Abstract
Introduction
There are no comparative studies available for hyperosmolar therapy in children. The present study is a prospective open label randomized control trial to compare the effect of equiosmolar doses of mannitol and hypertonic saline in reducing intracranial pressure in children who sustained severe traumatic brain injury.
Methods
This is a prospective open-label randomized controlled trial. Thirty children aged less than or equal to 16 years with severe traumatic brain injury and raised intracranial pressure as measured by ventricular catheter insertion were enrolled. Sixteen children received 20% mannitol, and 14 children received 3% saline as 2.5 ml/kg bolus for episodes of intracranial pressure above cutoff value for age. The mean reduction in intracranial pressure and Glasgow outcome scale at 6 months after injury was measured.
Results
The mean reduction in intracranial pressure in mannitol group was 7.13 mmHg and in hypertonic saline group was 5.67 mmHg, and the difference was not statistically significant, p = 0.33. The incidence of death or survival in vegetative state was 23.07% in mannitol group and 16.66% in hypertonic saline group, and the difference was not statistically significant, p = 0.69.
Conclusion
Both mannitol and hypertonic saline were equally effective for treatment of raised intracranial pressure in children with severe traumatic brain injury.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Traumatic brain injury (TBI) management protocols revolve around the monitoring and management of intracranial pressure (ICP). The use of hyperosmolar agents has a major role in management of raised ICP due to TBI. Mannitol is a standard hyperosmolar agent for reducing ICP. Hypertonic saline (HTS) was introduced later, and now both are used in contemporary management of raised ICP [1, 2]. Both agents exert an early effect on ICP by optimization of rheological properties of the blood resulting in decreased blood viscosity and hematocrit, increased cerebral blood flow (CBF), and oxygen delivery, resulting in reflex auto-regulatory vasoconstriction of cerebral arterioles resulting in reduction of cerebral blood volume (CBV) and ICP [2]. Despite being in clinical use for a long period of time, there is limited clinical data available for recommendation of choice of hyperosmolar therapy in children. Most of the evidence for hyperosmolar therapy is derived from studies in adult patients with TBI [3]. There is class II evidence supporting the use of 3% hypertonic saline for the acute treatment of severe pediatric TBI associated with intracranial hypertension and Class III evidence to support its use as a continuous infusion during the intensive care unit course. There is insufficient evidence to support or refute the use of mannitol, concentrations of hypertonic saline > 3%, or other hyperosmolar agents for the treatment of severe pediatric TBI [3]. The recommendation for the use of 3% hypertonic saline for the acute treatment of severe pediatric TBI associated with intracranial hypertension is based on availability of evidence for hypertonic saline rather than any direct comparisons of the two hyperosmolar agents. There is insufficient evidence about the preference and dosage of hyperosmolar agents. The direct effect on clinical outcome associated with use of a particular hyperosmolar agent is also unclear. One must thus weigh the value of longstanding clinical acceptance and safety of mannitol, which has no evidence to support its efficacy in pediatric age group, against hypertonic saline, for which there is less clinical experience but reasonably good performance in contemporary clinical trials and practice [3, 4]. The purpose of this prospective open randomized study was to compare the efficacy of equiosmolar dose of mannitol and hypertonic saline in reducing raised ICP in children with severe TBI.
Methods
The institute where the study was conducted is a major neurotrauma center in the country catering a large population of patients in southern India. This study was conducted between January 2012 and June 2014. The study received prior approval by the Institutional Ethics Committee and informed consent was obtained from patients’ legal relative. This trial was registered with Clinical Trials Registry of India (REF/2015/03/008696).
Study design and patient population
The present study was a prospective open-label randomized equivalence trial conducted in a neurosurgery intensive care unit of a tertiary neurosurgical center. The inclusion criteria were children in age group 1 to 16 years with severe TBI, defined as post-resuscitation Pediatric Glasgow Coma Scale (GCS) of 8 or less [5], and presenting within 24 h of trauma. Children with GCS 3, absent brain stem reflexes, systemic injuries requiring immediate treatment, clinical evidence of significant spinal cord injuries, and presentation after 24 h of injury were excluded. All children underwent initial resuscitation, and evaluation and treatment for TBI as required. The CT scan studies on admission were analyzed using criteria defined by Marshall et al. based on midline shift, cisternal compression, and volume of the bleeding [6].
