Abstract
Purpose
Kyphosis is the most severe spinal deformity associated with meningomyelocele (MMC) and is seen in approximately 15% of neonates. Our purpose is to present our clinical experience, to discuss the technique and deformity correction in kyphectomy in neonates with MMC, and to assess its long-term outcomes.
Method
In this prospective study, the authors reviewed eight cases submitted to surgery between 2013 and 2015. We evaluated clinical characteristics that were analyzed, as were the operative technique employed, and angle range of the kyphosis deformity postcorrection follow-up.
Results
Neonatal kyphectomy was performed of six females and two males. The mean birth weight was 2780 g, and the mean age at the time of surgery was 5.6 days. There were S-shaped type deformity in lumbar region in all neonates. In the correction of the kyphotic deformity, a total vertebrae were removed from four patient, whereas a partial vertebrectomy was done in four. The mean operative time was 116 min. No patients did not require the blood transfusion. There were no serious complications, and wound closure was successful in all patients. The mean follow-up period was 4 years and 3 months (range 36–61 months), except one patient who died 1 week after discharge. The mean preoperative kyphosis of 75.6° (range, 50°-90°) improved at last follow-up to 35° (range 15°–55°). All patients had surgical procedures for hydrocephalus. Three patients had surgery for Chiari type II malformation. The mean hospital stay was 27.7 days.
Conclusion
Kyphectomy performed at the time of dural sac closure in the neonate is a safe procedure with excellent correction.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Kyphosis is the most severe spinal deformity associated with meningomyelocele (MMC) and is seen in approximately 15% of neonates [4, 6, 7, 10, 11]. Severe lumbar kyphosis lead to epidermal ulceration, chronic nonhealing wounds and pyogenic spondylitis as well as functional impairment, decreased abdominal capacity, respiratory compromise, sitting imbalance [4, 8, 20]. Kyphotic deformity progresses 4° to 12° per year [9]. Kyphectomy in neonates with MMC is less frequently reported in the literature. An extensive review of the literature revealed that 26 cases have been reported [4, 6, 7, 18, 19].
Material and methods
This search identified eight neonates who underwent a kyphectomy at the time of dural sac closure from January 2013 to January 2015. In this prospective study, we performed a patient chart review for demographic data, the time and date of surgery, and presence of any additional congenital malformations. Estimated blood loss and length of surgery, number of vertebrae removed during surgery the kyphectomy, and length of hospitalization were recorded for each neonates. Postoperative follow-up evaluation focused on the need for a revision procedure and wound closure. Preoperative and postoperative kyphosis angle was measured on three-dimensional computed tomography (3D-CT). All patients had cranial and spinal magnetic resonance imaging (MRI) for preoperative preparation. All patients were examined by the first author for neurological evaluation. Muscle strength was assessed in both lower limbs by manual testing according to Bartonek et al. [2] (Table 1). All neonates were paraplegic from MMC level down.
Operative technique
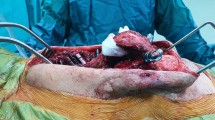
The neonate is positioned prone over bilateral chest rools. The membranous tissue connecting the neural plaque to the skin is excised. After exposure of the dura, we expose the transverse processes by releasing the lumbodorsal fascia, sacral spinalis, and quadratus lumborum. The gibbus deformity then is isolated by bipolar electrocautery dissection and subperiosteal elevation anteriorly and medially from the transverse processes and lateral sides of the corpus. The kyphosis is approached alternately right and left from lateral to midline. Blunt dissection with surgical sponges and cottonoids is carried forward, protecting the retroperitoneal structures and iliopsoas. The dural layer is very thin as it lies over prominent pedicles, and the dural layer is more adherent to the posterior longitudinal ligament. It may be safer to separate this ligament from the bone than from the dural layer if a cerebrospinal fluid fistula is to be avoided. The dural layer has been dissected from transverse processes. The three or four nerve roots associated with apical vertebra are shown as they leave the dura to pass through foramina in the bone. The lumbar fascia is incised at its attachment to the tips of the processes to expose their anterior surface. Lumbar nerve roots are shown passing from the foramina to enter the psoas muscle. The transverse processes and abnormal pedicles are removed, the nerves being protected by a blunt dissector. The nerve roots are then displayed in their full course from the spinal cord to the point at which they enter the psoas muscle anteriorly. Kyphotic deformity viewed from the side after removal of the transvers processes and the lateral aspects of the vertebral bodies can now be identified. By careful blunt dissection in the dural sac is mobilized from posterior longitudinal ligament, and the aorta and inferior vena cava are separated from anterior surfaces of the vertebral bodies. The sac was carefully retracted and not ligated to allow access to the vertebral body (Fig. 1a–e). After dissection of the entire area of the kyphotic deformity, the apex vertebra is removed through the disc spaces. A vertebrectomy is performed by removing a posterior dorsal wedge the superior space of the caudad segment and inferior space of the cephalad segment. It may be necessary to remove of about one and half vertebral bodies to achieve lordosis. A high-speed drill with 8-mm cutting burr was used to remove the vertebral bodies. The vertebra is removed by extracting it from between the roots above and below the level. The gap produced by this resection is easily closed by a finger maneuver. Holes and a large needle are used to place a #1 silk suture through the superior and inferior segments of the resected kyphos. An assistant is to bear on vertebral bodies finger to close up. It is important usually to four to six silk sutures through the endplates and disc space to achieve the strongest purchase on the vertebral bodies. Bone chips from the resected vertebrae are placed anteriorly into the retroperitoneal space (Fig. 1f). Then, vertebral bodies are being held together by silk sutures. After repair of the dura, the lateral muscles, quadratus lumborum, and lumbosacral fascia are closed (Fig. 1g, h). After surgery, the neonate is placed in the prone position. No additional splint has been used because restriction to respiration is not tolerated by a neonate (Fig. 1i).
