Abstract
Background
Total anomalous pulmonary venous connection (TAPVC) is a rare congenital heart disease of newborns characterized by impaired left ventricle growth and diastolic dysfunction. We hypothesized that the patients with TAPVC reduced blood flow into the left heart prenatally could affect left atrium (LA) not just growth but function. We compared the age-related changes in LA deformation using two-dimensional speckle-tracking echocardiography (2DSTE) in Patients with TAPVC.
Method
This single-center, retrospective cohort study was conducted on consecutive isolated TAPVC patients who underwent neonatal surgery between January 1, 2009 and January 1, 2022. The LA datasets in TAPVC patients were analyzed before surgery (n = 28) and follow-ups at 1−2 (n = 24) and 5−7 years of age (n = 13) and compared with those of age-matched healthy controls (January 2009−2022). The LA strain (ε), indicating LA function, was analyzed using QLAB represented by reservoir (εR), conduit (εCD), and contractile (εCT) strains. LA pressure was evaluated by periodic follow-up catheterization after repair.
Results
Compared to the controls, the TAPVC patients had significantly smaller LA maximum volume preoperatively, and with age, the LA maximal volumes reached normal levels, while the LA minimal volumes were larger. All 2DSTE-determined LA strains showed significant reductions at all time points in the TAPVC group compared to those in the control (median εR, εCD, and εCT; before surgery: 17.0% vs. 26.0%, 12.9% vs. 15.9%, and 6.3% vs. 10.4%; follow-up at 1−2 years: 30.0% vs. 45.7%, 23.2% vs. 29.6%, and 6.1% vs. 16.3%; follow-up at 5−7 years: 31.2% vs. 43.1%, 25.0% vs. 31.2%, and 5.2% vs. 10.8%, respectively; p < 0.05). Only εCT did not represented a significant change over time even though after correction of blood flow (median εCT: 6.0% → 5.9%). Patients with pulmonary venous obstruction (PVO) at birth showed significantly decreased εR and εCD and higher LA pressure compared to those without PVO.
Conclusion
This study showed that nevertheless maximum volume of LA was recovered within the normal range, reduced LA strains, especially contractile function lasted from birth even after repair in Patients with TAPVC.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Total anomalous pulmonary venous connection (TAPVC) is a rare congenital heart anomaly, representing approximately 1−3% of all the cardiovascular abnormalities in newborns. [1] Surgical intervention for TAPVC showed satisfactory results beyond the first decade of life, [2] however, exercise performance is mildly impaired at long-term follow-up after TAPVC repair during infancy [3, 4]. Moreover, the left atrium (LA) and left ventricle (LV) in the TAPVC patients are hypoplastic compared with normal is well documented [5, 6]. TAPVC is characterized by interruption in the communication between the splanchnic plexus and cardinal and umbilical-vitelline systems, and hence, all pulmonary veins directly or indirectly drain blood to the right atrium. [7] The left heart therefore do not receive blood supply from the splanchnic plexus directly during the embryonic stage; hence, blood flow into the left heart only through the foramen ovale [5, 8]. Based on the flow theory—reduced flow into the LV contributes to LV hypoplasticity and impairment in the patients with hypoplastic left heart syndrome (HLHS) at the fetus stage [9]—reduced flow into the left heart have affected not only LV but LA after birth. Impairment of LV function in the patients with TAPVC was discussed in previous reports [10, 11]. However, the LA function especially by the deformation analysis has not verified.
Recently, two-dimension speckle-tracking echocardiography (2DSTE) has been used to analyze the LA deformation in adult patients. 2DSTE is less affected by the angle dependency than Tissue Doppler Imaging to evaluate myocardial deformation, also reduced the influence of cardiac motion, loading condition and tethering effect. [12] A few studies have successfully implemented 2DSTE to assess the normal value of LA deformation in pediatric field, [13,14,15] and further studies analyzing congenital heart disease using this technique are expected.
This retrospective study aimed to assess the age-related changes in the LA deformation (from perioperative to follow-up period), analyzed by 2DSTE imaging, in Patients with TAPVC in comparison to those in the healthy age-matched controls.
Methods
Study design
This single-center, retrospective cohort study was conducted on consecutive isolated TAPVC patients who underwent neonatal surgery between January 1, 2009 and January 1, 2022. The LA volume and strains were compared between the TAPVC patients and healthy age-matched controls without functional and constructive anomaly before surgery and during the follow-ups at 1−2 and 5−7 years of age, respectively.