ICP monitor setup and treatment protocol
The patients were sedated and ventilated with head of bed elevated at 30°. The main aim of mechanical ventilation was to avoid hypoxia by maintaining O2 saturation of > 95%. The MAP was maintained with 0.9% saline, and ionotropes if required. The ICP monitoring was done using intraventricular device. The external ventricular drain was placed at Kocher’s point on right side unless there was contraindication like local scalp injury. Standard anatomic landmarks were used for deciding the trajectories for ventricular puncture. The ventricular catheter was connected to a closed external ventricular drainage (EVD) system connected to pediatric pressure transducers which is a strain gauge variable transducer. The zeroing of the transducer was at the level of tragus of the ear used as the external landmark of foramen of Monro. CSF sampling was done only if indicated otherwise a completely closed system was used with sampling of CSF done only during insertion and removal of EVD. The aim of the therapy was to maintain the ICP below 15 mmHg in children between 1 and 10 years of age and 18 mmHg in children age 11–16 years of age [7, 8]. When the ICP remained raised more than the cutoff value for more than 5 min in the absence of noxious stimuli like suction, positioning, etc., it qualified as an intracranial hypertensive (ICH) episode. For an ICH episode, the EVD was opened to drain CSF until it stopped flowing or up to 20 cc release of CSF whichever is first.
Randomization and intervention
After successful insertion of EVD and ICP monitoring, the patients were randomized to receive one of the interventional agent. The randomization was done through computer-generated random numbers. The patients received either 20% mannitol (1098 mOsm/l) as a bolus of 0.5 g/kg (2.5 ml/kg) or 3% hypertonic saline (1027 mOsm/l) as a bolus of 2.5 ml/kg through the central venous line over a period of 5 min. The dose calculated was equiosmolar dose of mannitol and hypertonic saline. The interventional agents were administered if ICP remained persistently above the cutoff value for more than 5 min in spite of CSF drainage. The ICU staff informed each episode of raised ICP to one of the investigators (AK or DS), who was available at bedside before initiating treatment for reduction of ICP. The investigator personally documented ICP before initiation and after completion of treatment, and measured reduction in ICP for each dose of medication. The aim was to decrease the ICP to cutoff value for the age. If the first infusion failed, a second infusion of the same agent was administered. If the ICP did not decrease even after two consecutive doses of the hyperosmolar agent, it was considered refractory to therapy.
Outcome variables
The following data were collected: age, gender, mode of injury, post-resuscitation GCS, pupillary reaction to light, head CT findings, interval between injury and insertion of EVD, duration of monitoring, duration of ventilation, duration of ICU stay, and duration of hospital stay. The ICP monitoring related data: Mean ICP, MAP, and CPP for each day; number of episodes of raised ICP requiring CSF releases, volume of CSF drained, and number of doses of osmotic drugs required per day were collected. Additional parameters during ICU stay like mean values of serum glucose, sodium, and creatinine, for each day, were collected. The number of patients who required ionotropes was also recorded. The primary outcome was mean reduction of ICP, which was defined as the difference between ICP value before administering hyperosmolar agent and lowest ICP value after completion of bolus for each dose. The mean reduction in ICP was obtained by summing the difference in ICP values before and after treatment divided by number of doses during the entire period of ICP monitoring. The secondary outcome was assessed using Glasgow outcome scale (GOS) modified for children. The outcome “work” was replaced with “activity or scholastic performance” for age and pre-injury status of child [9]. The assessment was done after 6 months of injury. The person who assessed outcome was blinded for the interventional agent. The functional outcome was analyzed as death or survival in vegetative state versus survival with or without disability. The analysis was intention-to-treat basis.
Statistical analysis
The aim of the study was to compare the effect of mannitol and hypertonic saline on the outcome. The baseline characteristics of the patients in the two groups were calculated as mean ± standard deviation (SD) or median and range for continuous variables and frequency for categorical variables. Differences in the baseline variables were analyzed using independent sample t test for continuous variables, chi-square, and Mann-Whitney test for non-parametric variables. The differences are analyzed using a p value of < 0.05 which was considered as statistically significant. A p value closer to 1 is mentioned as not significant (NS) in tables.
