Abstract
Incidence
Worldwide, the incidence of neural tube defects (NTDs) varies from 0.17 to 6.39 per 1,000 live births. The declining prevalence of myelomeningocele, the most common NTD, is secondary to several factors including folic acid fortification, prenatal diagnosis with termination of affected fetuses, and unknown factors.
Impact of changes
Of those born with myelomeningocele, survival during infancy and preschool years has improved over the last 25 years (Bowman et al., Pediatr Neurosurg 34:114–120, 4). Fewer newborns today require shunt placement, which will hopefully improve the long-term mortality associated with this disease (Chakraborty et al., J Neurosurg Pediatr 1(5):361–365, 13, unpublished data). Of a cohort born in 1975–1979 and treated at a single US institution, 74% have survived into young adulthood.
Clinical implications
One of the greatest challenges facing these young adults is the transitioning of their medical care into an adult medical community.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Neural tube defects (NTDs) affect approximately 3,000 pregnancies per year in the US [10]. Myelomeningocele (open spina bifida) is the most common NTD and the most severe birth defect compatible with long-term survival. Although the incidence of NTDs is declining, it continues to be the cause of significant chronic disability [29, 30].
In the early 1950s, the survival rate for individuals with myelomeningocele was about 10% [45]. Today, large numbers of children with myelomeningocele are surviving into adulthood because of advances in the management of several important complications [4, 29, 30]. However, optimal treatment requires multi-speciality care to prevent, monitor, and treat a variety of potential complications that can affect function, quality of life, and survival [35]. This care is ideally provided by a multi-disciplinary team with expertise in pediatric subspecialties of neurosurgery, orthopedics, neurology, urology, and rehabilitation. Access to physical and occupational therapists, nutritionists, social workers, wound specialists, and psychologists is also helpful.
Because of increased survival, many individuals with myelomeningocele now live long enough to transition to adult medical providers [30, 47]. This presents new challenges for many adult providers who may lack experience in managing these patients. The problem is compounded by the adults with spina bifida who have failed to acquire the skills needed to live independently and therefore remain dependent on aging parents or caregivers [30].
The aim of this paper is to review the etiology and incidence as well as the evolution in the diagnosis and management of children born with myelomeningocele. We will evaluate the impact these changes have had on this congenital disease. Lastly, we will discuss the effect of these changes on the pediatric multi-disciplinary spina bifida clinics.
Etiology
The majority of myelomeningoceles are isolated malformations of multi-factorial origin. NTDs also occur as part of syndromes, in association with chromosomal disorders, or as a result of an environmental exposure [51].
Genetics
A genetic factor is suggested by the observations that NTDs have a high concordance rate in monozygotic twins, are more frequent among siblings, and are more common in females compared to males [18, 53]. In addition, there is a high prevalence of karyotypic abnormalities among fetuses with NTDs, especially in the presence of other congenital anomalies. For example, a large study evaluating the frequency of aneuploidy in pregnancies with fetal NTDs found aneuploidy in 7% of affected cases [36]. The majority of the abnormal karyotypes were trisomies, and most of the trisomy fetuses also had multiple other congenital anomalies. A second series reported a similar rate (6.5%) of chromosomal abnormalities in fetuses with NTDs [16]. These data support the use of fetal karyotyping as an aid in diagnostic evaluation and recurrence risk counseling [16, 36]. For a woman delivered of a child with myelomeningocele, the risk of recurrence in subsequent pregnancies is 2.5% (about 20 times the rate in the general population) [16, 53]. A number of genes have now been isolated in animal models and humans that cause NTDs [27].
Folate deficiency
Folic acid deficiency has been implicated in the development of NTDs, although the exact mechanism by which it acts is largely unknown. Administrations of folic acid antagonists (dihydrofolate reductase inhibitors and others) increase the risk of NTDs. In a large case-control study, the risk of NTDs (spina bifida or anencephaly) was greater with exposure to folic acid antagonists (including aminopterin, carbamazepine, methotrexate, phenobarbital, phenytoin, primidone, sulfasalazine, triamterene, trimethoprim, and valproic acid) in the first or second month after the last menstrual period (adjusted odds ratio 2.8, 95% CI, from 1.7 to 4.6) [28]. Genetic abnormalities involving the metabolism of folate and homocysteine may account for some cases of NTDs [17, 37]. These disorders may explain why supplementation with folic acid significantly reduces but does not eliminate the risk of NTD.