In Patients with TAPVC, pulmonary venous obstruction (PVO) is a risk factor for long-term outcomes, and its presence may be a cause of less blood flow into the left heart in the fetal stage. Therefore, subgroup analysis was performed to determine significant changes in the LA function in Patients with or without PVO. PVO was defined as an obstruction to the vein draining pulmonary vein confluence before surgery, indication severe desaturation and low non-phasic vertical vein flow less than 1.2 m/s [16]. We routinely performed catheterization and echocardiography one year after surgery and around entering elementary school in the patients with repaired TAPVC. We also investigated the correlation between the LA pressure in follow-up catheterization and echocardiographic data. This study followed the tenets of the Declaration of Helsinki, and all patients were aware of an opt-out provision. The ethics committee in our hospital approved this study.
Patients
Of the total 70 patients with TAPVC referred to or born in our hospital for the intensive care and following surgery from January 1, 2009 to January 1, 2022, 37 patients were included in the present study. The inclusion criteria were isolated TAPVC without other cardiac abnormalities such as cases with functionally univentricular circulation or atrial isomerism and severe non-cardiac anomaly. The exclusion criteria included using other ultrasound device (We used IE33/EPIC/CX50 systems (Philips Medical Systems, Andover, MA)), inadequate echocardiography data or poor echocardiographic window precluding correct evaluation, and death or drop out during the follow-up period. Twenty-eight, twenty-four and thirteen patients were included before surgery, at 1−2 and 5−7 years of age, respectively. We also investigated age-matched healthy controls referred for the screening of the heart murmur, another non-cardiac anomaly, or evaluation of the cardiac function before chemotherapy, except for the patients with chromosomal abnormality, mitral valve insufficiency, and left ventricular impairment. Among the 28 patients, consecutive data could be obtained for 19 patients with isolated TAPVC, while five patients were excluded due to the use of other ultrasound systems (GE, Toshiba, Canon, and Siemens) and four patients were death during the 2 years follow-up period (median 1 year; range 0.8−2 years) (Fig. 1). Demographic data included gestational age, body weight, sex, type of TAPVC, operation date (day after birth), operation techniques, and PVO. Body surface area (BSA) was calculated using the Haycock formula [17].
Conventional echocardiography
All examinations were performed using IE33/EPIC/CX50 systems (Philips Medical Systems, Andover, MA). All the study participants underwent comprehensive two-dimensional echocardiography (2DE) with Doppler and tissue Doppler imaging. LV ejection fraction (LVEF) and LV end-diastolic volume index (LVEDVI) by modified Simpson method, peak early diastolic mitral inflow velocity E, and peak atrial filling velocity A during late diastole, E/A, peak early diastolic tissue Doppler velocity at the inter-ventricular septum (IVS) and lateral mitral annulus (e’), and E/e’ were obtained according to the reported guidelines [18, 19]. Then, average E/e’ was calculated.
Real-time three-dimensional echocardiography (RT3DE)
We performed transthoracic RT3DE using IE33/EPIC and acquired full volume images with a matrix array transducer (X7-2 or X5-1; Philips Medical Systems) of as many subjects as possible. The 3D datasets were transferred to an offline 3D software (TomTec-Arena®: TomTec Imaging Systems, Unterschleissheim, Germany) and analyzed by an experienced investigator using 4D cardio-view system to measure the maximum and minimum LA volume during the cardiac cycle, while excluding the LA appendage and pulmonary vein. The LA volume indexes to BSA was also calculated in both maximum and minimum.
2DSTE
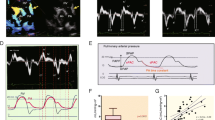
All images used for 2DSTE analysis were obtained at an average frame rate of 61.5 fps (54.8−87.0 fps) and expressed as a median (first and third quartile, Q1–Q3). Strain was analyzed by a single investigator using QLAB Advanced Quantification Software® version 13.0 (Philips Medical Systems, Bothell, WA). End-diastolic frame was determined in the apical 4-chamber view using one selected cardiac cycle. After initializing 3 points (septal and lateral corners of the mitral annulus and the LA roof) in the end-diastolic frame, the software automatically performed speckle-tracking analysis in 6 regions throughout the cardiac cycle. To avoid anatomic effects, the LA appendage and pulmonary vein were excluded. Manual adjustments of LA tracking points were performed when needed. The QRS complex (R–R gating) was used as the initiation of the strain value calculation. The longitudinal time strain curves were generated by the software for each atrial segment. The strain profile was acquired by averaging profiles computed at each of the 6 segments of the LA wall (Fig. 2A). LA reservoir function (εR (%)) was measured as the average peak atrial longitudinal strain calculated in all LA segments during LV systole (global peak atrial longitudinal strain). LA contractile function (εCT [%]) was obtained by the global LA wall strain just before atrial contraction in the LV early diastolic phase. Further, LA conduit function (εCD [%]) was calculated as follows: εCD (%) = εR – εCT. Global longitudinal time strain curve was reconstructed using the datasets in Microsoft Excel 2016 followed by the precise calculation of εR, εCD, and εCT (Fig. 2B). If patients with isolated TAPVC before surgery had a large atrial septal defect, we calculated the global longitudinal strain employing only five regions (excluding the mid inferoseptal) to minimize the effect of its defect.