Results
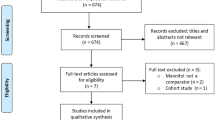
A total of 50 children were assessed for eligibility, out of which 30 children were enrolled and the remaining 20 were excluded for various reasons (Fig. 1). There were 16 patients in mannitol group and 14 patients in hypertonic saline group. There was no violation of treatment protocol. All subjects received one of the allocated agent without any crossovers.
Admission clinical parameters
The basic clinical parameters at presentation which were known to affect the clinical outcome were compared between the two groups. There was no difference in age, and gender distribution of the patient population (Table 1). There were 18 children less than 5 years, the youngest one being 22 months. The mode of injury was comparable between both groups. The commonest mode of injury being road traffic accidents either as a two wheeler passenger or pedestrian injury. All the patients were assessed and EVD inserted within 24 h and about 13 of them underwent EVD insertion and monitoring within 6 h of injury. The median (range) post-resuscitation GCS was 6 (5–7) and 7 (6–8) in mannitol and HTS group, respectively. The mean (SD) Marshal CT grade was 2.6 (0.8) and 2.3 (1.0), respectively, in mannitol and HTS group, respectively, with contusions being the most common pathology found in both the groups. The two groups were statistically comparable in their basic clinical parameters.
Effects on ICP and cerebral hemodynamics
The mean duration of ICP monitoring was of 4.1 (1.36) and 4.07 (1.38) days in mannitol and HTS group, respectively. The 24-h mean ICP from the day 1 to day 5 of monitoring for both the groups was comparable. The ICP tracings over this period showed a trend of higher mean ICP range on the first 2 days of monitoring plateauing down later during monitoring (Table 2).
The mean (SD) numbers of raised ICP episodes were 15.8 (9.04) and 18.4 (11.31) in mannitol and HTS group, respectively. All the raised ICP episodes were initially managed by CSF drainage. Therapeutic CSF drainage controlled almost more than two-thirds of raised ICP episodes. The episodes that did not respond to CSF drainage were treated with one of the hyperosmolar agents depending on the randomization. The mannitol group required a mean of 3.25 (1.39) doses and hypertonic saline group required 4.5 (3.75) doses. The amount of CSF drained for ICP reduction in both the groups was not statistically different. The daily serum sodium and creatinine concentrations were comparable in both the groups (Table 3).
Four patients in mannitol group underwent surgery: two for evacuation of extradural hematoma (EDH), one for depressed skull fracture, and one for contusion. Surgery was done before ICP monitoring in three patients, and during monitoring for one patient. One patient developed refractory ICP and neurological deterioration during ICP monitoring. A repeat imaging revealed increased size of contusion, hence underwent surgery. Only one patient in hypertonic saline group underwent surgery for EDH during monitoring.
Outcome
The primary outcome, mean (SD) reduction in ICP (ΔICP), was − 7.13 (2.9) in mannitol group and − 5.67 (3.9) in HTS group; the difference was not statistically different (Table 4). The mean (SD) change in CPP was 6.48 (5.4) mmHg and 5.89 (4.7) mmHg in mannitol and HTs group, respectively. The cerebral perfusion pressure showed a trend towards early improvement in HTS group than in the mannitol group, but the difference was not statistically significant (Fig. 2). The average duration of ventilation was 8.18 (3.7) days in mannitol and 8.64 (4.3) days in HTS group. The mean duration of hospital stay was also not significantly different in both the groups.
The dichotomized outcome assessment at 6 months post-injury was 3/12 (23.07%) in mannitol group and 2/12 (16.6%) in HTS group; the difference was not statistically significant.
Discussion
There are no clearly defined guidelines on the choice of hyperosmolar agents in the control of ICP in children with TBI. Although hypertonic saline is available in many different concentrations and there are no comparative studies available as to what concentration is better than the other, we used 3% hypertonic saline as it gives more control over maintaining the blood sodium levels. Moreover 3% hypertonic saline and 20% mannitol are equiosmolar, and can be given at equal volumes per dose.