Incidence
The incidence of NTDs (of which myelomeningocele is the most common) is highly variable and depends upon ethnic, geographic, and nutritional factors. Prior to the widespread use of folic acid, the overall incidence of NTDs was noted to be declining over the last 30 years [57, 63]. Several researchers documented “epidemics” of NTDs (wide variation in the incidence over a decade or more in a single region), notably in several major eastern US cities, England, Western Germany, and several other countries [19, 39]. In 1999, Kadir et al. reported a significant decline in the incidence of NTDs between 1972 and 1990 in England and Wales nearly two decades prior to the expanded use of folic acid (from 2.25/1,000 live births in 1972 to 0.48/1,000 live births in 1990) [34].
In the literature, the prevalence of spina bifida ranges from 0.17 to 6.39 per 1,000 live births [7, 10, 21, 26, 64]. Within the US, previous studies have noted a higher risk of NTDs in Hispanic population compared to non-Hispanic whites [7, 8, 62] although this finding is not supported by the recent report from the centers for disease control [12]. Prevalence throughout North America has been shown to be higher in the east and south compared to the west [57]. In many studies, girls are affected more often than boys [24].
The highest rates of birth defects reported in recent literature are in Northern China [64]. In a study conducted from 2002 to 2004 in the Shanxi province, the frequency of spina bifida was 6.39 per 1,000 live births. Mothers, younger than 20 years and older than 30 years, carried a much higher risk of delivering a child with an NTD (spina bifida or anencephaly), 40.9 and 20.5 per 1,000 live births, respectively. Zheng et al. noted a much higher prevalence of males born with birth defects than females [64].
The increased risk in mothers of advanced age is supported by an epidemiological study from Northern Iran from 1998 to 2003. In this report, mothers who were older than 35 years carried a risk of having a child with an NTD of 5.1 per 1,000 live births in comparison to younger mothers whose risk was 2.9 per 1,000 live births (1.6 per 1,000 live births for spina bifida) [23].
Folic acid supplementation/fortification
In 1991, the medical research council vitamin study research group (UK) demonstrated a significant decrease in NTDs with periconceptual multi-vitamin (folic acid) supplementation [50].This study was a double-blind, placebo-controlled trial in 33 centers across seven countries. It demonstrated that periconceptional folic acid supplementation (4 mg/day in mothers with a previous history of NTD) begun 6 weeks prior to conception was associated with a risk reduction of 72%.
Since 1992, the US center for disease control has recommended 400 μg of folic acid daily for all women of reproductive age [9]. Subsequently, the US food and drug administration mandated folic acid fortification of all enriched grain products by January 1998 [20]. Following this mandate, the incidence of spina bifida in the US decreased 22.9% when comparing the period of 1995–1996 to October 1998–December 1999 (from 2.62 to 2.02 per 10,000 live births) [12]. When comparing the early post-fortification period of 1999–2000 to 2003–2004, the prevalence further decreased to 1.9 per 10,000 live births. This risk reduction was noted in both the non-Hispanic white mothers and Hispanic mothers. The non-Hispanic blacks appreciated a significant decline in prevalence during this comparison period of 19.8% (from 2.17 to 1.74 cases per 10,000 live births).
Since this time, many population-based studies have demonstrated a similar impact on the prevalence of NTDs with periconceptual folate supplementation (as below). Interestingly, many studies have shown no decline in NTDs with government recommendations [3, 5, 22, 25, 54]. In a national survey completed annually between 2003 and 2007, women in the US 18–24 years of age had the least awareness regarding folic acid consumption (61%), the least knowledge regarding timing of folic acid consumption (6%), and the lowest daily consumption (30%) [11]. The protective effect of folic acid in reducing the incidence of NTDs is noted only after fortification programs are initiated [3, 5, 22, 25, 54].
This finding is in contrast to a recent study from Israel where a significant decline in the rate of spina bifida was recorded after the ministry of health recommended periconceptual folic acid supplementation [65]. In Jews, the rate of spina bifida decreased from 0.49 to 0.27 per 1,000 live births and from 0.95 to 0.62 in Arabs and Druze. Interestingly, a recent report by Mosley et al. found no additional protective effect of periconceptual folic acid supplementation after folate fortification was in effect in the US [52].