A Example of the assessment of LA strain (ε), and the longitudinal time strain curves were generated by the software for each atrial segment. The strain profile was acquired by averaging the strain profiles computed at all 6 segments. B The global longitudinal time strain curve was shown. The QRS complex (R–R gating) was used as the initiation of the ε calculation. LA reservoir function (εR (%)) was measured based on the average peak atrial longitudinal strain calculated in all LA segments during LV systole (global peak atrial longitudinal strain). LA contractile function (εCT (%)) was obtained by calculating the global LA wall strain just before atrial contraction in LV early diastole. LA conduit function (εCD (%)) was calculated as follows: εCD (%) = εR − εCT
Intra-observer and inter-observer variability
For the intra-observer variability, the same observer analyzed the LA functions obtained by 2DSTE (reservoir, conduit, and contractile function) again after one month in the same cardiac cycle in 20 subjects who were randomly selected and were blinded to the observer. Inter-observer variability was determined by a separate off-line analysis of the LA functions in 20 randomly selected subjects by two independent observers (pediatric cardiologists: N.R and T.K) who were blinded to patients’ clinical data at the time of analysis.
Statistical analysis
Continuous data are expressed as medians with the first and third quartile, Q1–Q3. Non-continuous variables are expressed as numbers and percentages. Comparisons between the continuous and categorical variables were performed using the Mann−Whitney U test and Fisher’s exact test, respectively. Paired and unpaired non-parametric sample comparisons (with the time-dependent continuous variables trends) were performed using the Friedman analysis and post-hoc analysis by the Bonferroni correction [15] and Kruskal−Wallis test and post-hoc analysis by Bonferroni correction, [15] respectively. Further, relationships between the continuous variables were evaluated using the Spearman’s rank correlation coefficient. Intra-observer and inter-observer variabilities were calculated as the absolute difference between the corresponding repeated measurements as a percent of the mean. Reliability was assessed using the Bland−Altman analysis and intraclass correlation coefficients (ICCs) with a 95% confidence interval limit. All analysis was performed using EZR version 1.4. [20] A P-value of less than 0.05 was considered statistically significant.
Results
Patient characteristics
Table 1 shows the demographic characteristics of the 28 patients with isolated TAPVC before surgery. TAPVC repair surgeries were performed at the median age of 9 days after birth (range: 0−180 days) by employing four different types of operative techniques; almost half of the patients underwent direct sutures (46.4%). Nine patients had PVO after birth, while only one of them had PVO after surgery.
Table 2 shows the clinical data of patients with isolated TAPVC and healthy age-matched controls collected before surgery and during the follow-up periods at 1−2 and 5−7 years of age. No significant difference was observed in the gestational age and sex between the two groups (isolated TAPVC and healthy controls). Body weight and BSA were significantly smaller in the TAPVC group than in the healthy control group during the follow-up period at 1−2 years of age; however, no difference was observed during the follow-up period at 5−7 years of age. Heart rate was slightly faster in the TAPVC group than that in the healthy control group before surgery, while no significant difference was observed in the frame rates between the two groups at any age. LVEDVI was significantly smaller than the control in neonates before surgery, however after correction of blood flow returned to the normal range. Similarly, LVEF was significantly decreased before surgery in the TAPVC group (median EF 58.1%) compared with those in the healthy controls (median EF 61.1%), but the median LVEF was normal, and LVEF was not significantly different after surgery between the two groups. While E/A and average E/e’ in the TAPVC group were significantly larger at the follow-ups in comparison to the controls. Similarly, E wave was significantly larger in the TAPVC group at the follow-ups while A wave was not different in both groups at any points.
Consecutive clinical data of patients with isolated TAPVC from the perioperative period (before and after surgery) to follow-up are also presented in Table 3.
Comparison of the LA volume by RT3DE
The LA maximum and minimum volume indexes measured by RT3DE, were significantly (p = 0.006) smaller in the TAPVC group before surgery than that in the healthy control group (Fig. 3A, B). Although the LA maximum volume indexes turned to normal range (in comparison to the healthy controls) during the follow-up period after repair, the LA minimum volume indexes were significantly (p < 0.001) larger in the TAPVC group during the follow-up period after repair than those in the healthy controls.
Comparison of the LA functions by 2DSTE
All 2DSTE-determined LA strains were significantly reduced in the TAPVC group compared to those in the healthy control group at all time points (median εR, εCD, and εCT; before surgery, 17.0 vs. 26.0, 12.9 vs. 15.9, and 6.3 vs. 10.4; follow-up at 1−2 years: 30.0 vs. 45.7, 23.2 vs. 29.6, and 6.1 vs. 16.3; and follow-up at 5−7 years: 31.2 vs. 43.1, 25.0 vs. 31.2, and 5.2 vs. 10.8, respectively (Fig. 4A–C and Table 4). Although εR and εCD showed significant improvements during the follow-up periods, εCT did not show any increase.
