECRA achieved only 37.3% and RESCUEicp 37.2% [6, 7, 21]. Possibly, despite having similar mortality rates, pediatric patients may present better functional outcome over time.
Overall, these results indicate that early surgical decompression associated with intensive medical therapy may indeed be life-saving and positively impact outcome in the pediatric age group. Nevertheless, in our cohort, 55.6% of the patients who survived the acute phase of TBI presented psychological and/or cognitive impairment at long-term follow-up, and this has to be considered and properly addressed with patient’s relatives and caregivers when discussing prognosis.
The prevalence of cognitive impairment across all age groups following intensive care treatment is estimated between 25 and 78% [22]. This is particularly alarming in the pediatric population, potentially leading to significant and far-reaching difficulties into adulthood, if not timely recognized and intervened [22, 23]. Apart from TBI itself, other factors associated to any critical illness and long stay in an intensive care unit (ICU) have been related to long-term cognitive impairment, such as hypoxia, glucose dysregulation, delirium, sedatives, and use of opioids [24]. Other factors, such as social economic status and education level of patient’s parents, may also contribute to patient’s long-term cognitive outcome [22].
To the best of our knowledge, long-term psychological and cognitive impairments following severe TBI and DC in children have not been specifically addressed so far. There are few studies discussing psychological aspects of patients submitted to DC. Andruszkow et al. in a cohort including 2602 TBI patients found increasing incidence of anxiety and depression associated with increasing age [25]. Furthermore, TBI sustained in younger age has been associated with cognitive impairment and cumulative effect on neuropsychologic development at older age, the “growing into deficits” hypothesis [2, 26, 27]. Brain damage during the phase of rapid maturation and neuronal pruning in young children is a possible explanation, as new functions rely on undamaged tissues [28]. In line with this, Max et al. found in a prospective study that 49% of a cohort of 75 children with TBI developed novel psychiatric disorder in comparison with only 13% of patients with isolated orthopedic trauma [29, 30], whereas Ahmadi et al. reported a high prevalence of major depression following TBI [31]. In our cohort, 22.2% of the children presented aggressiveness, 22.2% mild cognitive impairment, 22.2% vegetative state, and 11.1% severe cognitive dysfunction at long-term follow-up. None of the patients in this cohort developed anxiety or major depression. Interestingly, post-traumatic abnormal pupillary reaction at admission significantly not only increased the risk of unfavorable outcome and death but was also strongly associated with the development of long-term cognitive impairment among survivors.
The present study has some limitations, such as small sample size and the lack of a control group (i.e., patients submitted to intensive medical treatment only), which precluded more robust statistical analysis of the impact of DC on long-term outcome.
Conclusions
The present study indicates towards a potential benefit of DC in children with severe TBI; nevertheless, our data demonstrated a high incidence of neuropsychological impairment in the long-term follow-up. Post-traumatic pupillary dysfunction at admission is a predictor of unfavorable outcome and long-term neuropsychological disturbances among children. Rehabilitation goals have to be established by a multidisciplinary team upon admission and revisited throughout recovery, focusing on strategies to compensate for cognitive deficits and facilitate neurocognitive recovery, motor skill development, and social reintegration. Psychological and cognitive assessment should be computed in prognosis evaluation in future prospective studies.