Prenatal diagnosis
The other major impact on the declining birth rate of children with NTDs is elective termination of the pregnancy following prenatal diagnosis. The impact of termination on the overall incidence is impossible to determine in the US given the reason that termination is not reportable [57]. Chan et al. reported a 75.7% decrease in live births with spina bifida in South Australia from 1966 to 1991 secondary to elective termination [14].
Prenatal diagnosis is accomplished by maternal screening of serum alpha fetoprotein (AFP) levels and the diagnosis is established by ultrasonography. Usually, the obstetrician who suspects an NTD in the fetus will also recommend an amniocentesis in order to complete a chromosomal analysis and obtain amniotic fluid for AFP and acetylcholinesterase [56].
Maternal AFP screening
Maternal serum AFP screening for NTDs is performed in the second trimester between 15 and 20 weeks of gestation [56]. AFP screening is primarily intended for the detection of open myelomeningocele and anencephaly but can also detect several non-neural fetal abnormalities [56]. It does not detect skin covered lesions (spina bifida occulta). When the serum AFP is positive, it is important to repeat the serum AFP because repeat testing will be negative in many cases, and such findings are not associated with an increased frequency of false-negative NTD diagnoses.
Ultrasound findings
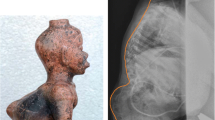
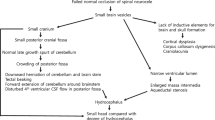
Myelomeningocele can be detected before the 12th postmenstrual week by noting irregularities of the bony spine or a bulging within the posterior contour of the fetal back [2]. After the 12th postmenstrual week, sonographic fetal markers for open NTDs include cranial findings: the “lemon” sign and the “banana” sign [2, 6]. The lemon sign refers to a concave shape of the frontal calvarium. The banana sign describes the posterior convexity of the cerebellum in the presence of spina bifida. These changes result from the Chiari II malformation (i.e., herniation of the cerebellum and brainstem through the foramen magnum). Other suggestive findings include ventriculomegaly, microcephaly, and obliteration of the cisterna magnum.
Fetal surgery
Fetal surgery for myelomeningocele can arrest leakage of spinal fluid from the back and might therefore prevent or reverse the Chiari II malformation and hydrocephalus. The surgery has been performed at a few centers since the late 1990s [1, 59]. Preliminary results show that fetal intervention improves the radiographic appearance of the hindbrain, including reducing the incidence of Chiari II malformation; it may also improve lower limb function, but this and other outcomes remain unproven because of varied selection criteria and lack of a comparable control group [15, 32]. These issues are being addressed in the management of myelomeningocele (MOMs) trial, which began in 2003 and is in progress at three institutions in the US [58]. Infants are randomized to fetal surgery at 18 to 25 weeks gestation or neonatal repair. Outcomes include the need for a shunt at 1 year, neurologic function, cognitive outcome, and maternal morbidity.
Diagnosis and management
Until the 1950s, hydrocephalus was an important cause of morbidity and mortality for individuals with myelomeningocele. The introduction of the valve for the ventricular cerebrospinal fluid (CSF) shunt in the 1950s offered effective treatment for hydrocephalus and presented the possibility of long-term survival for some infants [38].
At first, some providers, including John Lorber in England, advocated selective treatment for infants with myelomeningocele, reasoning that many of these children are a burden for themselves, their family, and society [40–42]. They advocated active treatment only for infants, whom they considered to have the best chance of a good neurologic outcome, amounting to 30% of newborns with myelomeningocele. The remainder was selected for non-treatment based on criteria that included hydrocephalus, paraplegia, severe kyphosis, and associated congenital defects. These children were given only supportive nursing care, and antibiotics and artificial feeding were withheld. Survival of an untreated child was considered an unacceptable outcome.
Today most centers treat all viable newborns aggressively without selection. Non-selective treatment increases overall survival several-fold, and there is little difference in functionality as compared with individuals surviving selective treatment protocols. Non-selective treatment is the current standard of care in the US [46, 48]. We await the outcome of the MOMs study to determine if in utero closure affords advantages to the child with myelomeningocele.