Hypertonic saline has gained significant support for its use in the traumatic brain injury. The current Brain Trauma Foundation (BTF) guidelines for pediatric brain injury have suggested the use of hypertonic saline [3]. However, studies supporting use of hypertonic saline in pediatric age group have shortcomings (Table 5). Two of these studies did not compare hypertonic saline with other drug [12, 13]. Other two studies compared hypertonic saline with normal saline or lactated Ringer’s solution [10, 11]. All of these studies showed superiority of hypertonic saline in reduction of ICP or improvement of outcome. These results are not surprising as it is well-known that hyperosmolar agents reduce ICP. Based on such evidence, BTF gave level 2 and 3 recommendation “hypertonic saline should be considered for the treatment of severe pediatric TBI associated with intracranial hypertension” [3]. This recommendation is probably an extrapolation from the studies in adults [16, 17]. The guideline also mentions that “although mannitol is commonly used in the management of raised ICP in pediatric TBI, no studies meeting inclusion criteria were identified for use as evidence for this topic” [3]. One study compared HTS with mannitol and concluded that duration of coma, and mortality was lower in hypertonic saline group [14]. However, this study was retrospective, and ICP was not measured. A more recent retrospective study comparing HTS with mannitol was inconclusive [15].
The strength of our current study was use of age-specific thresholds for ICP. We also used a multitier treatment with CSF drainage as the initial modality of reducing ICP. With therapeutic CSF drainage as the first line of management, the doses of hyperosmolar agents were reduced. We did not encounter any case of EVD-related infection as a closed system was used. Other strengths of our study were no cross overs, and follow-up of all patients. We assessed both ICP reduction and functional outcome, and we found that the outcome was equivalent for both hyperosmolar agents. The current study does not add significantly to evidence for choice of hyperosmolar agents for reduction of ICP in children. However, this study is an important initial step in the knowledge of hyperosmolar therapy for pediatric TBI. This study indicates the feasibility of a large randomized controlled trial of hyperosmolar therapy for pediatric TBI.
Limitations
The limitation of our study was small sample size. Other studies on hyperosmolar therapy in children with TBI are also underpowered (Table 4). For adequate power of study, a large multicenter study is warranted. The present underpowered study cannot generate a good class of evidence but demonstrates the feasibility of such studies at a larger scale. The second limitation was use of hyperosmolar therapy as second tier treatment after failure of CSF drainage to reduce the ICP. The EVD, when available, is recommended prior to hyperosmolar therapy [18]. The EVD as an initial treatment may dilute the effect of hyperosmolar therapy. It is not known, whether there will be any difference in ICP reduction between mannitol and hypertonic saline if any of these agents are administered as first-line therapy. Many centers do not use EVD for ICP monitoring. When ICP monitoring is done using parenchymal sensor, option of CSF drainage is not available, and true effect of hyperosmolar therapy can be assessed. The third limitation was that we did not measure time to peak effect and duration of effect, cerebral blood flow, cerebral tissue oxygen, cerebral metabolism, cerebral injury biomarkers, cerebrospinal compliance, and pressure reactivity. The multimodal monitoring is labor intensive, and is not available in our set up.
Conclusion
There was no significant difference in reduction in ICP between mannitol and HTS group. Moreover, both groups had similar functional outcome. Pending further larger studies, the choice of hyperosmolar therapy for children with severe TBI should be individualized based on one’s practice and experience. The issues that remain to be clarified with hyperosmolar therapy are duration of effect, possibility of rebound hypertension, effect on blood flow and metabolism, and outcome.
