Abstract
Pulmonary arterial hypertension (PAH) is characterized by a progressive increase in pulmonary arterial pressure and vascular resistance. Despite advances in therapy for PAH, its treatment and prognosis remain poor. We aimed to investigate whether the transplantation of bone marrow mesenchymal stem cells (MSCs) overexpressing hepatocyte growth factor (HGF), alone or in combination with granulocyte colony-stimulating factor (G-CSF), attenuates the development of experimental monocrotaline (MCT)-induced PAH. Three weeks after MCT administration, rats were divided into the following groups: (1) untreated (PAH); (2) HGF treated; (3) MSCs administered; (4) HGF-MSCs treated; and (5) HGF-MSCs plus G-CSF treated. After 3 weeks, hemodynamic changes, histomorphology, and angiogenesis were evaluated. To elucidate the molecular mechanisms of vascular remodeling and angiogenesis, serum levels of transforming growth factor (TGF)-β and endothelin-1 (ET-1) were measured, and the gene and protein expression levels of vascular cell adhesion molecule-1 (VCAM-1) and matrix metalloproteinase-9 (MMP-9) were determined. Compared with the PAH, MSC, and G-CSF groups, the HGF and HGF+G-CSF groups exhibited significantly reduced right ventricular hypertrophy and mean pulmonary arterial pressure (P < 0.05). Histologically, vessel muscularization or thickening and collagen deposition were also significantly decreased (P < 0.05). The number of vessels in the HGF+G-CSF group was higher than that in the other groups (P < 0.05). The TGF-β and ET-1 concentrations in the plasma of pulmonary hypertensive rats were markedly lower in the HGF and HGF+G-CSF groups (P < 0.05). Furthermore, HGF induced the expression of VCAM-1, and HGF treatment together with G-CSF synergistically stimulated MMP-9 expression. Transplanted HGF-MSCs combined with G-CSF potentially offer synergistic therapeutic benefit for the treatment of PAH.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pulmonary arterial hypertension (PAH) is a progressive disease caused by a variety of pulmonary and/or cardiac disorders, and is characterized by a progressive increase in pulmonary arterial pressure and vascular resistance that leads to right ventricular failure. Idiopathic PAH is the most severe form, characterized by uncertain etiology and features, including pulmonary precapillary lesions [1, 2] that lead to right heart failure and death within a median of 2.8 years after diagnosis [3, 4]. At present no effective drug intervention exists, and despite significant advances in drug therapies for PAH during the last decade, the prognosis of idiopathic PAH remains poor. Vascular contraction, microvascular occlusive remodeling, inflammation, and thrombosis have been shown to contribute to the pathogenesis of PAH [5–9].
Evidence from animal and human lung specimens indicates that endothelial dysfunction may play a central role in the development of PAH [10]. Pulmonary vasoconstriction and vascular smooth muscle cell proliferation are strongly linked to endothelial cell dysfunction [11]. Several lines of evidence suggest that endothelial progenitor cells (EPCs) are involved in the process of endothelial repair [12, 13]. Moreover, these precursors may participate in postnatal neovascularization and re-endothelialization [12–16]. Granulocyte colony-stimulating factor (G-CSF), already widely used in the clinical setting, regulates the recruitment of stem cells and progenitor cells from the bone marrow into the peripheral circulation. G-CSF has been reported to enhance angiogenesis by increasing total marrow and circulating levels of EPCs immediately after G-CSF administration, lasting for at least 1 week [13]. Maruyama et al. [17] demonstrated that G-CSF prevented the progression of monocrotaline (MCT)-induced PAH in rats by promoting vascular regeneration. Hepatocyte growth factor (HGF), a pleiotropic growth factor, has been shown to have mitogenic, morphogenic, and antiapoptotic activities in various cell types [9]. The pluripotent activities of HGF are mediated by a membrane-spanning tyrosine kinase receptor encoded by the proto-oncogene c-Met [18]. c-Met has been shown to be expressed in pulmonary arterial smooth muscle cells in MCT-induced PAH rats, and a number of studies have shown that HGF improves pulmonary hemodynamic parameters and recovery from right ventricular and pulmonary remodeling in pulmonary hypertension (PH) models [19, 20]. Furthermore, HGF is involved in the adhesion of stem cells to microenvironmental components [21]. HGF has been reported to mobilize and recruit Lin-c-kit+Sca-1+CD34+ progenitor cells from the bone marrow to the injured organ [22], and we have previously shown that HGF creates an adhesive microenvironment after stem cells are recruited by G-CSF [23]. Therefore, we hypothesized that the combination of G-CSF and HGF may have a significant beneficial effect and would increase the efficacy of PH treatment. In this study, we transplanted bone marrow mesenchymal stem cells (MSCs) overexpressing HGF alone or in combination with G-CSF to determine whether either treatment can attenuate the development of experimental MCT-induced PAH in rats.
Materials and methods
Animals
All animal studies and procedures were approved by the Institutional Animal Care Committee of the Chinese PLA General Hospital, Chinese PLA Postgraduate Medical School, China (approval No. 20101201). All adult male Sprague–Dawley (SD) rats (250–300 g) and younger rats (80–100 g) were obtained from the experimental animal department of the Chinese PLA General Hospital (Beijing, China). The investigation conformed to the Guidelines for the Care and Use of Laboratory Animals, as published by the National Academy Press (NIH Publication No. 85-23, revised 1985). The number of animals needed for the study was minimized.