References
Walker PA, Harting MT, Baumgartner JE, Fletcher S, Strobel N, Cox CS Jr (2009) Modern approaches to pediatric brain injury therapy. J Trauma 67:S120–S127. https://doi.org/10.1097/TA.0b013e3181ad323a
Popernack ML, Gray N, Reuter-Rice K (2015) Moderate-to-severe traumatic brain injury in children: complications and rehabilitation strategies. J Pediatr Health Care Off Publ Natl Assoc Pediatr Nurse Assoc Pract 29:e1–e7. https://doi.org/10.1016/j.pedhc.2014.09.003
TBI Data and Statistics | Concussion | Traumatic brain injury | CDC Injury Center. https://www.cdc.gov/traumaticbraininjury/data/index.html. Accessed 23 Aug 2018
Brain Trauma Foundation, American Association of Neurological Surgeons, Congress of Neurological Surgeons et al (2007) Guidelines for the management of severe traumatic brain injury. Introduction. J Neurotrauma 24(Suppl 1):S1–S2. https://doi.org/10.1089/neu.2007.9997
Miller JD, Becker DP, Ward JD, Sullivan HG, Adams WE, Rosner MJ (1977) Significance of intracranial hypertension in severe head injury. J Neurosurg 47:503–516. https://doi.org/10.3171/jns.1977.47.4.0503
Hutchinson PJ, Kolias AG, Timofeev IS, Corteen EA, Czosnyka M, Timothy J, Anderson I, Bulters DO, Belli A, Eynon CA, Wadley J, Mendelow AD, Mitchell PM, Wilson MH, Critchley G, Sahuquillo J, Unterberg A, Servadei F, Teasdale GM, Pickard JD, Menon DK, Murray GD, Kirkpatrick PJ (2016) Trial of decompressive craniectomy for traumatic intracranial hypertension. N Engl J Med 375:1119–1130. https://doi.org/10.1056/NEJMoa1605215
Cooper DJ, Rosenfeld JV, Murray L, Arabi YM, Davies AR, D’Urso P, Kossmann T, Ponsford J, Seppelt I, Reilly P, Wolfe R, DECRA Trial Investigators, Australian and New Zealand Intensive Care Society Clinical Trials Group (2011) Decompressive craniectomy in diffuse traumatic brain injury. N Engl J Med 364:1493–1502. https://doi.org/10.1056/NEJMoa1102077
Ardissino M, Tang A, Muttoni E, Tsang K (2019) Decompressive craniectomy in paediatric traumatic brain injury: a systematic review of current evidence. Childs Nerv Syst ChNS Off J Int Soc Pediatr Neurosurg 35:209–216. https://doi.org/10.1007/s00381-018-3977-5
Young AMH, Kolias AG, Hutchinson PJ (2017) Decompressive craniectomy for traumatic intracranial hypertension: application in children. Childs Nerv Syst ChNS Off J Int Soc Pediatr Neurosurg 33:1745–1750. https://doi.org/10.1007/s00381-017-3534-7
Marshall LF, Marshall SB, Klauber MR et al (1992) The diagnosis of head injury requires a classification based on computed axial tomography. J Neurotrauma 9(Suppl 1):S287–S292
Morrissey K, Fairbrother H (2016) Severe traumatic brain injury in children: an evidence-based review of emergency department management. Pediatr Emerg Med Pract 13:1–28
Pinto PS, Poretti A, Meoded A, Tekes A, Huisman TAGM (2012) The unique features of traumatic brain injury in children. Review of the characteristics of the pediatric skull and brain, mechanisms of trauma, patterns of injury, complications and their imaging findings--part 1. J Neuroimaging Off J Am Soc Neuroimaging 22:e1–e17. https://doi.org/10.1111/j.1552-6569.2011.00688.x
Jankovic J, Mazziotta JC, Pomeroy SL, Daroff RB (2015) Bradley’s neurology in clinical practice, 2-volume set, 7th edn. Elsevier, London
Youmans JR, Winn HR (2011) Youmans neurological surgery. Elsevier - Health Sciences Division, St. Louis
Badri S, Chen J, Barber J, Temkin NR, Dikmen SS, Chesnut RM, Deem S, Yanez ND, Treggiari MM (2012) Mortality and long-term functional outcome associated with intracranial pressure after traumatic brain injury. Intensive Care Med 38:1800–1809. https://doi.org/10.1007/s00134-012-2655-4
Moon JW, Hyun DK (2017) Decompressive craniectomy in traumatic brain injury: a review article. Korean J Neurotrauma 13:1–8. https://doi.org/10.13004/kjnt.2017.13.1.1
Hutchinson PJ, Kolias AG, Timofeev IS et al (2016) Trial of decompressive craniectomy for traumatic intracranial hypertension. In: https://doi.org/10.1056/NEJMoa1605215. https://www.nejm.org/doi/10.1056/NEJMoa1605215?url_ver=Z39.88-2003&rfr_id=ori%3Arid%3Acrossref.org&rfr_dat=cr_pub%3Dwww.ncbi.nlm.nih.gov. Accessed 23 Aug 2018
Strnad M, Borovnik Lesjak V, Vujanović V, Križmarić M (2017) Predictors of mortality in patients with isolated severe traumatic brain injury. Wien Klin Wochenschr 129:110–114. https://doi.org/10.1007/s00508-016-0974-0
