Abstract
Aneurysmal degeneration of a saphenous vein graft (SVG) is a rare, but potentially fatal complication of coronary artery bypass graft (CABG) surgery. In this case report, a patient that had undergone prior CABG surgery and bare metal stent (BMS) implantation at the site of a stenotic SVG lesion presented at our hospital with chest pain, and an SVG aneurysm was detected at the previous BMS implantation site. In addition, the implanted BMS was fractured and floating in the SVG aneurysm. The SVG aneurysm was successfully occluded by percutaneous intervention, using a combination of distal covered stent deployment at the site of the anastomosis between the native coronary artery and the SVG and proximal coil embolization of the aneurysm.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Aneurysmal degeneration of a saphenous vein graft (SVG) is a rare, but potentially fatal complication of coronary artery bypass graft (CABG) surgery. In this case report, a patient that had undergone prior CABG surgery and bare metal stent (BMS) implantation at the site of a stenotic SVG lesion presented at our hospital with chest pain, and an SVG aneurysm was detected at the previous BMS implantation site. In addition, the implanted BMS was fractured and floating in the SVG aneurysm. We describe a novel percutaneous approach for occluding the proximal and distal ends of SVG aneurysms; i.e., using a combination of distal covered stent deployment and proximal coil embolization.
Case report
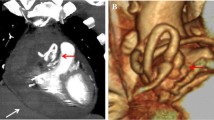
An 82-year-old female was referred to our hospital with exertional dyspnea and chest pain. She had a past medical history of CABG surgery involving a SVG to the left anterior descending artery (LAD) and a SVG to the left circumflex artery (LCX) 20 years ago. Then, 8 years ago she suffered severe stenosis of the SVG to LAD, which caused angina pectoris, and BMS (5.0 mm × 28 mm) implantation was performed (Fig. 1a–c, Movie 1). A SVG aneurysm (30 mm × 29 mm) at the previous BMS implantation site was detected by a contrast enhanced multi-slice computed tomography (MSCT) in our hospital (Fig. 2a–c). In addition, MSCT images demonstrated that the implanted BMS had fractured and was floating in the SVG aneurysm (Fig. 2c), and coronary angiography confirmed this finding (Fig. 3a). The blood flow through the SVG was sluggish and turbulent, and the native LAD was occluded at the proximal portion of the anastomosis between the LAD and SVG (Fig. 3b, Movie 2). Endoluminal exclusion of the SVG aneurysm with a covered stent and coil embolization were planned because the SVG aneurysm increased in size, and so the risk of subsequent rupturing was considered to be too high. The patient was elderly and had a low exercise capacity and multiple comorbidities, including dermatomyositis and osteoarthritis, and so was not considered a candidate for repeat open heart surgery. Thus, we planned to revascularize the occluded native LAD using a retrograde approach and to block SVG blood flow with a covered stent and coil embolization. Retrograde wiring of the occluded proximal native LAD via the SVG aneurysms was successful. After passing the antegrade wire into the occluded LAD using the reverse controlled antegrade and retrograde subintimal tracking (CART) technique, antegrade LAD blood flow was reinstituted (Fig. 3c). A covered stent (3.0 mm × 12 mm) was implanted at the site of the anastomosis between the native LAD and SVG to occlude retrograde blood flow from the native LAD into the SVG aneurysm (Fig. 3d). Three additional drug-eluting stents were implanted at the site of the occlusion in the native LAD. Furthermore, four embolic coils were inserted using a microcatheter in order to abolish antegrade blood flow into the SVG aneurysm and ensure that there was no residual flow into the SVG aneurysm (Fig. 3f). The patient was discharged and experienced an uneventful postoperative course.