MCT (Sigma Chemical, St Louis, MO, USA) was peritoneally injected into the rats at a dose of 60 mg/kg. Three weeks later, the rats were randomly divided into five groups: the PAH group (n = 10); the MSC group (transplantation of 5 × 106 MSCs transfected with an empty adenovirus vector (Ad), n = 10); the G-CSF group (peritoneal injection of 100 μg/kg G-CSF daily for 5 days, n = 10); the HGF group (transplantation of 5 × 106 MSCs transfected with Ad-HGF into the jugular veins, n = 10); and the HGF+G-CSF group (transplantation of 5 × 106 MSCs transfected with Ad-HGF into the jugular veins and peritoneal injection of 100 μg/kg G-CSF daily for 5 days, n = 10). Upon euthanasia or in cases of accidental death, the rats were replaced by new experimental rats to maintain a consistent number of animals, following the original experimental design.
Preparation of mesenchymal stem cells
Bone marrow cells were aspirated from the femur and tibia of SD rats weighing 80–100 g. The cell suspension was loaded onto a 20–60 % Percoll gradient (Sigma) and centrifuged at 900 g for 30 min. The top two-thirds of the total volume was transferred into a separate tube and washed with phosphate-buffered saline (PBS; Sigma) to remove the Percoll. The cells were cultured in Dulbecco’s modified Eagle’s medium (Gibco, Carlsbad, CA, USA) containing 10 % (v/v) fetal bovine serum (Hyclone, Logan, UT, USA) and streptomycin (10,000 IU/100 ml). The cells were cultured for 24 h in an incubator maintained at 37 °C in a saturated humidified atmosphere with 5 % CO2, after which the non-adherent debris was removed and the remaining adherent cells were maintained in culture.
Flow cytometry was performed to assay for the cell surface antigens CD29, CD34, CD44, and CD45 in rat MSCs, as described previously [24, 25]. The cells were collected in a solution containing 0.25 % (w/v) trypsin and 1 mM ethylenediaminetetraacetic acid (Sigma). The cells (5 × 105) were then washed in 3 ml PBS. After centrifugation, the pellet was resuspended in 200 μl primary antibody solution for 30 min at room temperature in the dark. The cell analysis was performed with a FACSCalibur flow cytometer (Becton–Dickinson Immunocytometry Systems, San Jose, CA, USA).
MSCs were seeded on coverslips in six-well plates and cultured in complete medium to 100 % confluence. At confluence, the cells were transferred to an adipogenic medium (OriCell SD Rat Mesenchymal Stem Cell Adipogenic Differentiation Medium; Cyagen Biosciences, Sunnyvale, CA, USA), an osteogenic medium (OriCell SD Rat Mesenchymal Stem Cell Osteogenic Differentiation Medium; Cyagen), or a chondrogenic medium (OriCell SD Rat Mesenchymal Stem Cell Chondrogenic Differentiation Medium; Cyagen) to allow for the selection of adipocytes, osteocytes, and chondrocytes, respectively. The cells were further cultured according to the manufacturer’s specifications. Prior to light microscopy and imaging, the adipogenic, osteogenic, and chondrogenic cultures were stained with fresh Oil Red-O working solution, Alizarin Red working solution, and Alcian Blue working solution, respectively.
Adenoviral vectors, and gene transfection and expression
The HGF construct (Ad-HGF) was generated by the Chinese Academy of Military Medical Sciences and is a replication-deficient recombinant adenovirus vector carrying human HGF [26–28]. Passage-5 MSCs, which are undifferentiated, were infected with the recombinant replication-defective adenovirus at different multiplicities of infection (MOI) in the range of 0–200. Infection efficiency was determined by flow cytometry. An MOI of 150 was ideal for infection of MSCs with high efficiency and low toxicity [29]. Therefore, the MSCs were transfected with Ad-HGF at an MOI of 150 in all of the subsequent experiments.
Hemodynamic measurements and measurement of right ventricular hypertrophy
PAH rats were anesthetized with chloral hydrate (2.5 mg/kg; Chinese PLA General Hospital) 3 weeks after MSC transplantation. The right jugular veins were cannulated with a 2 % (v/v) heparin-saline-filled polyethylene catheter (PE50; 0.97 mm outer diameter, 0.58 mm inner diameter) connected to a pressure transducer and polygraph system (RM-6240; Instrument Plant of Chengdu, Chengdu, China) to measure hemodynamics [30]. Hemodynamics, meaning literally “blood movement,” is the study of blood flow or the circulation. It explains the physical laws that govern the flow of blood in the blood vessels. Hemodynamic parameters include blood pressure (BP), mean pulmonary arterial pressure (mPAP), aortic systolic pressure (AoSP), and right ventricular systolic pressure (RVSP). These data were recorded using a polyethylene catheter inserted into the ascending aorta via the right carotid artery. After hemodynamic measurements, the rats were euthanized. The weights of the right ventricle (RV) and left ventricle (LV) plus the septum (S) were measured to evaluate right ventricular hypertrophy. The RV/LV+S weight ratio and the RVSP/AoSP pressure ratio were considered as indices of RV hypertrophy.