References
White H, Cook D, Venkatesh B (2006) The use of hypertonic saline for treating intracranial hypertension after traumatic brain injury. Anesth Analg 102:1836–1846. https://doi.org/10.1213/01.ane.0000217208.51017.56
Ziai WC, Toung TJK, Bhardwaj A (2007) Hypertonic saline: first-line therapy for cerebral edema? J Neurol Sci 261:157–166. https://doi.org/10.1016/j.jns.2007.04.048
Kochanek PM, Carney N, Adelson PD et al (2012) Guidelines for the acute medical management of severe traumatic brain injury in infants, children, and adolescents—second edition. Pediatr Crit Care Med 13(Suppl 1):S1–S82. https://doi.org/10.1097/PCC.0b013e31823f435c
Bell MJ, Adelson PD, Hutchison JS, Kochanek PM, Tasker RC, Vavilala MS, Beers SR, Fabio A, Kelsey SF, Wisniewski SR (2013) Differences in medical therapy goals for children with severe traumatic brain injury—an international study. Pediatr Crit Care Med 14:811–818. https://doi.org/10.1097/PCC.0b013e3182975e2f
Kirkham FJ, Newton CRJC, Whitehouse W (2008) Paediatric coma scales. Dev Med Child Neurol 50:267–274. https://doi.org/10.1111/j.1469-8749.2008.02042.x
Marshall LF, Marshall SB, Klauber MR et al (1992) The diagnosis of head injury requires a classification based on computed axial tomography. J Neurotrauma 9(Suppl 1):S287–S292
Avellino A, Carson Sr B (2005) Increased intracranial pressure. In: Maria B (ed) Current Management in Child Neurology, 3rd ed. BC Decker, Connecticut, pp 563–568
Chambers IR, Jones PA, Lo TYM et al (2006) Critical thresholds of intracranial pressure and cerebral perfusion pressure related to age in paediatric head injury. J Neurol Neurosurg Psychiatry 77:234–240. https://doi.org/10.1136/jnnp.2005.072215
Beers SR, Wisniewski SR, Garcia-Filion P, Tian Y, Hahner T, Berger RP, Bell MJ, Adelson PD (2012) Validity of a pediatric version of the Glasgow outcome scale-extended. J Neurotrauma 29:1126–1139. https://doi.org/10.1089/neu.2011.2272
Simma B, Burger R, Falk M, Sacher P, Fanconi S (1998) A prospective, randomized, and controlled study of fluid management in children with severe head injury: lactated Ringer’s solution versus hypertonic saline. Crit Care Med 26:1265–1270
Fisher B, Thomas D, Peterson B (1992) Hypertonic saline lowers raised intracranial pressure in children after head trauma. J Neurosurg Anesthesiol 4:4–10
Khanna S, Davis D, Peterson B, Fisher B, Tung H, O’Quigley J, Deutsch R (2000) Use of hypertonic saline in the treatment of severe refractory posttraumatic intracranial hypertension in pediatric traumatic brain injury. Crit Care Med 28:1144–1151
Peterson B, Khanna S, Fisher B, Marshall L (2000) Prolonged hypernatremia controls elevated intracranial pressure in head-injured pediatric patients. Crit Care Med 28:1136–1143
Yildizdas D, Altunbasak S, Celik U, Herguner O (2006) Hypertonic saline treatment in children with cerebral edema. Indian Pediatr 43:771–779
Roumeliotis N, Dong C, Pettersen G, Crevier L, Emeriaud G (2016) Hyperosmolar therapy in pediatric traumatic brain injury: a retrospective study. Childs Nerv Syst 32:2363–2368. https://doi.org/10.1007/s00381-016-3231-y
Gu J, Huang H, Huang Y, Sun H, Xu H (2018) Hypertonic saline or mannitol for treating elevated intracranial pressure in traumatic brain injury: a meta-analysis of randomized controlled trials. Neurosurg Rev. https://doi.org/10.1007/s10143-018-0991-8
Harutjunyan L, Holz C, Rieger A, Menzel M, Grond S, Soukup J (2005) Efficiency of 7.2% hypertonic saline hydroxyethyl starch 200/0.5 versus mannitol 15% in the treatment of increased intracranial pressure in neurosurgical patients - a randomized clinical trial [ISRCTN62699180]. Crit Care 9:R530–R540. https://doi.org/10.1186/cc3767
Stocchetti N, Maas AIR (2014) Traumatic intracranial hypertension. N Engl J Med 370:2121–2130
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The study received prior approval by the Institutional Ethics Committee and informed consent was obtained from patients’ legal relative. This trial was registered with Clinical Trials Registry of India (REF/2015/03/008696).
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kumar, S.A., Devi, B.I., Reddy, M. et al. Comparison of equiosmolar dose of hyperosmolar agents in reducing intracranial pressure—a randomized control study in pediatric traumatic brain injury. Childs Nerv Syst 35, 999–1005 (2019). https://doi.org/10.1007/s00381-019-04121-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00381-019-04121-3