In the Netherlands between 1997 and 2004, 22 children with spina bifida were euthanized during the neonatal period, following a protocol intended to reduce suffering in terminally ill infants known as the Groningen protocol [60, 61]. This form of active euthanasia has prompted intense controversy and criticism, in part because of the difficulties of objectively determining suffering in an infant, predicting survival and quality of life, and because the infant patient cannot participate in the decision about his or her treatment [33, 43].
Impact of changes
Birth rate/mortality
The long-term outcome of non-selective treatment for myelomeningocele was outlined in a review of 118 infants with myelomeningocele born between 1975 and 1979 who had their original back closure at the Children’s Memorial Hospital in Chicago, IL, USA [4]. This cohort has been followed prospectively throughout childhood, and their continual progress is reported at regular intervals [4, 44, 49].
When comparing this older cohort to children born between 2000 and 2004 treated at the same institution, two notable differences are apparent (unpublished data). As anticipated, there is a decline in the number of live births with myelomeningocele who present for initial back closure to our tertiary care medical center. During 1975–1979, 118 children (range of 16–32 live births/year) presented for closure as compared to the younger cohort consisting of 40 children (range of 1–13 live births/year).
In the older cohort, the overall mortality at the 20–25-year follow-up was 24% and continued to increase into young adulthood [4]. A majority of the deaths occurred during infancy and the preschool years (18/28). Most of the children died secondary to hindbrain dysfunction (13/18). As expected, the cohort who died had a higher rate of posterior cervical decompression with tracheostomies and/or gastrostomy tubes.
In comparison, in the cohort born in 2000–2004, none of the patients have died during infancy or the preschool years (unpublished data). Four children have undergone a posterior cervical decompression, but only one child required a tracheostomy placement with long-term ventilator support. She will soon be decanulated. Four children have required gastrostomy tube placement, three have been removed.
Shunt dependency
In the older cohort, 86% of patients had undergone CSF diversion and 95% of those with shunts had undergone at least one shunt revision.
In the children born between 2000 and 2004, the rate of shunt placement is 65% (26/40 patients). Of those shunted, 71% have undergone at least one revision. This decreased rate of shunt placement is not as low as the one recently reported by Chakraborty et al. [13]. The group from Great Ormond Street, London has been able to decrease the rate of shunt placement to 51%. As with us, they are reserving shunt placement for those children with symptoms, massive hydrocephalus, or significant progressive ventriculomegaly after back closure.
Clinical implications
Over the last 25 years, as the incidence of live births with myelomeningocele decreased, fortunately, the severity of the disease has also lessened. Far fewer children now die during infancy and early childhood. The outcome of children born with myelomeningocele has improved. Not only do we have a very large group of patients who are becoming young adults, but we have also noted a significant decline in the mortality rate within the first few years of life. Given the decrease in shunt dependency, it is our hope that the overall mortality rate associated with this disease will decline. In older cohort of children born between 1975 and 1979, the most common cause of death was an unrecognized shunt malfunction.
Although it is a success for the children, families, and medical team, the increased survival now presents a challenging issue for adult medical providers. Many adult medical practitioners are uncomfortable caring for individuals with chronic, congenital illnesses, yet their pediatric colleagues are uncomfortable caring for an adult patient. This presents a quandary for the young adult with myelomeningocele.
One of the biggest challenges facing the pediatric multi-disciplinary spina bifida clinics is the transitioning of the young adults into an appropriate medical setting/care. Kaufman et al. documented a significant correlation of increased morbidity (amputation and nephrectomy) in spina bifida patients not treated in a multi-disciplinary clinic [35]. Unfortunately, few adult multi-disciplinary clinics are established [55]. Most of the care of these older patients is provided by primary care physicians [29, 30].
Not only are few clinics established, but the patients are quite hesitant to transition to an adult care team [55]. Many young adults have difficulty leaving the treatment team they have known throughout their lifetime, and in many situations, have supported them through life-threatening illnesses. The biggest insecurity expressed by patients and their families in transitioning to an adult care team is a lack of appreciation for the varied symptoms of a shunt malfunction. As shown by Dr. Hunt and colleagues, the ongoing disability associated with myelomeningocele is related to the child’s original neurologic deficit and episodes of increased intracranial pressure related to their shunts [29–31]. It is the responsibility of the pediatric multi-disciplinary medical and surgical subspecialists to assist our adult colleagues in attaining the skills necessary to care for this population of patients who are surviving this complex chronic congenital disease.