Gene-expression detection
Total RNA was extracted from lung tissue using TRIzol reagent (Invitrogen, Carlsbad, CA, USA). Reverse transcription was performed using SuperScript III (Invitrogen). The polymerase chain reaction (PCR) was performed using the iCycler Thermal Cycler (Bio-Rad, Hercules, CA, USA). The primer sequences were as follows: human HGF, forward primer 5′-ATG ATG TCC ACG GAA GAG GAG A-3′, reverse primer 5′-CAC TCG TAA TAG GCC ATC ATA GTT GA-3′; GAPDH, forward primer 5′-CCA TCA CTG CCA CTC AGA AGA C-3′, reverse primer 5′-TCA TAC TTG GCA GGT TTC TCC A-3′; matrix metalloproteinase-9 (MMP-9), forward primer 5′-AAG TAT TTG TCA TGG CAG AAA TAG GC-3′, reverse primer 5′-CCA GAG CGT TAC TCG CTT GG-3′; and vascular cell adhesion molecule-1 (VCAM-1), forward primer 5′-ACA CCT CCC CCA AGA ATA CAG-3′, reverse primer 5′-GCT CAT CCT CAA CAC CCA CAG-3′.
Histologic analysis
The tissue specimens obtained from the left lung were fixed in ethanol, embedded in paraffin, and sectioned (4-μm sections). The tissue sections were stained with hematoxylin and eosin. The sections were also immunostained with antibodies against human HGF (Abcam, Cambridge, UK). The capillary density was determined as described previously [31]. Medial wall thickening was assessed as the percent medial wall thickness measured using an α-actin antibody (Santa Cruz Biotechnology, Santa Cruz, CA, USA) and Sirius Red in saturated carbazotic acid staining, as described previously [32]. A blinded observer measured all of the vessels with perceptible media (>35 muscular arteries/rat) under ×200 magnification. To evaluate the pulmonary arterial lumen, the internal and external areas of nine to ten pulmonary arteries (100–150 μm external diameter) in each rat were measured using a Microanalyzer (BX-45; Olympus, Tokyo, Japan). The percentage of the internal area of the pulmonary artery (vascular lumen ratio) was calculated using the following formula: vascular lumen ratio (%) = internal area/whole (external) area of pulmonary artery × 100.
Enzyme immunoassays
We measured the levels of transforming growth factor-β (TGF-β) and endothelin-1 (ET-1) in blood serum from pulmonary hypertensive rats 3 weeks after MSC transplantation. Serum was purified from whole blood samples by centrifugation and added to a 96-well microplate for enzyme-linked immunosorbent assay analysis using commercial kits for TGF-β (R&D Systems, Minneapolis, MN, USA) and ET-1 (Cayman Chemical, Ann Arbor, MI, USA).
Western blot detection for MMP-9 and VCAM-1 expression in injured lungs
Small lung specimens for Western blotting were carefully dissected from the right lung and snap-frozen in liquid nitrogen. Total protein was extracted from the samples using RIPA Lysis Buffer (Applygen Gene Technology, Beijing, China), and total protein concentration was quantified using the bicinchoninic acid method. Immunoblotting was performed using antibodies against MMP-9 (1:500; Santa Cruz Biotechnology) and VCAM-1 (1:500; Santa Cruz Biotechnology). Detection was performed using an ECL kit (Applygen Gene Technology), and quantification was performed using the AlphaEaseFC Image program (Alpha Innotech, San Leandro, CA, USA) in a blinded fashion.
Statistical analysis
All data are expressed as the mean ± standard error of the mean. SPSS 16.0 software was used for all analyses. Values of P < 0.05 were considered statistically significant. The significant differences among the five groups were evaluated by one-way analysis of variance followed by Fisher’s protected least significant difference test.
Results
Characterization of bone marrow MSCs
Bone marrow MSCs were imaged using light (Fig. 1a) and fluorescence microscopy (Fig. 1b). Flow cytometry data demonstrated that these cells were positive for CD29 and CD44 but negative for CD34 and CD45 (Fig. 1c). Bone marrow MSCs were differentiated in vitro using adipogenic, osteogenic, and chondrogenic induction media (Fig. 1d). In terms of morphology, marker expression, and cell differentiation capacity, the cells used in this study were classified as bone marrow MSCs.
The characterization of bone marrow mesenchymal stem cells (MSCs). The same field viewed under a an inverted microscope and b a fluorescence microscope (×400). c Phenotypic characterization of MSCs. MSCs were positive for CD29 and CD44 but negative for CD34 and CD45. d Adipogenic induction, osteogenic induction, and chondrogenic induction of rat bone marrow MSCs
Engraftment of MSCs into the lung and the expression of exogenous human HGF in vivo
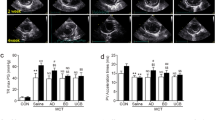
Three days after transfection, PCR revealed significant pulmonary expression of the human HGF gene in the HGF and HGF+G-CSF groups (Fig. 2a). Human HGF gene expression was observed in only the HGF and HGF+G-CSF groups. Likewise, 3 days after transfection, immunohistochemical examination using an antihuman HGF polyclonal antibody revealed the expression of human HGF in the HGF and HGF+G-CSF groups (Fig. 2b). To determine whether MSCs engrafted in the lung expressed HGF, we examined vascular endothelial cells by immunohistochemistry to identify green fluorescent protein (GFP)-positive MSCs 3 days after the transplantation of MSCs transfected with Ad-GFP. Indeed, GFP-positive MSCs (green fluorescence) were observed in the endothelial cell zone of the lung (Fig. 2c).