a Anterior view of MMC during operation. b Lateral view of MMC during operation. c After initial incision and exposure of the dura, we exposes the transverse processes by releasing the lumbodorsal fascia, sacral spinalis and quadratus lumborum. d, e The gibbus deformity then is isolated by bipolar electrocautery dissection and subperiosteal elevation anteriorly and medially from the transverse processes and lateral sides of the corpus The kyphosis is approached alternately right and left from lateral to midline. A periostal elevator and sac were dissected free of the posterior longitudinal ligament. The sac was carefully retracted and not ligated to allow access to the vertebral body. f A high-speed drill with 8 mm cutting burr was used to remove the vertebral bodies. g, h After repair of the dura, the lateral muscles, quadratus lumborum and lumbosacral fascia is closed. i After surgery, the neonate is placed in the prone position
Case 1
A term female neonate presented with a large neural tube defect (4 × 6 cm in diameter) in the lumbar region with a birth weight of 2700 g (Fig. 2A). She was delivered by caesarean section. Neurological examination was reported as paraplegic. Preoperative MRI demonstrated a significant kyphosis at L2–L3 level with MMC (Fig. 2B). Surgical repair of the MMC by closing neural placode and correction of lumbar kyphosis with L3 total vertebrectomy was performed 10 days after birth. Surgical time was 90 min. There was no need for blood transfusion. Postoperative period was uneventful (Fig. 2c, d). The mean hospital stay, determined by the neonatology and neurosurgery team, was 32 days, which also included the placement of a ventriculoperitoneal (VP) shunt for hydrocephaly. At 61 months follow-up, the wound was weel-healed. Mental development was good. There were no kyphotic deformity and VP shunt problem. She was a paraplegic. Her preoperative kyphosis was 90° by the Cobb method. Two years after surgery, it improved to 45°. The sitting posture and position was normal (Figs. 2e and 3) (Table 2, patient no. 1).
Case 2
A term female neonate was born via caesarean section with birth weight of 2780 g. Examination upon admission revealed the size of MMC sac at approximately 5 × 5 cm and was cystic, covered by healthy skin except at fundus, where it was ulcerated (Fig. 1a–c). There was no spontaneous lower limb movement. 3D-CT and MRI showed a significant kyphosis at L2–L3 level with MMC (Fig. 4). On day 3 of life, we made closure of the MMC and correction of lumbar kyphosis with L2–L3 total vertebrectomy. Surgical time was 2 h. There was no need for blood transfusion. The rest of the hospital stay was uneventful. VP shunt was performed nine days. She was discharged on the seventeenth day. At 59 months follow-up, the child is doing well without any wound problem and kyphotic deformity. VP shunt failure and mental deficiency has not been seen in the patient’s follow-up. She had preoperative kyphosis measuring 85° that was corrected 30° postoperatively (at 2 years follow-up, 55° correction) (Figs. 5, 6, and 7). Although she was a paraplegic, sitting position and posture were good (Table 2, patient no. 2).