References
Adzick NS, Sutton LN, Crombleholme TM, Flake AW (1998) Successful fetal surgery for spina bifida. Lancet 352:1675–1676
Blumenfeld Z, Siegler E, Bronstein M (1993) The early diagnosis of neural tube defects. Prenat Diagn 13:863–871
Botto LD, Lisi A, Robert-Gnansia E, Erickson JD, Vollset SE, Mastroiacovo P, Botting B, Cocchi G, de Vigan C, de Walle H, Feijoo M, Irgens LM, McDonnell B, Merlob P, Ritvanen A, Scarano G, Siffel C, Metneki J, Stoll C, Smithells R, Goujard J (2005) International retrospective cohort study of neural tube defects in relation to folic acid recommendations: are the recommendations working? BMJ 330(7491):571
Bowman RM, McLone DG, Grant JA, Tomita T, Ito JA (2001) Spina bifida outcome: a 25-year prospective. Pediatr Neurosurg 34:114–120
Busby A, Abramsky L, Dolk H, Armstrong B, Eurocat Folic Acid Working Group (2005) Preventing neural tube defects in Europe: population based study. BMJ 330(7491):574–575
Campbell J, Gilbert WM, Nicolaides KH, Campbell S (1987) Ultrasound screening for spina bifida: cranial and cerebellar signs in a high-risk population. Obstet Gynecol 70:247–250
Canfield MA, Annegers JF, Brender JD, Cooper SP, Greenberg F (1996) Hispanic origin and neural tube defects in Houston/Harris County. Texas. II. Risk factors. Am J Epidemiol 143:12
Carmichael SL, Shaw GM, Song J, Abrams B (2008) Markers of acculturation and risk of NTDs among Hispanic women in California. Birth Defects Res A Clin Mol Teratol 82(11):755–762
Centers for Disease Control (1992) Recommendations for the use of folic acid to reduce the number of cases of spina bifida and other neural tube defects. MMWR Recomm Rep 41(RR-14):1–7
Centers for Disease Control (2004) Spina bifida and anencephaly before and after folic acid mandate—United States, 1995–1996 and 1999–2000. MMWR Morb Mortal Wkly Rep 52:362–365
Centers for Disease Control (2008) Use of supplements containing folic acid among women of childbearing age—United States 2007. MMWR Morb Mortal Wkly Rep 57(1):5–8
Centers for Disease Control (2009) Racial/ethnic differences in the birth prevalence of spina bifida—United States, 1995–2005. MMWR Morb Mortal Wkly Rep 57(53):1409–1413
Chakraborty A, Crimmins D, Hayward R, Thompson D (2008) Toward reducing shunt placement rates in patients with myelomeningocele. J Neurosurg Pediatr 1(5):361–365
Chan A, Robertson EF, Haan EA, Keane RJ, Ranieri E, Carney A (1993) Prevalence of neural tube defects in South Australia, 1966–91, effectiveness and impact on prenatal diagnosis. BMJ 307(6906):703–706
Chen CP (2008) Prenatal diagnosis, fetal surgery, recurrence risk and differential diagnosis of neural tube defects. Taiwan J Obstet Gynecol 47:283–290
Cowchock S, Ainbender E, Prescott G, Crandall B, Lau L, Heller R, Muir WA, Kloza E, Feigelson M, Mennuti M, Cederquist L (1980) The recurrence risk for neural tube defects in the United States: a collaborative study. Am J Med Genet 5:309–314
De Marco P, Calevo MG, Moroni A, Arata L, Merello E, Finnell RH, Zhu H, Andreussi L, Cama A, Capra V (2002) Study of MTHFR and MS polymorphisms as risk factors for NTD in the Italian population. J Hum Genet 47:319–324
Deak KL, Siegel DG, George TM, Gregory S, Ashley-Koch A, Speer MC, NTD Collaborative Group (2008) Further evidence for a maternal genetic effect and a sex-influenced effect contributing to risk for human neural tube defects. Birth Defects Res A Clin Mol Teratol 82(10):662–9
Elwood JM, Elwood JH (1980) Epidemiology of anenecephalus and spina bifida. Oxford University Press, New York, Toronto, pp 85–119