The expression of exogenous human hepatocyte growth factor (HGF) in vivo. a Electrophoresis results of human HGF transfection. Gene expression in the HGF-only group (a) and the HGF + granulocyte colony-stimulating factor (G-CSF) group (b). The G-CSF group (c), the mesenchymal stem cells (MSC) group (d), and the pulmonary arterial hypertension (PAH) group (e) all show no expression of the human HGF gene. GAPDH glyceraldehyde 3-phosphate dehydrogenase. b Representative photomicrographs of immunohistochemical staining for HGF at 3 days after HGF-MSC administration (×200). c Representative photomicrographs of immunofluorescence staining for green fluorescent protein at 3 days after adenovirus vector-GFP administration. GFP-positive MSCs (green fluorescence) were observed in the endothelial cell zone of the lung. Nuclei were stained with diamidino-2-phenylindole (blue fluorescence; arrow) (×400)
Improvement in the hemodynamic parameters
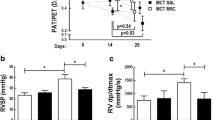
To determine the effect of HGF and G-CSF on the hemodynamic parameters, rats with PAH were catheterized, and systemic BP and mPAP were investigated after 3 weeks of treatment (Fig. 3). The HGF and HGF+G-CSF groups had lower PASP and mPAP values than the other groups (P < 0.05), particularly the HGF+G-CSF group, which had the lowest PASP values.
The hemodynamic parameters of the pulmonary arterial hypertension (PAH), bone marrow mesenchymal stem cells (MSC), granulocyte colony-stimulating factor (G-CSF), hepatocyte growth factor (HGF), and HGF+G-CSF groups, including blood pressure (BP), mean pulmonary arterial pressure (mPAP), and right ventricular systolic pressure (RVSP)/aortic systolic pressure (AoSP). There were no differences in BP among the PAH, MSC, G-CSF, HGF and HGF+G-CSF groups (P > 0.05). There were no differences in mPAP among the PAH, MSC, and G-CSF groups (P > 0.05). Compared with the PAH group, the MSC, G-CSF, HGF, and HGF+G-CSF groups had lower mPAP (P < 0.05). No significant difference was observed in mPAP between the HGF group and the HGF+G-CSF group (P > 0.05). Compared with the PAH group, the MSC group, and the G-CSF group, the HGF+G-CSF group had a significantly lower pressure ratio of RVSP/AoSP (P < 0.05). There were no differences in pressure ratio among the PAH, MSC, and G-CSF groups. *P < 0.05 vs PAH group or MSC group or G-CSF group; ^P < 0.05 vs HGF+G-CSF group
Assessment of right ventricular hypertrophy
Three weeks after MCT administration, transplantation, and/or treatment, right ventricular hypertrophy was assessed by the RVSP/AoSP pressure ratio (Fig. 3) and the RV/LV+S weight ratio (Fig. 4). The results showed that the MSC group, the G-CSF group, and the HGF+G-CSF group all had significantly less right ventricular hypertrophy than the PAH group (P < 0.05).
The assessment of right ventricular hypertrophy. a Images of hearts showing thickening of the right ventricle in different groups. b Weight ratio of right ventricle/left ventricle + septum (RV/LV+S). Compared with the PAH group, the MSC group and the G-CSF group, the HGF+G-CSF group had significantly less right ventricular hypertrophy (P < 0.05). There were no differences in the weight ratios among the PAH, MSC, and G-CSF groups. Four-pointed star indicates the right ventricle. *P < 0.05 vs the PAH, MSC, or G-CSF group; ^P < 0.05 vs the HGF+G-CSF group
Inhibition of pulmonary arteriole remodeling
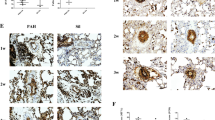
We evaluated the effects of HGF and G-CSF on MCT-induced PAH in rats using histologic analyses (Fig. 5a). In the HGF+G-CSF group, vessel muscularization or thickening and collagen deposition were decreased. Moreover, there was an increase in the vascular lumen ratio and a marked decrease in the medial wall thickness (Fig. 5b). In addition, light microscopy revealed an obvious vascular occlusion and inflammatory response, particularly in the PAH group.
Inhibitory effects on pulmonary arteriole remodeling. a Representative photomicrograph of hematoxylin–eosin staining (1), α-actin antibody staining (2), and Sirius Red in saturated carbazotic acid staining (3) in rats 3 weeks after treatment. Vascular occlusion and the inflammatory response were also obvious by microscopy. The PAH, MSC, and G-CSF groups (a) showed similar morphologic features; b HGF group; c HGF+G-CSF group. b Representative wall thickness of the pulmonary artery. In the PAH, MSC, and G-CSF groups, vessel muscularization and thickening, as well as collagen accumulation leading to vascular medial thickness and marked pulmonary arterial stenosis, were observed. However, the vascular medial thickness and collagen deposition was attenuated, and the vascular lumen ratio was significantly decreased in the HGF+G-CSF group (PAH group = 32.95 % ± 4.96 %; MSC group = 33.54 % ± 3.90 %; G-CSF group = 31.84 % ± 2.81 %; HGF group = 29.77 % ± 4.97 %; HGF+G-CSF group = 25.69 % ± 5.14 %). *P < 0.05 vs the PAH group, MSC group, and G-CSF group; ^P < 0.05 vs the HGF+G-CSF group
Changes in serum TGF-β and ET-1 concentrations
The TGF-β concentrations in the plasma of pulmonary hypertensive rats were markedly lower in the HGF (2513.32 ± 1255.156 ng/ml) and HGF+G-CSF (1869.94 ± 1317.297 ng/ml) groups than in the other groups (PAH group = 3347.31 ± 1873.32 ng/ml; MSC group = 3674.31 ± 1690.80 ng/ml; G-CSF group = 4410.79 ± 1375.43 ng/ml). There was no statistically significant difference between the HGF and HGF+G-CSF groups; however, there was a decrease in the HGF+G-CSF group (Fig. 6a). The concentration of ET-1 levels in the plasma of pulmonary hypertensive rats was significantly lower in the HGF+G-CSF group (15.16 ± 6.52 pg/ml) than in the PAH (28.37 ± 4.40 pg/ml), MSC (31.65 ± 7.74 pg/ml), G-CSF (40.42 ± 19.12 pg/ml), and HGF (25.07 ± 6.77 pg/ml) groups (Fig. 6b).
