Abstract
Organic materials with low C/N ratio, such as animal manure and compost, have been largely applied to orchard soil to maintain soil organic matter and improve soil fertility. However, little is known about the decomposition characteristics and nitrogen (N) mineralization of added organic materials. Thus, a laboratory incubation study using 15N tracing technique was carried out to investigate the effects of organic materials with low C/N ratio (rapeseed meal and chicken manure) on gross N transformations in a vineyard soil. Our result showed that carbon (C) mineralization of organic material depended on C/N ratio and lignin/N ratio of organic material, while N mineralization was associated with substrate N concentration. The application of organic material with low C/N ratio increased gross N mineralization, NH4 + immobilization, autotrophic nitrification rates, and CO2, N2O, and NO emissions. Heterotrophic nitrification and NO3 − immobilization did not occur, irrespective of organic material amendments. Organic material amendments increased more total inorganic N production (mineralization + heterotrophic nitrification) than total inorganic N consumption (immobilization of NH4 + and NO3 −), leading to increasing net N mineralization rates. In addition, NO3 − consumption (NO3 − immobilization + dissimilatory NO3 − reduction to NH4 +) increased to a lesser extent than NO3 − production (heterotrophic + autotrophic nitrification) following organic material amendments, leading to more rapid accumulation of NO3 − in soils. Our results suggest that organic material with a low C/N ratio can provide readily available N as N fertilizers but accompanied by enhanced risk of N losses through gaseous N emissions and possibly NO3 − leaching and runoff.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The Taihu Lake region is one of the most intensive agricultural regions and the most economically developed areas in China. This region is characterized by large inputs of synthetic N fertilizer (Zhu and Chen 2002; Ju et al. 2009). As a result, the excessive input of synthetic N fertilizer induced environmental problems, such as eutrophication of surface waters (non-point source pollution), nitrate pollution of groundwater, acid rain and soil acidification, and greenhouse gas emissions (Guo et al. 2004; Jin et al. 2005). Traditionally, a rice–wheat rotation is the main cropping system adopted in this region (Ju et al. 2009). However, the region is experiencing the rapid conversion of rice–wheat rotation to orchard, which is predicted to increase in future due to the high economic benefit of fruits (Cheng et al. 2014). In contrast to rice–wheat rotation, which relies on inorganic fertilizers, the application of organic fertilizers is most commonly used in orchard systems (Ji et al. 2008; Ju et al. 2009; Cheng et al. 2014). However, the effects of organic fertilizers addition on soil N dynamics and N losses are largely unknown in these orchard systems.
For acid soil, organic fertilizer amendments can increase soil pH by providing ash alkalinity and enhancing mineralization of organic N (de Boer et al. 1988; Cai et al. 2015). Increasing pH has been demonstrated to stimulate autotrophic nitrification (Ste-Marie and Pare 1999; Cheng et al. 2013). Thus, organic fertilizer amendments would be expected to promote the conversion of NH4 + to NO3 − through autotrophic nitrification. However, increasing production of NO3 − is not always associated with rapid accumulation of NO3 − and increasing risk of NO3 − losses, as NO3 − consumption processes (e.g., NO3 − immobilization) may completely or partly counteract the production of NO3 − (Stark and Hart 1997). Numerous studies have shown that microbial NO3 − immobilization was non-existent in agricultural soils (Jansson et al. 1955; Rice and Tiedje 1989; Recous et al 1990; Shi and Norton 2000; Shi et al. 2004; Zhang et al. 2013), whereas it did occur in forest and grassland soils as well as in the organic farm systems under high C availability (Davidson et al. 1990; Bradley 2001; Burger and Jackson 2003). It has been suggested that available C is likely an important factor controlling microbial NO3 − immobilization (Shi and Norton 2000; Shi et al. 2004). Therefore, organic material applications may enhance NO3 − immobilization in agricultural soils by providing C source and increasing soil microbial biomass and activity. However, net rates calculated by the change of soil NO3 − concentrations over time cannot differentiate if both productive and consumptive processes are taking place simultaneously, which makes it unsuitable to understand the actual N cycles in soils. Alternatively, measuring gross N transformation rates using 15N isotope techniques has been demonstrated as useful for quantifying individual microbial N processes (Stark and Hart 1997; Nannipieri and Paul 2009; Li and Lang 2014).