Time-dependent paired comparisons of LA strains
The LA strains were traced among same patients over 1–2 years after surgery (n = 19). The εR and εCD significantly (εR, p = 0.015; εCD, p < 0.001) increased over time (before surgery vs. after surgery vs. follow-up; median; εR: 16.8% vs. 17.5% vs. 27.5%, εCD: 13.1% vs. 12.7% vs. 23.0%) (Fig. 5A, B), while εCT showed no significant difference (before surgery vs. after surgery vs. follow-up; median; εCT: 6.0% vs. 6.0% vs. 5.9%) (Fig. 5C).
Comparison of LA deformation and LV diastolic function between obstructed and unobstructed TAPVC before surgery and with different operative techniques
In the subgroup analysis conducted before surgery, patients with PVO (PVO group) showed significantly (εR p = 0.013 and εCD p = 0.043) decreased εR and εCD than those without PVO (PVO vs. non-PVO; median; εR: 15.2% [12.7–16.0] vs. 20.3% [16.8–25.4], εCD: 8.5% (7.6 − 11.0) vs. 14.5% [10.9–15.3]). Further, between these two groups, LVEF and average E/e’ were not significantly different (Table 5). Finally, patients who underwent direct suture (n = 11) did not show significantly different LA function and LV diastolic parameters in comparison to the patients who underwent other operative techniques (n = 8).
Correlation of LA pressure in the patients with repaired TAPVC by catheterization
Patients with repaired TAPVC underwent 32 catheterizations during the follow-up period. The mean age was 3.16 (± 3.0) years and all the patients were administered with general anesthesia and were under intubation during the examination. The catheterization showed that the mean LA pressure and pulmonary artery pressure were 8.91 (± 2.35) and 16.1 (± 4.28) mm Hg, respectively. Only four of thirty-two patients (12.5%) showed an LA pressure of 12 mm Hg or greater. The LA pressure had significantly negative correlations with both the LA maximum (r = – 0.53, p = 0.0017) and minimum (r = – 0.46, p = 0.0080) volume indexes and age at the repair (r = – 0.43, p = 0.0128). In contrast, any LA strain determined by 2DSTE had no significant correlation with the LA pressure. Furthermore, the LA pressure was significantly (p = 0.046) elevated in the PVO group (n = 11; median: 10 [9−11] mmHg) compared to that in the non-PVO group (n = 21; median: 8 [7−9] mm Hg) before surgery.
intra-observer and inter-observer variability
The intra-observer and inter-observer variabilities were assessed in 20 randomly selected patients (median age: 13.5 days [day 0–2 years], 8 male patients). The intra-observer and inter-observer coefficients of variation showed acceptable reproducibility (Table 6). The linear correlations, results of the Bland–Altman analyses, and ICCs are showed in Supplemental data 1 and 2. The 2DSTE method for the analysis of each LA function was proved to have favorable intra-observer (εR: r = 0.91, bias ± limits of agreement [LOA] = 1.29 ± 5.9%, ICC = 0.95; εCD: r = 0.95, bias ± LOA = 1.51 ± 4.4%, ICC = 0.94; εCT: r = 0.92, bias ± LOA = 0.09 ± 4.3%, ICC = 0.95) and inter-observer (εR: r = 0.82, bias ± LOA = – 2.37 ± 9.1%, ICC = 0.82; εCD: r = 0.95, bias ± LOA = – 0.8 ± 6.5%, ICC = 0.85; εCT: r = 0.85, bias ± LOA = – 1.24 ± 5.3%, ICC = 0.92) agreements.
Discussion
This study demonstrated that in patients with isolated TAPVC, maximum volume of LA and LV end-diastolic volume developed to the normal range after surgery, however, LA reservoir, conduit, and contractile strains were reduced; especially, reservoir and conduit strains tended to increase while notably the contractile strain did not recover over time.
As previous reports have shown, small LV and LA in the patients with TAPVC returned to the normal volume after correction [6, 11]. Our data also consistent with those of these studies and PVO may accelerate its hypoplasia. This supported that the blood flow into the left heart affected the growth of ventricle and atrium, reported as a flow theory previously [9]. On the other hand, LV diastolic dysfunction after TAPVC repair was demonstrated in comparison with both normal controls and patients with transposition of the great arteries, and this report may rule out potential adverse effects of cardiopulmonary bypass [10]. In the present study, LV diastolic function was deteriorated represented by increased E/A and average E/e’ after repair compared with normal controls as well. Besides, LV diastolic impairment was not clear before TAPVC repair. Relative unloading LV during cardiac development may cause impairment of LV diastolic function, [10] but cardiopulmonary bypass and cardioplegic arrest can lead to LV fibrosis and accelerate diastolic dysfunction [21]. We speculate there is a possibility that both of them affects the LV diastolic impairment in repaired TAPVC.