Angiogenesis promotion
To evaluate the effect of HGF on endothelial cells, we calculated the number of blood vessels observed following immunohistochemical staining with the α-actin antibody and Sirius Red in saturated carbazotic acid (Fig. 7a). We found a significant increase in the capillary density of the lung in the HGF+G-CSF group (P < 0.05), which was approximately 1.2-fold greater than that in the HGF group and 1.6-fold greater than that in the control group (Fig. 7b).
Representative vascular densities. a Representative photomicrograph of α-actin antibody staining (1) and Sirius Red in saturated carbazotic acid staining (2) in rats 3 weeks after treatment. a The PAH, MSC, and G-CSF groups exhibit similar morphologic features; b HGF group; c HGF+G-CSF group. b The number of vessels of the HGF+G-CSF group was higher than that in the other groups (P < 0.05). The number of vessels in each group was as follows: HGF+G-CSF group = 4.97 ± 1.94 per mm2, HGF group = 4.06 ± 1.9 per mm2, PAH group = 2.88 ± 1.89 per mm2, MSC group = 2.78 ± 0.53 per mm2, and G-CSF group = 3.31 ± 0.81 per mm2. There was no significant difference in density between the PAH and MSC group or between the G-CSF and HGF group. *P < 0.05 vs the PAH group, MSC group, G-CSF group, and HGF group
MMP-9 and VCAM-1 expression in lung tissue
To examine the mechanisms underlying the synergistic effect of HGF and G-CSF, we investigated gene and protein expression in the lung (Fig. 8). VCAM-1 expression was significantly induced by HGF+G-CSF (P < 0.05). There was no difference in the expression of VCAM-1 among the PAH, MSC, G-CSF, and HGF groups (P > 0.05). Both the HGF group and the HGF+G-CSF group showed increased MMP-9 expression in the lung compared with the other three groups. However, there was no difference in MMP-9 expression between the HGF group and the HGF+G-CSF group (P > 0.05).
Matrix metalloproteinase-9 (MMP-9) and vascular cell adhesion molecule-1 (VCAM-1) expression in lung tissue. A represents the gene expression of MMP-9 and VCAM-1 using electrophoresis; B and C represent the relative gene expression of MMP-9 and VCAM-1, respectively. a represents the protein expression of MMP-9 and VCAM-1 using Western blotting; b and c represent the relative protein expression of MMP-9 and VCAM-1, respectively. *P < 0.05 vs the PAH group, MSC group, and G-CSF group; ^P < 0.05 vs the HGF+G-CSF group
Discussion
PAH is characterized by the deregulated proliferation of pulmonary arterial endothelial and intimal smooth muscle cells, resulting in progressive pulmonary vascular remodeling and an increase in pulmonary arterial pressure. At present, the treatment for PH is limited and has poor efficacy. Although the subcutaneous injection of G-CSF mobilized CD34+ cells from the bone marrow to the peripheral circulation, bone marrow stimulation with G-CSF alone is unlikely to yield sufficient pulmonary-specific targeting of EPCs [23]. HGF is a mediator of stem cell homing to the bone marrow, and has been shown to induce the recruitment of EPCs in an ischemic heart model and a valvular heart disease model [33, 34]. However, there is little information available in the literature regarding the combination of G-CSF and HGF in the treatment of PH. In this study, we investigated the physiologic and therapeutic value of HGF and G-CSF combination therapy. Our study demonstrated that the expression of HGF following the transplantation of genetically modified MSCs, combined with the subcutaneous injection of G-CSF, ameliorates pulmonary hemodynamics in MCT-induced PAH rats by attenuating adverse pulmonary vascular remodeling and enhancing neovascularization.
In this study, we found that implanted MSCs could be incorporated into the lung and express human HGF, which suggests that MSC vectors could be used to maintain HGF at relatively high levels. The combination of G-CSF and HGF had a significant effect. Our results indicate that both hemodynamics and right ventricular hypertrophy were better preserved in the HGF+G-CSF group than in any of the other groups studied (Figs. 3 and 4). The histologic improvements following the addition of G-CSF may be attributed to the ability of G-CSF to stimulate and mobilize stem cells [35, 36].
Furthermore, our findings demonstrate that the mechanisms responsible for the beneficial effects of HGF+G-CSF included improved effects on pulmonary arteriole remodeling and angiogenesis. EPCs play an important role in endothelial repair and thus inhibit pulmonary vascular remodeling caused by endothelial dysfunction [10, 12, 14, 37, 38]. Laboratory evidence suggests that these precursors participate in postnatal neovascularization and re-endothelialization [12–16]. Xia et al. [39] reported that EPCs from PAH beagles were sluggish and senescent, and that the reduction and functional impairment of EPCs played a critical role in the onset of PAH. G-CSF has been shown to mobilize EPCs to the peripheral circulation, whereas HGF recruits EPCs to the injured organ [39]. In the present study, we found that the combination of G-CSF and HGF maintained endothelial homeostasis and inhibited pulmonary arteriole remodeling. In the combined-therapy group, vascular medial thickness and inflammatory cell infiltration were attenuated, and the vascular lumen ratio was significantly increased (Figs. 4 and 5).