a Coronary angiography (right anterior oblique view) demonstrated a stenotic lesion of saphenous vein graft to the left anterior descending artery (arrow). b Small aneurysm in a saphenous vein graft to the left anterior descending artery (arrows). c A bare metal stent was deployed at the stenotic lesion
a Contrast-enhanced computed tomographic axial image revealed a 30 × 29 mm mass in the saphenous vein graft (SVG). The mass was compressing the pulmonary valve (arrow). b Three-dimensional computed tomographic image showing the aneurysm (arrow). c Curved multi-planar reconstruction image demonstrated that the implanted bare metal stent had fractured and was floating in the SVG aneurysm (arrow)
a CAG demonstrates the SVG aneurysm occurred at the previous BMS implantation site (arrows). Graft flow is sluggish and turbulent. b The proximal portion of the native LAD is occluded (arrow). c Antegrade LAD blood flow is reinstituted using the reverse controlled antegrade and retrograde subintimal tracking technique. d, e The covered stent was implanted at the site of the anastomosis between the native LAD and SVG (arrow). Subsequent angiography found that retrograde flow into the SVG aneurysm had been abolished. f The four embolic coils were deployed in the aneurysm (arrow). Subsequent angiography showed that the proximal SVG had been occluded
Discussion
Aneurysmal dilatation of an SVG is an infrequent (<1 %), but potentially fatal complication of CABG surgery [1]. SVG aneurysms are classified into true and pseudo-aneurysms, and pseudo-aneurysms occur more frequently. True aneurysms tend to occur long after bypass surgery (more than 5 years) and to be located in the midportion of the SVG [1]. True SVG aneurysm formation is thought to involve atherosclerotic degeneration and is associated with the valve area, which is weaker than other areas due to its lack of circular smooth muscle [1–4]. On the other hand, pseudo-aneurysms involve dilatation of the graft with disruption of one or more of its wall layers, rather than the expansion of all layers of the wall. Pseudo-aneurysms are not lined by endothelial tissue and are caused by focal distension and hematoma formation. They tend to occur soon after bypass surgery (from 4 to 6 months) at the sites of leaking or infected anastomosis [1]. In this case, it was difficult to determine whether the lesion was a true or pseudo-aneurysm because no histopathological examination was performed, and the patient’s intravascular ultrasound findings did not clearly demonstrate the layers of the wall.
Several reports have described the mechanisms responsible for the fracturing of drug-eluting stents. Positive vessel remodeling with aneurysm formation and malapposition caused by biological reactions to the eluted drug can cause the stent to kink within the aneurysm, leading to stent fracture. Alternatively, stent fracturing can lead to aneurysm formation and, as a result, present late [5–7]. It was thought that the SVG aneurysm in this case might have been caused by one of these mechanisms. Coronary angiography before BMS implantation in the SVG to LAD demonstrated a small SVG aneurysm, and a BMS was deployed in that segment (Fig. 1a–c). The motion and kinking of the stent within the aneurysm might have caused it to fracture, which could have led to aneurysm formation.
The appropriate timing of surgical or percutaneous intervention for patients with a SVG aneurysm is unknown. For symptomatic patients, Sareyyupoglu et al. suggested prompt treatment to eliminate the risk of subsequent rupturing [8–11]. A more conservative, observational approach for asymptomatic patients seems to be reasonable for SVG aneurysms of less than 1 cm in diameter with brisk graft flow. However, prompt treatment is recommended for SVG aneurysms with a diameter exceeding 1 cm, as was found in the present case [8]. Traditionally, SVG aneurysms are managed with surgical excision and CABG, but the treatment strategy has recently evolved to include endoluminal exclusion with a covered stent or coil for patients such as the patient in this case. In this case, endoluminal exclusion was selected because of the patient’s general condition. Instead of high restenosis rate of covered stent, a covered stent was deployed at the site of the anastomosis between the native LAD and the SVG in order to prevent retrograde flow from the native LAD into the SVG, which might lead to SVG aneurysm rupture. As mentioned above, the rate of restenosis following placement of a covered stent is high, and that this is of concern as it’s the mid-LAD, but that at present the patient is angina free. And, endovascular coil embolization of the SVG aneurysm was performed to prevent antegrade flow from the aorto-saphenous anastomosis. We decided not to deploy an embolic coil in the distal graft because it might have shifted in the native LAD and prevented blood flow to the distal LAD.