Similar to present study, previous report suggested that volume of LA returned to normal range after repair of TAPVC in five cases [6]. However, perioperative functional analysis of LA had not performed before in repaired TAPVC patients. LA analysis using deformation method by 2DSTE was feasible to assess the details of LA function like a reservoir, conduit and contractile function. Reservoir function defined as LA compliance, affected by mitral annular movement and end-systolic LV volume. In the early diastolic phase, LA volume shrinks mostly (~ 75%) and εCD for transport of blood from the pulmonary vein to the LA is influenced by LV relaxation. The remaining volume (~ 25%) shifts into LV during late diastolic phase by active contracts of LA (εCT) [12, 21, 22]. In the present study, LV systolic function affecting mitral valve movement was normal at birth, and LV volume returned to normal but εR was sustained to reduce after surgery but shown a mild improvement in the patients with TAPVC. This may suggest reduction of LA compliance in TAPVC patients from birth and lasting. Also, abnormal LV relaxation after repair could have influenced the persistent decrease in εCD. And the reservoir and conduit function improved mildly as the left heart volume became adequate. On the other hands, εCT remained to reduce until follow-up period and had not increased over time. This may suggest that reduction of native LA contractility lasts even after correction of blood supply in the case without appropriate LA volume loading in utero.
Previously, greater prognosis benefits of the LA myocardial deformation assessment were suggested in adult patients, in comparison to those of the LA volume and LA ejection fraction [23]. Also, LA strains were recommended as an additional value for evaluation of a LA pressure [24]. Moreover, several studies demonstrated that LA reservoir function was strictly correlated with LV filling pressure [25] [26]. Regarding to εCT, reduced εCT was a useful predictor for newly developing and recurrent atrial fibrillation (AF) [27, 28]. Moreover, previously reported that there is a clinically feasible potential to predict LV filling pressure in εCT, which is comparable to εR. The optimal cut-off to differentiate between normal and elevated LV filling pressure was 18% for εR and 8% for εCT when defining LV filling pressure > 12 mmHg as elevated, and 16% and 6% when using LV filling pressure > 15 mmHg [29]. However, our results showed no correlation between any LA strains and LA pressure nevertheless any LA strains were reduced. On the other hands, LA 3D volume was strongly correlated with LA pressure. It is possible that the reasons why no correlation between LA deformation and LA pressure are because of small sample size, few patients with high LA pressure (LA pressure over 12 mmHg in those of 12.5%) and no patients with LA enlargement (≧34 ml/m2). Moreover, in the last European Association of Cardiovascular Imaging (EACVI) consensus document, LA deformation was recommended as the third parameter following the traditional indices like a E/e’ (> 14), tricuspid valve regurgitation (> 2.8 m/s) and LA volume index (≧34 ml/m2)[30]. LA deformation, therefore, may hard to predict elevation of LV filling pressure under the condition within the normal range of LAVI and weaker independent determinant. However, several studies have shown that εR strain can detect elevated LV filling pressure even when LAVI is normal, [31, 32] hence more studies in TAPVC patients are needed. Conversely, the present study showed a significant reduction of LA strains in patients with TAPVC before LA pressure increased significantly. These results may imply that LA 2D speckle tracking analysis can detect the potential deterioration of LA earlier than conventional Doppler parameters. Also, although periodic catheterization may not be necessary in postoperative patients with TAPVC, the present study suggests that careful periodic observation of left ventricular diastolic function including LA function by non-invasive methods, such as echocardiography, is important.
On the other hand, TAPVC patients have similar pathophysiology “but not exactly same” to patients with heart failure with preserved ejection fraction (HFpEF) in terms of diastolic impairment and reduction of LA reservoir and contractile strains. HFpEF is well known, particular among elderly patients, female and hypertensive patients. This condition has been reported the increase mortality and hospital readmission [33,34,35]. A patient group with HFpEF without LA enlargement showed significantly decrease of εR and εCT compared with controls in a previous study. Also, lower εR was associated with higher prevalence of prior heart failure and history of AF [33, 36]. Reduced all LA strains therefore may cause cardiovascular events in the future, for example early progression to HFpEF and arrythmia in adulthood even they were asymptomatic now. Hence, regular monitoring of even the asymptomatic patients with repaired TAPVC is crucial.