Results
From January 2013 to January 2015, a total of 50 neonates were diagnosed as MMC in our hospital, and 8 of them a kyphectomy was performed. Six of the patients were males. Two of the neonates were preterm. The mean birth weight of the neonates was 2780 g (1950–3510 g). The mean age at the time of surgery was 5.6 days, ranging from 2 to 11 days. There were S-shaped type deformity in lumbar region in all neonates. In the correction of kyphotic deformity, a total vertebrae were removed from four patients, whereas a partial vertebrectomy (decancellation) were done in four. The mean length of surgery procedure was 116 min (90–180 min). No patients did not require the blood transfusion. There were no serious complications and wound healing problems. All patients had surgical procedures for hydrocephalus with 7 VP shunt and 1 third ventriculostomy. Chiari type II malformation was present in all patients and three of them operated with suboccipital craniectomy, cervical laminectomy, and duraplasty with fascial greft (SOC + CL + DP). The mean hospital stay, determined by the neonatology and neurosurgery team, was 27.7 days, which also included the surgery for hydrocephaly and Chiari II malformation. The mean follow-up period was 4 years and 3 months (range 36–61 months), except one patient who died 1 week after discharged. The mean preoperative kyphosis angle was 75.6° (range 50°–90°) and improved at last follow-up at 35° (range 15°–55°). Patient 8 was not included in the calculation of the averages because of the death at first week after the discharge (Table 2).
Discussion
Kyphotic deformity of different severity at the lumbar or thoracolumbar area occurs in approximately 15% of neonates with a MMC [4, 6, 8,9,10]. These neonates usually are more severely neurologically involved, have a higher prevalence of hydrocephalus, and have a poorer quality of life. Mintz et al. [17] reviewed 51 children who had a rigid kyphosis at birth; 40 of these patients had a thoracic level paralysis, and 9 of the remaining 11 children had grade 3 motor strength in the quadriceps. In our study, neurological examination of the lower limbs was level 5 at birth in all neonates according to the Bartonek et al. [2]. Marreiros et al. [16] reported that the distribution of spine surgery across neurological levels was not equal, and a higher proportion of patients with a thoracic level of involvement was submitted to an intervention on the spine compared to these lower neurological levels; thus, a higher level of neurological involvement is a good predictor of greater need for spinal surgery over time. There were clinical and radiological hydrocephaly in all patients. VP shunt was performed on seven cases. Third ventriculostomy only was done on one neonate (Table 2, patient no. 7). Radiological Chiari type II malformation was present in all neonates. But, clinical symptoms were seen in our three neonates (Table 2, patient nos. 3, 6, and 8) during follow-up and operated with SOC + CL + DP.
Kyphosis in MMC should realistically be reserved 50° on the preoperative radiographic examination in cases of surgery. We chose to define kyphosis as a generalized for curves 50°. The kyphotic curve may be initially large at birth, and progression can range from 4° and 12° per year [1, 5, 9, 10, 17]. Banta and Hamada [1] found that 46 of 457 patients had developmental kyphosis, rigid congenital kyphosis, or kyphoscoliosis that progressed a mean of 8°, 8.3°, and 6.8° per year, respectively. Mintz et al. [17] followed 51 children with congenital kyphosis and MMC for a mean of 4.8 years. Thirty-five children had initial radiographs at 1 year of age or less. Sixteen children had radiographs taken after the age 1 year. Kyphotic curves less than or equal 90° in 35 children progressed 7.7° per year; those greater than 90° progressed 12.1° per year. Curves less than or equal to 90° and greater than 90° progressed at similar rates, regardless of initial curve magnitude: 6.4° per year and 6.7° per year, respectively. Doers et al. [5] reported that radiographs of 37 patients with untreated kyphosis without congenital vertebral anomalies associated with MMC. With a mean interval between radiographs of 6.2 years, the kyphotic curves were noted to increase at a mean rate of 4.3°/year without correlation to its initial magnitude. Progression becomes more rapid after the first year of life, when the child begins to sit. The fixed compensatory thoracic lordosis, so commonly seen in older patients, is not present at birth and progresses by approximately 2.5°/year.
Kyphosis in MMC patients may have a dramatic impact on the patients’ quality of life. The rationale for surgical treatment of kyphosis is based on many functional factors, but absolute criteria remain ill-defined. Surgical management is often the only option to prevent progression and improve function in these patients. Martin et al. [12] showed that surgery improving kyphosis in patients with MMC correlated with improved wound healing. Although surgical intervention is typically offered to help with sitting balance or to treat chronic persistent skin breakdown, other surgical indications include reducing respiratory impairment, increasing intraabdominal space, halting deformity progression, managing associated pain, and improving function [7, 9, 12, 15]. The most appropriate timing and optimal type of surgery are areas of controversy. As the child ages, the deformity becomes rigid, and a compensatory fixed thoracic lordosis develops. Initial attempts at correction involve resection or osteotomy of the apical vertebrae, with little attention directed to the proximal thoracic lordosis. Proponents of neonatal kyphectomy at the time of closure of the MMC report that procedure is safe and provides good initial correction. Although recurrence of the kyphosis was common, the new deformity was less rigid and easier to address. More extensive fusion and instrumentation are required in the older child, and complication rates are higher [9].