Food and Drug Administration (1996) Food standards: amendment of standards of identity for enriched grain products to require addition of folic acid. Fed Regist 61:8781–8797
Frey L, Hauser WA (2003) Epidemiology of neural tube defects. Epilepsia 44(Suppl 3):4
Godwin KA, Sibbald B, Bedard T, Kuzeljevic B, Lowry RB, Arbour L (2008) Changes in frequencies of select congenital anomalies since the onset of folic acid fortification in a Canadian birth defect registry. Can J Public Health 99(4):271–275
Golalipour MJ, Mobasheri E, Vakili MA, Keshtkar AA (2007) Epidemiology of neural tube defects in Northern Iran, 1998–2003. East Mediterr Health J 13(3):560–566
Greene W, Terry R, Demasi R, Herrrington R (1991) Effect of race and gender on neurological level in myelomeningocele. Dev Med Child Neurol 33(2):110–117
Gucciardi E, Pietrusiak MA, Reynolds DL, Rouleau J (2002) Incidence of neural tube defects in Ontario, 1986–1999. Can Med Assoc J 167(3):237–240
Harmon JP, Hiett AK, Palmer CG, Golichowski AM (1980) Prenatal ultrasound detection of isolated neural tube defects: is cytogenetic evaluation warranted? Am J Med Genet 5:595
Harris MJ, Juriloff DM (2007) Mouse mutants with neural tube closure defects and their role in understanding human neural tube defects. Birth Defects Res A Clin Mol Teratol 79:187–210
Hernandez-Dias S, Werler MM, Walker AM, Mitchell AA (2001) Neural tube defects in relation to use of folic acid antagonists during pregnancy. Am J Epidemiol 153(10):961–968
Hunt GM, Oakeshott P (2003) Long-term outcome in open spina bifida. Br J Gen Pract 53(493):632–636
Hunt GM, Oakeshott P (2003) Outcome in people with open spina bifida at age 35: prospective community based cohort study. BMJ 326(7403):1365–1366
Hunt G, Oakeshott P, Kerry S (1999) Link between the CSF shunt and achievement in adults with spina bifida. J Neurol Neurosurg Psychiatry 67(5):591–595
Johnson MP, Sutton LN, Rintoul N, Crombleholme TM, Flake AW, Howell LJ, Hedrick HL, Wilson RD (2003) Fetal myelomeningocele repair: short-term clinical outcomes. Am J Obstet Gynecol 189:482–487
Jotkowitz AB, Glick S (2006) The Groningen protocol: another perspective. J Med Ethics 32:157
Kadir RA, Sabin C, Whitlow B, Brockbank E, Economides D (1999) Neural tube defects and periconception folic acid in England and Wales: retrospective study. BMJ 319:92–93
Kaufman BA, Terbrock A, Winters N, Ito JA, Klosterman A, Park TS (1994) Disbanding a multidisciplinary clinic: effects on the healthy care of myelomeningocele patients. Pediatr Neurosurg 21:36–44
Kennedy D, Chitayat D, Winsor EJ, Silver M, Toi A (1998) Prenatally diagnosed neural tube defects: ultrasound, chromosome and autopsy or postnatal findings in 212 cases. Am J Med Genet 77:317
Kirke PN, Mills JL, Molloy AM, Brody LC, O’Leary VB, Daly L, Murray S, Conley M, Mayne PD, Smith O, Scott JM (2004) Impact of the MTHFR C677T polymorphism on risk of neural tube defects: case control study. BMJ 328:1535–1536
Laurence K (1974) Effect of early surgery for spina bifida on survival and quality of life. Lancet 1:301–304
Leck I (1966) Changes in the incidence of neural-tube defects. Lancet 2:791–793
Lorber J (1973) Early results of selective treatment of spina bifida cystica. BMJ 4:201
Lorber J (1974) Selective treatment of myelomeningocele: to treat or not to treat? Pediatrics 53:307
Lorber J, Salfield SA (1981) Results of selective treatment of spina bifida cystica. Arch Dis Child 56:822
Manninen BA (2006) A case for justified non-voluntary active euthanasia: exploring the ethics of the Groningen protocol. J Med Ethics 32:643