Limitations
First, LA measurement by 2DSTE was analyzed only in one view (obtained from apical 4 chamber), which failed to address the complexities of LA geometry and motion. Moreover, the far-field location of the atrium, reduced signal-to-noise ratio, and presence of the appendage and pulmonary vein were challenges in conducting the LA deformation analysis. Second, some of the patients may have insufficient frame rate to be analyzed by 2DSTE (typically 50 to 70 fps are thought to be sufficient for correct analysis). Third, the maximum velocity of tricuspid regurgitation is recognized as an important factor in the estimation of left atrial pressure in the adult cardiovascular field. However, there was no significant tricuspid regurgitation in most patients in this study, and the association with estimated pulmonary hypertension could not be clarified. Furthermore, regarding other surrogate markers, the analysis of these patients with respect to LA pre-components and left atrial function was limited, because these markers vary widely during neonatal and child developmental period, and their correlation with definite left atrial pressure is still unknown. Fourth, we could not observe the long-term outcome of the LA function and pressure. Further, we were not able to establish whether a reduced LA function predicts any clinical symptoms such as arrhythmia and functional capacity. Fifth, we could not remove an influence of bypass-induced injury in the patients with repaired TAPVC. However, we speculate that the preoperative parameters were based on the LA innate function. Further large scale, multi-center, prospective studies are required to validate findings of this study.
Conclusions
TAPVC patients have reduced LA function from birth lasting even repaired. Notably, instead of restoring the maximum volume of the LA, the contractile function of the LA has not developed to a normal level over time, even after surgery. Early progression to HFpEF like cardiac condition may concerned because of sustained reduction of the native LA function in the patients with repaired TAPVC.
Data availability
The data that support the findings of this study are available from the corresponding author, Dr Kiyohiro Takigiku, upon reasonable request.
Abbreviations
- TAPVC:
-
Total anomalous pulmonary venous connection
- LA:
-
Left atrial; 2DSTE, two-dimensional speckle-tracking echocardiography
- εR:
-
Reservoir function; εCD, conduit function
- εCT:
-
Contractile function
- PVO:
-
Pulmonary venous obstruction
- LV:
-
Left ventricle
- HLHS:
-
Hypoplastic left heart syndrome
- 2DE:
-
Two-dimensional echocardiography
- LVEF:
-
Left ventricle ejection fraction
- E:
-
Peak early diastolic mitral inflow velocity
- A:
-
Peak atrial filling velocity during late diastole
- IVS:
-
Inter-ventricular septum
- e’:
-
Peak early diastolic tissue Doppler velocity at the mitral annulus
- BSA:
-
Body surface area
- RT3DE:
-
Real-time three-dimensional echocardiography
- ICC:
-
Intraclass correlation coefficient
- AF:
-
Atrial fibrillation
- HFpEF:
-
Heart failure with preserved ejection fraction
- EACVI:
-
European Association of Cardiovascular Imaging
References
Karamlou T, Gurofsky R, Sukhni EA, Coles JG, Williams WG, Caldarone CA, Arsdell GSV, McCrindle BW (2007) Factors associated with mortality and reoperation in 377 children with total anomalous pulmonary venous connection. Circulation 115(12):1591–1598
Talwar S, Arora Y, Gupta SK, Kothari SS, Ramakrishnan S, Saxena A, Choudhary SK (2019) Total anomalous pulmonary venous connection beyond the first decade of life. World J Pediatr Congenit Heart Surg 10(2):185–191
McBride MG, Kirshbom PM, Gaynor JW, Ittenbach RF, Wernovsky G, Clancy RR, Flynn TB, Hartman DM, Spray TL, Tanel RE, Santiago MC, Paridon SM (2007) Late cardiopulmonary and musculoskeletal exercise performance after repair for total anomalous pulmonary venous connection during infancy. J Thorac Cardiovasc Surg 133(6):1533–1539
Paridon SM, Sullivan NM, Schneider J, Pinsky WW (1993) Cardiopulmonary performance at rest and exercise after repair of total anomalous pulmonary venous connection. Am J Cardiol 72(18):1444–1447
Rosenquist GC, Kelly JL, Chandra R, Ruckman RN, Galioto FM, Midgley FM, Scott LP (1985) Small left atrium and change in contour of the ventricular septum in total anomalous pulmonary venous connection: a morphometric analysis of 22 infant hearts. Am J Cardiol 55(6):777–782