Our study showed that the overexpression of HGF, together with G-CSF treatment, inhibited TGF-β expression in the pulmonary hypertensive lung (Fig. 6a). TGF-β is well established as a critical molecule involved in cell proliferation, vascular remodeling, and lung fibrosis. The inhibition of TGF-β expression may be another mechanism that prevents pulmonary vascular remodeling. In fact, histologic analysis of the pulmonary arteries in the HGF+G-CSF group showed better suppression of vessel muscularization or thickening and collagen deposition in the pulmonary arteries than did any of the other groups. These data indicate that combination therapy with HGF and G-CSF induces a hemodynamic effect via the inhibition of pulmonary arteriole remodeling.
Endothelial dysfunction could result in an imbalance between vasodilators and vasoconstrictors, and this imbalance is crucial in the progression of PAH [12]. ET-1, a vasoconstrictor produced by endothelial cells, plays an essential role in the pathogenesis of PAH by enhancing vasoconstriction and hypertension. Haug et al. [40] showed that HGF inhibited ET-1 release in cultured human coronary artery endothelial cells. In our study, we found that HGF inhibited ET-1 expression in the serum and that combination therapy with HGF and G-CSF could further decrease ET-1 expression. The suppression of ET-1 may contribute, in part, to the inhibition of PH remodeling (Fig. 6b).
Furthermore, our findings demonstrate that the mechanisms responsible for the beneficial effects of HGF+G-CSF are involved in angiogenesis. In advanced PH, decreased pulmonary blood flow becomes evident and leads to lung hypoxia. Under ischemic conditions, parenchymal destruction is further aggravated, and this damage is associated with the expansion of interstitial fibrotic spaces [1–4]. Therefore, strategies to increase pulmonary blood beds should be considered for stopping these pathologic cycles. We have established successful techniques for inducing angiogenesis in lung tissue using HGF and G-CSF combination therapy. In the present study, the number of lung vessels was significantly increased after HGF and G-CSF combination therapy, concomitant with the enhanced proliferation of endothelial cells (Fig. 7). Thus, HGF-mediated angiogenesis in PH could be responsible for not only a decline in peripheral blood pressure but also improved hypoxia.
Integrin α4β1 (CD49d/CD29) is a receptor for VCAM-1. In stem cells, HGF has been reported to induce VCAM-1 expression and promote the coexpression of CD49d and CD34, which have been shown to be mobilized by G-CSF in an ischemic hind limb model [41]. Consistent with these data, we found that VCAM-1 expression was significantly induced by HGF in the lungs of PH rats (Fig. 8). These findings suggest that one mechanism accounting for this enhanced expression is that the circulating CD49d+CD34+ cells mobilized by G-CSF adhere to VCAM-1, which is induced by the local expression of HGF in the pulmonary hypertensive lung.
MMPs, a family of Zn2+ proteases, are able to degrade a wide spectrum of extracellular matrix proteins. Among these proteases, MMP-9 (gelatinase B) plays a specific role in angiogenesis [42]. MMP-9 has been shown to stimulate angiogenesis, not only by degrading the endothelial basement membrane and the interstitial matrix that enhances endothelial cell migration and that promotes the ability of endothelial cells to form tubes on Matrigel [43], but also by promoting the mobilization and recruitment of EPCs [44]. Our results demonstrated that G-CSF treatment significantly enhanced the migration of bone marrow cells into the lungs of pulmonary hypertensive rats and increased MMP-9 expression (Fig. 8). These findings suggest that G-CSF not only recruits bone marrow cells into the peripheral blood but also affects their migration and phenotype in the lung. Our study also showed that HGF overexpression by itself did not enhance MMP-9 expression. However, the overexpression of HGF together with G-CSF treatment stimulated MMP-9 expression in the lung. The enhanced MMP-9 expression stimulates angiogenesis by promoting endothelial cell migration through the degradation of the endothelial basement membrane and interstitial matrix. In fact, the G-CSF+HGF group had higher vascular densities than any of the other groups studied.
In summary, HGF-MSC transplantation combined with G-CSF treatment is able to offer a therapeutic benefit. Future basic experimental studies including a larger sample size and a different PAH model will be needed to define the utility of this combination therapy.