McLone DG (1983) Results of treatment of children born with a myelomeningocele. Clin Neurosurg 30:407–412
McLone DG (1986) Treatment of myelomeningocele: arguments against selection. Clin Neurosurg 33:359–370
McLone DG (1986) The diagnosis, prognosis and outcome for the handicapped newborn: a neonatal view. Issues Law Med 2:15
McLone DG (1989) Spina bifida today: problems adults face. Semin Neurol 9(3):169–175
McLone DG (2008) Deliberate termination of life of newborns with spina bifida. Childs Nerv Syst 24:33
McLone DG, Dias L, Kaplan WE, Sommers MW (1985) Concepts in the management of spina bifida. In: Humphreys RP (ed) Concepts in Pediatric Neurosurgery, vol 5. Karger, Basel, pp 97–106
Medical Research Council Vitamin Study Research Group (1991) Prevention of neural tube defects: results of the medical research council vitamin study. Lancet 338:131–137
Moretti MD, Bar-Oz B, Fried S, Koren G (2005) Maternal hyperthermia and the risk for neural tube defects in offspring: systematic review and meta-analysis. Epidemiology 16:216
Mosley BS, Cleves MA, Siega-Riz AM, Shaw GM, Canfield MA, Waller DK, Werler MM, Hobbs CA (2009) Neural tube defects and maternal folate intake among pregnancies conceived after folic acid fortification in the United States. Am J Epidemiol 169(1):9–17
Papp C, Adam Z, Toth-Pal E, Török O, Váradi V, Papp Z (1997) Risk of recurrence of craniospinal anomalies. J Matern Fetal Med 6:57
Persad VL, Van den Hof MC, Dube JM, Zimmer P (2002) Incidence of open neural tube defects in Nova Scotia after folic acid fortification. CMAJ 167(3):241–245
Sawyer SM, Collins N, Bryan D, Brown D, Hope MA, Bowes G (1998) Young people with spina bifida: transfer from paediatric to adult health care. J Paediatr Child Health 34(5):414–417
Shaer CM, Chescheir N, Schulkin J (2007) Myelomeningocele: a review of the epidemiology, genetics, risk factors for conception, prenatal diagnosis and prognosis for affected individuals. Obstet Gynecol 62(7):471–479
Shurtleff DB (2004) Epidemiology of neural tube defects and folic acid. Cerebrospinal Fluid Res 1:5–9
Sutton LN (2008) Fetal surgery for neural tube defects. Best Pract Res Clin Obstet Gynaecol 22:175
Tulipan N, Bruner JP (1998) Myelomeningocele repair in utero: a report of three cases. Pediatr Neurosurg 28:177
Verhagen AA, Sauer PJ (2005) The Groningen protocol—euthanasia in severely ill newborns. N Engl J Med 352:959
Verhagen AA, Sol JJ, Brouwer OF, Sauer PJ (2005) Deliberate termination of life in newborns in the Netherlands: review of all 22 reported cases between 1997 and 2004. Ned Tijdschr Geneeskd 149:183
Williams LJ, Rasmussen SA, Flores A, Kirby RS, Edmonds LD (2005) Decline in the prevalence of spina bifida and anencephaly by race/ethnicity: 1995–2002. Pediatrics 116:580–586
Woodhouse CRJ (2008) Myelomeningocele: neglected aspects. Pediatr Nephrol 23(8):1223–1231
Zheng XY, Song XM, Chen G, Ji Y, Wu JL, Liu JF, Zhang L, Fan XH (2007) Epidemiology of birth defects in high-prevalence areas of China. Zhonghua Liu Xing Bing Xue Za Zhi 28:5–9
Zlotogora J, Amitai Y, Leventhal A (2006) Surveillance of neural tube defects in Israel: the effect of the recommendation for periconceptional folic acid. Isr Med Assoc J 8(9):601–604
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bowman, R.M., Boshnjaku, V. & McLone, D.G. The changing incidence of myelomeningocele and its impact on pediatric neurosurgery: a review from the Children’s Memorial Hospital. Childs Nerv Syst 25, 801–806 (2009). https://doi.org/10.1007/s00381-009-0865-z
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00381-009-0865-z