Mathew R, Thilenius OG, Replogle RL, Arcilla RA (1977) Cardiac function in total anomalous pulmonary venous return before and after surgery. Circulation 55(2):361–370
Bălgrădean M, Cinteză E, Cîrstoveanu C, Enculescu A, Pleşca D (2013) Abnormalities in embryological development in total anomalous pulmonary venous connection. A case report. Rom J Morphol Embryol 54(3):635–637
Lima CO, Valdes-Cruz LM, Allen HD, Horowitz S, Sahn DJ, Goldberg SJ, Barron JV, Grenadier E (1983) Prognostic value of left ventricular size measured by echocardiography in infants with total anomalous pulmonary venous drainage. Am J Cardiol 51(7):1155–1159
Gobergs R, Salputra E, Lubaua I (2016) Hypoplastic left heart syndrome: a review. Acta Med Litu 23(2):86–98
Marcondes LD, Galati JC, Jones BO, Konstantinov IE, d’Udekem Y, Brizard CP, Cheung MM (2014) Abnormal left ventricular diastolic function at late follow-up after repair of total anomalous pulmonary venous drainage: the impact of altered ventricular loading in utero. J Thorac Cardiovasc Surg 148(1):238–244
Nakamura Y, Hoashi T, Nakata T, Shimada M, Ozawa H, Kurosaki K, Ichikawa H (2019) Left ventricular function after repair of totally anomalous pulmonary venous connection. Ann Thorac Surg 107(1):151–156
Sun BJ, Park J-H (2021) Echocardiographic measurement of left atrial strain- a key requirement in clinical practice. Circ J 86(1):6–13
Kutty S, Padiyath A, Li L, Peng Q, Rangamani S, Schuster A, Danford DA (2013) Functional maturation of left and right atrial systolic and diastolic performance in infants, children, and adolescents. J Am Soc Echocardiogr 26(4):398-409.e392
Ghelani SJ, Brown DW, Kuebler JD, Perrin D, Shakti D, Williams DN, Marx GR, Colan SD, Geva T, Harrild DM (2018) Left Atrial Volumes and strain in healthy children measured by three-dimensional echocardiography: normal values and maturational changes. J Am Soc Echocardiogr 31(2):187-193.e181
Cantinotti M, Scalese M, Giordano R, Franchi E, Assanta N, Molinaro S, Iervasi G, Santoro G, Koestenberger M, Kutty S (2019) Left and right atrial strain in healthy caucasian children by two-dimensional speckle-tracking echocardiography. J Am Soc Echocardiogr 32(1):165-168.e3
White BR, Ho DY, Faerber JA, Katcoff H, Glatz AC, Mascio CE, Stephens P Jr, Cohen MS (2019) Repair of total anomalous pulmonary venous connection: risk factors for postoperative obstruction. Ann Thorac Surg 108(1):122–129
Haycock GB, Schwartz GJ, Wisotsky DH (1978) Geometric method for measuring body surface area: a height-weight formula validated in infants, children, and adults. J Pediatr 93(1):62–66
Lopez L, Colan SD, Frommelt PC, Ensing GJ, Kendall K, Younoszai AK, Lai WW, Geva T (2010) Recommendations for quantification methods during the performance of a pediatric echocardiogram: a report from the pediatric measurements writing group of the American society of echocardiography pediatric and congenital heart disease council. J Am Soc Echocardiogr 23(5):465–495
Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, Flachskampf FA, Foster E, Goldstein SA, Kuznetsova T, Lancellotti P, Muraru D, Picard MH, Rietzschel ER, Rudski L, Spencer KT, Tsang W, Voigt JU (2015) Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American society of echocardiography and the European association of cardiovascular imaging. J Am Soc Echocardiogr 28(1):1-39.e14
Kanda Y (2013) Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant 48(3):452–458
Grotenhuis HB, Cifra B, Mertens LL, Riessenkampff E, Manlhiot C, Seed M, Yoo S-J, Grosse-Wortmann L (2018) Left ventricular remodelling in long-term survivors after the arterial switch operation for transposition of the great arteries. Eur Heart J Cardiovasc Imaging 20(1):101–107
Mondillo S, Cameli M, Caputo ML, Lisi M, Palmerini E, Padeletti M, Ballo P (2011) Early detection of left atrial strain abnormalities by speckle-tracking in hypertensive and diabetic patients with normal left atrial size. J Am Soc Echocardiogr 24(8):898–908
Cameli M, Lisi M, Focardi M, Reccia R, Natali BM, Sparla S, Mondillo S (2012) Left atrial deformation analysis by speckle tracking echocardiography for prediction of cardiovascular outcomes. Am J Cardiol 110(2):264–269
Smiseth OA, Baron T, Marino PN, Marwick TH, Flachskampf FA (2021) Imaging of the left atrium: pathophysiology insights and clinical utility. Eur Heart J Cardiovasc Imaging 23(1):2–13
Wakami K, Ohte N, Asada K, Fukuta H, Goto T, Mukai S, Narita H, Kimura G (2009) Correlation between left ventricular end-diastolic pressure and peak left atrial wall strain during left ventricular systole. J Am Soc Echocardiogr 22(7):847–851