Neonatal kyphectomy in MMC is less frequently reported in the literature. The total number of reported neonatal kypectomy cases in MMC was 26 previously [4, 6, 7, 18, 19]. It was first reported by Sharrard [18] in 1968, with the vertebral bodies held together being by silk sutures. He said that the inevitability of progressive increase in the kyphosis and the difficulty in closure of an open MMC associated with kyphosis at birth make it advisable that osteotomy-resection of the spine should be done in the newborn at the time of surgical closure [18]. Sharrard [18] reported three deaths in his initial series of six neonates. He reported seven further cases of neonatal kyphectomy in 1972. However, he failed to provide any follow-up data on the second group of seven neonates [19]. Eckstein and Vora [7] operated on one neonate for severe kyphosis with MMC in 1972. In this case, the authors give no details of the surgery and follow-up. Crawford et al. [4] had reported the largest series with 11 cases in 2003, and they proposed a surgical maneuver for kyphotic deformity correction without instrumentation. In 2013, Duddy et al. [6] first described a case of neonatal kyphectomy and posterior fixation with a cervical plate at the time of dural closure. Our study is the second largest series of kyphectomy in neonates with MMC in the literature.
In a retrospective research, Crawford et al. [4] reported a group of 11 patients who underwent neonatal kyphectomy with dural sac closure between 1980 and 2000. Mean age at time of surgery was 36 h. A #1 nonabsorbable suture was placed through the cephalad and caudad segments of the resected kyphos, and no instrumentation was used. An assistant then proceeds underneath the drapes before suturation for vertebral bodies and lifts both femurs in an effort to extend the thoracolumbosacral region. After surgery, each neonate is placed in a plaster-molded splint. For this cohort of patients, the mean preoperative kyphotic angle was 67°, with a the mean follow-up period of 7 years and 4 months. The mean initial correction was 77°, and the loss of correction at follow-up of 55°. No serious complications were noted. Wound closure was successful in all neonates, and primary wound healing occurred in 7 to 10 days. One patient required a repeat kyphectomy and posterior spinal fusion at 9 years and 2 months of age. The authors concluded that neonatal kyphectomy performed at time of dural sac closure is a safe procedure and provides excellent initial correction. Recurrence of the deformity is expected, but the resultant kyphotic deformity that occurs is longer and less angulated, which theoretically should be less technically demanding.
Vertebrectomy and vertebral body decancellation are two surgical methods for kyphotic deformity. Vertebrectomy was the original surgical treatment [10]. It was first described in 1968 by Sharrard [18], who advocated correction in the neonate by resection of 1.5 vertebra; he and others noted a high complication rate with loss correction over time [3, 7, 11, 12, 14, 15, 19]. Since then, major advances have been made in our understanding and surgery of neonatal kyphosis with MMC. Lindseth [13] classified the kyphosis into a collapsing C-shaped deformity and a more rigid S-shaped deformity. In the S-shaped type, vertebral resection is usually required. Vertebrectomy can be used for S- and C-shaped type of kyphotic deformity with MMC. The vertebrectomy method was refined by Lindseth and Stelzer [11], who demonstrated improved results when the lordotic cephalad region, along with a region of the apex vertebra, was resected [14]. The less rigid C-shaped type of the deformity would seem ideal for the decancellation technique. Lindseth [13] has advocated surgery by decancellation, the removal of the cancellous vertebral bone, of the bodies below and above the apex vertebra. Theoretically, vertebral body decancellation procedure spares the growth centers of the vertebral bodies, allowing continued growth. If the severity of the kyphotic deformity precludes skin closure or if the philosophy of the treating surgeon is for earliest correction, these two surgical methods can be performed in the neonates. The procedure is accomplished at the time of dural sac closure. Compared with vertebrectomy, the neural structures are much less at risk with vertebral body decancellation, making it having priority in the rare patient with normal or minor loss neurological function distal to the kyphotic deformity. Also, the anterior column is not lengthened, so vascular structures are not put into hazard, and the decancellation method is less extensive and may be associated with less blood loss. Although serious complications have been demonstrated in older series for vertebrectomy with S-shaped type deformity in the neonates, Crawford et al. [4] have reported excellent results with limited complications. In our cases, there were S-shaped type deformity in lumbar region. The total vertebrectomy procedure was performed on four neonates (Table 2, patient nos. 1, 2, 3, 8). Two level total vertebrectomy only was done on one neonate for correction of the kyphotic deformity (Table 2, patient no. 2). The partial vertebrectomy (decancellation) was performed on four neonates (Table 2, two level → three patients and three level → one patient (patient no. 6)). The mean length of surgery procedure was 116 min. No patients did not require the blood transfusion. There was no serious complication. The wounds were weel-healed. Postoperative periods were uneventful. The mean follow-up period was 4 years and 3 months, except one patient who died 1 week after discharge. The mean preoperative kyphosis angle was 75.6° improved at last follow-up 35°.