References
Runo JR, Loyd JE (2003) Primary pulmonary hypertension. Lancet 361(9368):1533–1544
Rubin LJ (1997) Primary pulmonary hypertension. N Engl J Med 336(2):111–117
D’Alonzo GE, Barst RJ, Ayres SM, Bergofsky EH, Brundage BH, Detre KM, Fishman AP, Goldring RM, Groves BM, Kernis JT, Levy PS, Pietra GG, Reid LM, Reeves JT, Rich S, Vreim CE, Williams GW, Wu M (1991) Survival in patients with primary pulmonary hypertension. Results from a national prospective registry. Ann Intern Med 115(5):343–349
Fuster V, Steele PM, Edwards WD, Gersh BJ, McGoon MD, Frye RL (1984) Primary pulmonary hypertension: natural history and the importance of thrombosis. Circulation 70(4):580–587
Cowan KN, Heilbut A, Humpl T, Lam C, Ito S, Rabinovitch M (2000) Complete reversal of fatal pulmonary hypertension in rats by a serine elastase inhibitor. Nat Med 6(6):698–702
Budhiraja R, Tuder RM, Hassoun PM (2004) Endothelial dysfunction in pulmonary hypertension. Circulation 109(2):159–165
Morse JH, Deng Z, Knowles JA (2001) Genetic aspects of pulmonary arterial hypertension. Ann Med 33(9):596–603
Voelkel NF, Cool C (2004) Pathology of pulmonary hypertension. Cardiol Clin 22(3):343–351 v
Taraseviciene-Stewart L, Kasahara Y, Alger L, Hirth P, McMahon G, Waltenberger J, Voelkel NF, Tuder RM (2001) Inhibition of the VEGF receptor 2 combined with chronic hypoxia causes cell death-dependent pulmonary endothelial cell proliferation and severe pulmonary hypertension. FASEB J 15(2):427–438
Tuder RM, Cool CD, Yeager M, Taraseviciene-Stewart L, Bull TM, Voelkel NF (2001) The pathobiology of pulmonary hypertension. Endothelium. Clin Chest Med 22(3):405–418
Perros F, Dorfmuller P, Humbert M (2005) Current insights on the pathogenesis of pulmonary arterial hypertension. Semin Resp Crit Care Med 26(4):355–364
Hill JM, Zalos G, Halcox JP, Schenke WH, Waclawiw MA, Quyyumi AA, Finkel T (2003) Circulating endothelial progenitor cells, vascular function, and cardiovascular risk. N Engl J Med 348(7):593–600
Walter DH, Rittig K, Bahlmann FH, Kirchmair R, Silver M, Murayama T, Nishimura H, Losordo DW, Asahara T, Isner JM (2002) Statin therapy accelerates reendothelialization: a novel effect involving mobilization and incorporation of bone marrow-derived endothelial progenitor cells. Circulation 105(25):3017–3024
Takahashi T, Kalka C, Masuda H, Chen D, Silver M, Kearney M, Magner M, Isner JM, Asahara T (1999) Ischemia- and cytokine-induced mobilization of bone marrow-derived endothelial progenitor cells for neovascularization. Nat Med 5(4):434–438
Nagaya N, Kangawa K, Kanda M, Uematsu M, Horio T, Fukuyama N, Hino J, Harada-Shiba M, Okumura H, Tabata Y, Mochizuki N, Chiba Y, Nishioka K, Miyatake K, Asahara T, Hara H, Mori H (2003) Hybrid cell-gene therapy for pulmonary hypertension based on phagocytosing action of endothelial progenitor cells. Circulation 108(7):889–895
Zhao YD, Courtman DW, Deng Y, Kugathasan L, Zhang Q, Stewart DJ (2005) Rescue of monocrotaline-induced pulmonary arterial hypertension using bone marrow-derived endothelial-like progenitor cells: efficacy of combined cell and eNOS gene therapy in established disease. Circ Res 96(4):442–450
Maruyama H, Watanabe S, Kimura T, Liang J, Nagasawa T, Onodera M, Aonuma K, Yamaguchi I (2007) Granulocyte colony-stimulating factor prevents progression of monocrotaline-induced pulmonary arterial hypertension in rats. Circ J 71(1):138–143
Bottaro DP, Rubin JS, Faletto DL, Chan AM, Kmiecik TE, Vande Woude GF, Aaronson SA (1991) Identification of the hepatocyte growth factor receptor as the c-Met proto-oncogene product. Science 251(4995):802–804
Ono M, Sawa Y, Mizuno S, Fukushima N, Ichikawa H, Bessho K, Nakamura T, Matsuda H (2004) Hepatocyte growth factor suppresses vascular medial hyperplasia and matrix accumulation in advanced pulmonary hypertension of rats. Circulation 110(18):2896–2902
Hiramine K, Sata N, Ido A, Kamimura R, Setoyama K, Arai K, Nuruki N, Tanaka Y, Uto H, Tsubouchi H (2011) Hepatocyte growth factor improves the survival of rats with pulmonary arterial hypertension via the amelioration of pulmonary hemodynamics. Int J Mol Med 27(4):497–502
Weimar IS, Miranda N, Muller EJ, Hekman A, Kerst JM, de Gast GC, Gerritsen WR (1998) Hepatocyte growth factor/scatter factor (HGF/SF) is produced by human bone marrow stromal cells and promotes proliferation, adhesion and survival of human hematopoietic progenitor cells (CD34+). Exp Hematol 26(9):885–894
Tajima F, Tsuchiya H, Nishikawa K, Kataoka M, Hisatome I, Shiota G (2010) Hepatocyte growth factor mobilizes and recruits hematopoietic progenitor cells into liver through a stem cell factor-mediated mechanism. Hepatol Res 40(7):711–719
Guo YH, He JG, Wu JL, Yang L, Zhang DS, Tan XY, Qi RD (2008) Hepatocyte growth factor and granulocyte colony-stimulating factor form a combined neovasculogenic therapy for ischemic cardiomyopathy. Cytotherapy 10(8):857–867
Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR (1999) Multilineage potential of adult human mesenchymal stem cells. Science 284(5411):143–147