Cameli M, Lisi M, Mondillo S, Padeletti M, Ballo P, Tsioulpas C, Bernazzali S, Maccherini M (2010) Left atrial longitudinal strain by speckle tracking echocardiography correlates well with left ventricular filling pressures in patients with heart failure. Cardiovasc Ultrasound 8:14
Hirose T, Kawasaki M, Tanaka R, Ono K, Watanabe T, Iwama M, Noda T, Watanabe S, Takemura G, Minatoguchi S (2012) Left atrial function assessed by speckle tracking echocardiography as a predictor of new-onset non-valvular atrial fibrillation: results from a prospective study in 580 adults. Eur Heart J Cardiovasc Imaging 13(3):243–250
Yasuda R, Murata M, Roberts R, Tokuda H, Minakata Y, Suzuki K, Tsuruta H, Kimura T, Nishiyama N, Fukumoto K, Aizawa Y, Tanimoto K, Takatsuki S, Abe T, Fukuda K (2015) Left atrial strain is a powerful predictor of atrial fibrillation recurrence after catheter ablation: study of a heterogeneous population with sinus rhythm or atrial fibrillation. Eur Heart J Cardiovasc Imaging 16(9):1008–1014
Inoue K, Khan FH, Remme EW, Ohte N, García-Izquierdo E, Chetrit M, Moñivas-Palomero V, Mingo-Santos S, Andersen ØS, Gude E, Andreassen AK, Wang TKM, Kikuchi S, Stugaard M, Ha JW, Klein AL, Nagueh SF, Smiseth OA (2021) Determinants of left atrial reservoir and pump strain and use of atrial strain for evaluation of left ventricular filling pressure. Eur Heart J Cardiovasc Imaging 23(1):61–70
Smiseth OA, Morris DA, Cardim N, Cikes M, Delgado V, Donal E, Flachskampf FA, Galderisi M, Gerber BL, Gimelli A, Klein AL, Knuuti J, Lancellotti P, Mascherbauer J, Milicic D, Seferovic P, Solomon S, Edvardsen T, Popescu BA, Committee RTdwrbmotESD (2021) Multimodality imaging in patients with heart failure and preserved ejection fraction: an expert consensus document of the European association of cardiovascular imaging. Eur Heart J Cardiovasc Imaging 23(2):e34–e61
Morris DA, Belyavskiy E, Aravind-Kumar R, Kropf M, Frydas A, Braunauer K, Marquez E, Krisper M, Lindhorst R, Osmanoglou E, Boldt LH, Blaschke F, Haverkamp W, Tschöpe C, Edelmann F, Pieske B, Pieske-Kraigher E (2018) Potential usefulness and clinical relevance of adding left atrial strain to left atrial volume index in the detection of left ventricular diastolic dysfunction. JACC Cardiovasc Imaging 11(10):1405–1415
Morris DA, Takeuchi M, Krisper M, Köhncke C, Bekfani T, Carstensen T, Hassfeld S, Dorenkamp M, Otani K, Takigiku K, Izumi C, Yuda S, Sakata K, Ohte N, Tanabe K, Osmanoglou E, Kühnle Y, Düngen HD, Nakatani S, Otsuji Y, Haverkamp W, Boldt LH (2015) Normal values and clinical relevance of left atrial myocardial function analysed by speckle-tracking echocardiography: multicentre study. Eur Heart J Cardiovasc Imaging 16(4):364–372
Santos AB, Kraigher-Krainer E, Gupta DK, Claggett B, Zile MR, Pieske B, Voors AA, Lefkowitz M, Bransford T, Shi V, Packer M, McMurray JJ, Shah AM, Solomon SD (2014) Impaired left atrial function in heart failure with preserved ejection fraction. Eur J Heart Fail 16(10):1096–1103
Sakaguchi E, Yamada A, Naruse H, Hattori H, Nishimura H, Kawai H, Muramatsu T, Ishii J, Hata T, Saito K, Izawa H (2022) Long-term prognostic value of changes in left ventricular global longitudinal strain in patients with heart failure with preserved ejection fraction. Heart Vessels. https://doi.org/10.1007/s00380-022-02211-y
van Woerden G, van Veldhuisen DJ, Gorter TM, Willems TP, van Empel VPM, Peters A, Pundziute G, den Akker op JW, Rienstra M, Westenbrink BD (2022) The clinical and prognostic value of late gadolinium enhancement imaging in heart failure with mid-range and preserved ejection fraction. Heart Vessels 37(2):273–281
Santos AB, Roca GQ, Claggett B, Sweitzer NK, Shah SJ, Anand IS, Fang JC, Zile MR, Pitt B, Solomon SD, Shah AM (2016) Prognostic relevance of left atrial dysfunction in heart failure with preserved ejection fraction. Circ Heart Fail 9(4):e002763
Acknowledgements
We thank Editage (www.editage.jp) for editing a draft of this manuscript.
Funding
The authors have no funding concerning this article.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Numata, R., Takigiku, K., Obinata, H. et al. Assessment of left atrial deformation in patients with total anomalous pulmonary venous connection by two-dimensional speckle-tracking echocardiography. Heart Vessels 38, 825–838 (2023). https://doi.org/10.1007/s00380-023-02232-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00380-023-02232-1