Conclusions
Kyphectomy performed at the time of dural sac closure in the neonate is a safe procedure with excellent correction. Primary wound healing is better than without kyphectomy. Recurrent deformity does occur but seems to be well tolerated and can be addressed by less demanding procedures than those required in the older child.
References
Banta JV, Hamada JS (1976) Natural history of the kyphotic deformity in myelomeningocele. J Bone Joint Surg Am 58:279
Bartonek A, Saraste H (2001) Factors influencing ambulation in myelomeningocele: a cross-sectional study. Dev Med Child Neurol 43:353–260
Christofersen MR, Brooks AL (1985) Excision and wire fixation of rigid myelomeningocele kyphosis. J Pediatr Orthop 5:691–696
Crawford AH, Strub WM, Lewis R, Gabriel KR, Billmire DA, Berger T, Crone K (2003) Neonatal kyphectomy in the patient with myelomeningocele. Spine 28:260–266
Doers T, Walker JL, van den Brink KD, Stevens DB, Heavilon J (1997) The progression of untreated lumbar kyphosis and compensatory thoracic lordosis in myelomeningocele. Dev Med Child Neurol 39:326–330
Duddy JC, Caird J, Connolly P (2013) Repair of a large thoracolumbar myelomeningocele with associated lumbar kyphosis. Acta Neurochir 155:1965–1968
Eckstein HB, Vora RM (1972) Spinal osteotomy for severe kyphosis in children with myelomeningocele. J Bone Joint Surg Br 54:328–333
Gepp RA, Quiroga MRS, Gomes CR, Araujo HJ (2013) Kyphectomy in meningomyelocele children: surgical technique, risk analysis, and improvement of kyphosis. Child Nerv Syst 29:1137–1141
Guille JT, Sarwark JF, Sherk HH, Kumar SJ (2006) Congenital and developmental deformities of the spine in children with meningomyelocele. J Am Acad Orthop Surg 14:294–302
Karlin MI (2007) Kyphectomy for myelodysplasia. Neurosurg Clin N Am 18:357–364
Lindseth RE, Stelzer L (1979) Vertebral excision for kyphosis in children with myelomeningocele. J Bone Joint Surg Am 61:699–704
Martin J, Kimar SJ, Guille JT, Ger D, Gibbs M (1994) Congenital kyphosis in myelomeningocele: results following operative and nonoperative treatment. J Pediatr Orthop 14:323–328
Lindseth RE (1991) Spine deformity in myelomeningocele. Instr Course Lec 40:273–286
Linther SA, Lindseth RE (1994) Kyphotic deformity in patients who have a myelomeningocele. J Bone Joint Surg Am 76:1302–1307
Lowe GP, Menelaus MB (1978) The surgical management of kyphosis in older children with myelomeningocele. J Bone Joint Surg Br 60:40–45
Marreiros H, Loff C, Calado E (2015) Who needs surgery for pediatric myelomeningocele? A retrospective study and literature review. J Spinal Cord Med 38:626–640
Mintz LJ, Sarwark JF, Dias LS, Schafer MF (1991) The natural history of congenital kyphosis in myelomeningocele: a review of 51 children. Spine 16:S348–S350
Sharrard WJW (1968) Spinal osteotomy for congenital kyphosis in myelomeningocele. J Bone Joint Surg Br 50:466–471
Sharrard WJW, Drennan JC (1972) Osteotomy-excision of the spine for lumbar kyphosis in older children with myelomeningocele. J Bone Joint Surg Br 54:50–60
Yoshioka K, Watanabe K, Toyama Y, Chiba K, Matsumoto M (2011) Kypectomy for severe kyphosis with pyogenic spondylitis associated with myelomeningocele: a case report. Scoliosis 6:5
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Özdemir, N., Özdemir, S.A. & Özer, E.A. Kyphectomy in neonates with meningomyelocele. Childs Nerv Syst 35, 673–681 (2019). https://doi.org/10.1007/s00381-018-4006-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00381-018-4006-4