Pillai S, Praharaj SS, Mohanty A, Kolluri VR (2001) Prognostic factors in children with severe diffuse brain injuries: a study of 74 patients. Pediatr Neurosurg 34:98–103. https://doi.org/10.1159/000056002
Fulkerson DH, White IK, Rees JM, Baumanis MM, Smith JL, Ackerman LL, Boaz JC, Luerssen TG (2015) Analysis of long-term (median 10.5 years) outcomes in children presenting with traumatic brain injury and an initial Glasgow Coma Scale score of 3 or 4. J Neurosurg Pediatr 16:410–419. https://doi.org/10.3171/2015.3.PEDS14679
Taylor A, Butt W, Rosenfeld J, Shann F, Ditchfield M, Lewis E, Klug G, Wallace D, Henning R, Tibballs J (2001) A randomized trial of very early decompressive craniectomy in children with traumatic brain injury and sustained intracranial hypertension. Childs Nerv Syst ChNS Off J Int Soc Pediatr Neurosurg 17:154–162. https://doi.org/10.1007/s003810000410
Kachmar AG, Irving SY, Connolly CA, Curley MAQ (2018) A systematic review of risk factors associated with cognitive impairment after pediatric critical illness. Pediatr Crit Care Med J Soc Crit Care Med World Fed Pediatr Intensive Crit Care Soc 19:e164–e171. https://doi.org/10.1097/PCC.0000000000001430
Cermak CA, Scratch SE, Reed NP, Bradley K, Quinn de Launay KL, Beal DS (2019) Cognitive communication impairments in children with traumatic brain injury: a scoping review. J Head Trauma Rehabil 34:E13–E20. https://doi.org/10.1097/HTR.0000000000000419
Jackson JC, Ely EW (2013) Cognitive impairment after critical illness: etiologies, risk factors, and future directions. Semin Respir Crit Care Med 34:216–222. https://doi.org/10.1055/s-0033-1342984
Andruszkow H, Deniz E, Urner J, Probst C, Grün O, Lohse R, Frink M, Krettek C, Zeckey C, Hildebrand F (2014) Physical and psychological long-term outcome after traumatic brain injury in children and adult patients. Health Qual Life Outcomes 12:26. https://doi.org/10.1186/1477-7525-12-26
Fletcher JM, Miner ME, Ewing-Cobbs L (1987) Age and recovery from head injury in children: developmental issues. In: Levin HS, Grafman J, Eisenberg HM (eds) Neurobehavioral recovery from head injury. Oxford University Press, New York, pp 279–291
Resch C, Anderson VA, Beauchamp MH, Crossley L, Hearps SJC, van Heugten CM, Hurks PPM, Ryan NP, Catroppa C (2019) Age-dependent differences in the impact of paediatric traumatic brain injury on executive functions: a prospective study using susceptibility-weighted imaging. Neuropsychologia 124:236–245. https://doi.org/10.1016/j.neuropsychologia.2018.12.004
Krasny-Pacini A, Chevignard M, Lancien S, Escolano S, Laurent-Vannier A, de Agostini M, Meyer P (2017) Executive function after severe childhood traumatic brain injury - age-at-injury vulnerability periods: the TGE prospective longitudinal study. Ann Phys Rehabil Med 60:74–82. https://doi.org/10.1016/j.rehab.2016.06.001
Max JE, Wilde EA, Bigler ED, MacLeod M, Vasquez AC, Schmidt AT, Chapman SB, Hotz G, Yang TT, Levin HS (2012) Psychiatric disorders after pediatric traumatic brain injury: a prospective, longitudinal, controlled study. J Neuropsychiatr Clin Neurosci 24:427–436. https://doi.org/10.1176/appi.neuropsych.12060149
Max JE, Wilde EA, Bigler ED, Thompson WK, MacLeod M, Vasquez AC, Merkley TL, Hunter JV, Chu ZD, Yallampalli R, Hotz G, Chapman SB, Yang TT, Levin HS (2012) Neuroimaging correlates of novel psychiatric disorders after pediatric traumatic brain injury. J Am Acad Child Adolesc Psychiatry 51:1208–1217. https://doi.org/10.1016/j.jaac.2012.08.026
Ahmadi SA, Meier U, Lemcke J (2010) Detailed long-term outcome analysis after decompressive craniectomy for severe traumatic brain injury. Brain Inj 24:1539–1549. https://doi.org/10.3109/02699052.2010.523049
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors report no conflict of interest concerning the materials or methods used in this study or the findings specified in this paper.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ballestero, M.F.M., Furlanetti, L.L., Augusto, L.P. et al. Decompressive craniectomy for severe traumatic brain injury in children: analysis of long-term neuropsychological impairment and review of the literature. Childs Nerv Syst 35, 1507–1515 (2019). https://doi.org/10.1007/s00381-019-04274-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00381-019-04274-1