Conget PA, Minguell JJ (1999) Phenotypical and functional properties of human bone marrow mesenchymal progenitor cells. J Cell Physiol 181(1):67–73
Duan HF, Wu CT, Wu DL, Lu Y, Liu HJ, Ha XQ, Zhang QW, Wang H, Jia XX, Wang LS (2003) Treatment of myocardial ischemia with bone marrow-derived mesenchymal stem cells overexpressing hepatocyte growth factor. Mol Ther 8(3):467–474
Zhang YR, Wu DL, Lao MF, Bi JJ, Wu CT (2003) The comparative efficacy of recombinant adenovirus and plasmid in treatment of myocardial ischemia. Bull Acad Military Med Sci 27:16–18
Lu ZZ, Ni F, Hu ZB, Wang L, Wang H, Zhang QW, Huang WR, Wu CT, Wang LS (2006) Efficient gene transfer into hematopoietic cells by a retargeting adenoviral vector system with a chimeric fiber of adenovirus serotype 5 and 11p. Exp Hematol 34(9):1171–1182
Guo Y, He J, Wu J, Yang L, Dai S, Tan X, Liang L (2008) Locally overexpressing hepatocyte growth factor prevents post-ischemic heart failure by inhibition of apoptosis via calcineurin-mediated pathway and angiogenesis. Arch Med Res 39(2):179–188
Tan XY, He JG (2009) The remodeling of connexin in the hypertrophied right ventricular in pulmonary arterial hypertension and the effect of a dual ET receptor antagonist (bosentan). Pathol Res Pract 205(7):473–482
Ono M, Sawa Y, Matsumoto K, Nakamura T, Kaneda Y, Matsuda H (2002) In vivo gene transfection with hepatocyte growth factor via the pulmonary artery induces angiogenesis in the rat lung. Circulation 106(12 Suppl 1):I264–I269
O’Blenes SB, Fischer S, McIntyre B, Keshavjee S, Rabinovitch M (2001) Hemodynamic unloading leads to regression of pulmonary vascular disease in rats. J Thorac Cardiovasc Surg 121(2):279–289
Urbich C, Dimmeler S (2004) Endothelial progenitor cells: characterization and role in vascular biology. Circ Res 95(4):343–353
Huang SD, Liu XH, Bai CG, Lu FL, Yuan Y, Gong DJ, Xu ZY (2007) Synergistic effect of fibronectin and hepatocyte growth factor on stable cell-matrix adhesion, re-endothelialization, and reconstitution in developing tissue-engineered heart valves. Heart Vessels 22(2):116–122
Kmiecik TE, Keller JR, Rosen E, Vande Woude GF (1992) Hepatocyte growth factor is a synergistic factor for the growth of hematopoietic progenitor cells. Blood 80(10):2454–2457
Jalili A, Shirvaikar N, Marquez-Curtis LA, Turner AR, Janowska-Wieczorek A (2010) The HGF/c-Met axis synergizes with G-CSF in the mobilization of hematopoietic stem/progenitor cells. Stem Cells Dev 19(8):1143–1151
Rafii S (2000) Circulating endothelial precursors: mystery, reality, and promise. J Clin Invest 105(1):17–19
Szmitko PE, Fedak PW, Weisel RD, Stewart DJ, Kutryk MJ, Verma S (2003) Endothelial progenitor cells: new hope for a broken heart. Circulation 107(24):3093–3100
Xia L, Zhu JH, Qiu FY, Yang Y, Xie XD, Wang XX, Chen JZ, Fu GS (2009) Senescent endothelial progenitor cells from dogs with pulmonary arterial hypertension: a before-after self-controlled study. J Physiol Sci 59(6):429–437
Haug C, Schmid-Kotsas A, Zorn U, Bachem MG, Schuett S, Gruenert A, Rozdzinski E (2000) Hepatocyte growth factor is upregulated by low-density lipoproteins and inhibits endothelin-1 release. Am J Physiol-Heart C 279(6):H2865–H2871
Jayasankar V, Woo YJ, Bish LT, Pirolli TJ, Chatterjee S, Berry MF, Burdick J, Gardner TJ, Sweeney HL (2003) Gene transfer of hepatocyte growth factor attenuates postinfarction heart failure. Circulation 108(Suppl 1):II230–II236
Van den Steen PE, Dubois B, Nelissen I, Rudd PM, Dwek RA, Opdenakker G (2002) Biochemistry and molecular biology of gelatinase B or matrix metalloproteinase-9 (MMP-9). Crit Rev Biochem Mol 37(6):375–536
Pepper MS (2001) Role of the matrix metalloproteinase and plasminogen activator-plasmin systems in angiogenesis. Arterioscler Thromb Vasc Biol 21(7):1104–1117
Renault MA, Losordo DW (2007) The matrix revolutions: matrix metalloproteinase, vasculogenesis, and ischemic tissue repair. Circ Res 100(6):749–750
Acknowledgments
This study was supported by the National Natural Science Foundation of China (No. 81000018), the Major Program of the Chinese PLA General Hospital Nursery funds (No. 10KMZ04), the opening foundation of the State Key Laboratory of Space Medicine Fundamentals and Application, Chinese Astronaut Research and Training Center (No. SMFA11K02), a Special Financial Grant from the China Postdoctoral Science Foundation (Grant No. 201104776), and the Beijing Nova Program (No. Z12111000250000).
Conflict of interest
The authors declare that they have no competing interests.
Author information
Authors and Affiliations
Corresponding author
Additional information
Yinghua Guo, Longxiang Su, and Yinghui Li contributed equally to this work.
Rights and permissions
About this article
Cite this article
Guo, Y., Su, L., Li, Y. et al. The synergistic therapeutic effect of hepatocyte growth factor and granulocyte colony-stimulating factor on pulmonary hypertension in rats. Heart Vessels 29, 520–531 (2014). https://doi.org/10.1007/s00380-013-0395-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00380-013-0395-1