To the best of our knowledge, this is the first report of an SVG aneurysm occurring at a previous BMS implantation site, with the subsequent finding that the implanted BMS was fractured. In addition, various percutaneous approaches to the occlusion of SVG aneurysms have been described, and this is the first case report in which distal covered stent occlusion was used in combination with proximal coil occlusion to occlude both ends of an SVG aneurysm, which eliminated the subsequent risk for rupture.
References
Doyle MT, Spizarny DL, Baker DE (1997) Saphenous vein graft aneurysm after coronary artery bypass surgery. AJR 168:747–749
Wyatt DA, Gay SB, Gimple LW, Spotnitz WD (1993) Successful preoperative diagnosis and treatment of a saphenous vein coronary artery bypass graft aneurysm. Chest 104:283–284
Tanaka A, Ishii H, Oshima H, Shibata Y, Tatami Y, Osugi N, Ota T, Kawamura Y, Suzuki S, Usui A, Murohara T (2015) Progression from stenosis to occlusion in the proximal native coronary artery after coronary artery bypass grafting. Heart Vessels. doi:10.1007/s00380-015-0715-8
Gatti G, Maschietto L, Dell’Angela L, Benussi B, Forti G, Dreas L, Soso P, Russo M, Sinagra G, Pappalardo A (2015) Predictors of immediate and long-term outcomes of coronary bypass surgery in patients with left ventricular dysfunction. Heart Vessels. doi:10.1007/s00380-015-0714-9
Doi H, Maehara A, Mintz GS, Tsujita K, Kubo T, Castellanos C, Liu J, Yang J, Oviedo C, Aoki J, Franklin-Bond T, Dasgupta N, Lansky AJ, Dangas GD, Stone GW, Moses JW, Mehran R, Leon MB (2009) Classification and potential mechanism of intravascular ultrasound patterns of stent fracture. Am J Cardiol 103:818–823
Kim U, Seol SH, Kim DI, Kim DK, Jang JS, Yang TH, Kim DK, Kim DS, Min HK, Choi KJ (2011) Clinical outcomes and the risk factors of coronary artery aneurysms that developed after drug-eluting stent implantation. Circ J 75:861–867
Akin I, Kische S, Rehders TC, Schneider H, Turan GR, Kleinfeldt T, Ortak J, Ince H, Nienaber CA (2011) Very late coronary aneurysm formation with subsequent stent thrombosis secondary to drug-eluting stent. Chin Med J 124:3427–3429
Sareyyupoglu B, Schaff HV, Ucar I, Sundt TM 3rd, Dearani JA, Park SJ (2009) Surgical treatment of saphenous vein graft aneurysms after coronary artery revascularization. Ann Thorac Surg 88:1801–1805
Riahi M, Vasu CM, Tomatis LA, Schlosser RJ, Zimmerman G (1975) Aneurysm of saphenous vein bypass graft to coronary artery. J Thorac Cardiovasc Surg 70:358–359
Dabboussi M, Saade YA, Poncet A, Baehrel B (2001) Fistula between a saphenous vein graft aneurysm and the pulmonary artery trunk. Ann Thorac Surg 71:1356–1358
Richardson MP, Thuraisingham SI, Dunning J (1992) Apparent obstruction of the superior vena cava and a continuous murmur: signs of a fistula between a vein graft aneurysm and the right atrium. Br Heart J 68:412–413
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical standards
The current paper was approved by the institutional review board of our hospital, and the person in this case report gave informed consent.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kodama, A., Kurita, T., Kato, O. et al. Impending rupture of saphenous vein graft aneurysm with floating fractured bare metal stent treated by coil embolization and covered stent implantation. Heart Vessels 31, 1882–1885 (2016). https://doi.org/10.1007/s00380-016-0799-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00380-016-0799-9