The decomposition rate and N release from organic fertilizer are largely determined by the C/N ratio of organic fertilizer. C/N ratio of organic fertilizer has been demonstrated effectively to indicate net N mineralization and immobilization, with the threshold value of approximately 20-40 (Whitmore 1996; Van Kessel et al. 2000). However, net N mineralization was calculated as the difference between gross N mineralization and immobilization. Thus, the factor controlling gross and net N mineralization from organic fertilizer may be different. In addition, N mineralization from organic fertilizer can be immobilized into the soil organic N pool, which subsequently gradually re-mineralize, resulting in a net increase in soil N mineralization and N availability for plant (Choi et al. 2004; Sørensen 2004). Luxhøi et al. (2007) suggested that mineral N in the transition between gross N mineralization and immobilization is available for assimilation by plants. Consequently, understanding the decomposition rate and gross N release capacity of organic fertilizer could potentially improve our capacity to synchronize N supply with plant demand, and thus minimize N losses to the environment.
The application of organic fertilizers characterized by low C/N ratio (<20) is commonly prevalent in the vineyard soil of the Taihu Lake region (Cheng et al. 2014). Thus, the objective of this study was to quantify the effects of the added organic fertilizers with low C/N ratio on gross N transformation rates in a vineyard soil in the Taihu Lake region. Two contrasting types of organic fertilizers (rapeseed meal vs. chicken manure) used in this region were chosen to examine the effects of organic fertilizer characteristics on decomposition and N release. Gross N transformation rates were quantified with a numeric 15N tracing model in combination with a Markov chain Monte Carlo sampling algorithm for parameter estimation (Müller et al. 2007).
Materials and methods
Soil and amendment characteristics
The study site was located in Zhoutie town in southern Jiangsu Province, China, on the west side of Taihu Lake. This region is characterized by a subtropical monsoon climate, an annual average temperature of 15.7 °C and annual rainfall of 1,177 mm. A vineyard soil with a pH of 5.1 and containing 13 % sand, 70 % silt, 17 % clay, 20 g kg−1 organic C, and 2.0 g kg−1 organic N was collected from a depth of 0–20 cm. The vineyard soil was converted from rice paddy soil about 5 years ago. The vineyard soil had received both organic and chemical fertilizer, with annual rates of about 150 and 224 kg of N ha−1, respectively. The fresh soil was sieved (2 mm mesh) to remove roots and other debris, and then stored at 4 °C in closed plastic bags less than a week before being used in the experiment.
In the studied region, rapeseed meal and chicken manure were applied widely into the vineyard soil. Thus, rapeseed meal and two types of chicken manure differing in biochemical characteristics were selected in this study. Those residues were dried at 60 °C and ground to less than 1 mm. Selected characteristics of the organic amendments used are listed in Table 1.
15N tracing experiment
Gross N transformation rates were determined by the 15N isotope tracing technique, using a paired labeling method with one receiving 15NH4NO3 and the other receiving NH4 15NO3 (Müller et al. 2007). The experiment comprised four treatments in total: no organic material added (abbreviated as CK); rapeseed meal added (RM); chicken manure 1 added (CM1); and chicken manure 2 added (CM2). The treatments were replicated three times. For each treatment, 20 g (dry weight basis) of fresh soil were placed inside a 250-mL flask. The soils in sealed flasks were acclimated to 25 °C for 1 day to allow equilibration. Then, the soil sample from each flask in the organic material amendments treatments was mixed thoroughly with ground organic material. The application rate of various organic materials was equivalent to 100 mg N kg−1 soil, a typical incorporation rate in the field in this region. The corresponding amounts of C provided were 836 for RM, 752 for CM1, and 1,618 mg kg−1 for CM2. For all flasks, an ammonium nitrate solution containing either ammonium (15NH4NO3) or nitrate (NH4 15NO3) labeled with 15N at 10 atom% excess was applied to the soil at 100 mg N kg−1 soil (oven-dried weight), which is equivalent to a moderate rate of N application in the studied region. The 15N-labeled solution was added uniformly over the soil surface with a pipette, and the final soil moisture contents were adjusted to 60 % water holding capacity (WHC) using deionized water. Subsequently, all flasks containing the soil samples were sealed with rubber stoppers and incubated at 25 °C in the dark for an additional 14 days. During incubation, the samples were aerated for 30 min each day to maintain aerobic conditions inside the flasks, and any lost water was replaced every 3 days with deionized water as required.
Gas samples (three replicates) were taken from the headspace of the flasks on days 1, 2, 5, 9, and 14. Before each gas sampling event, the flasks were opened for 30 min to renew the atmosphere inside and immediately sealed for 6 h using a silicone sealant. Two sets of flasks with the same soil samples were used for N2O and NO sampling, respectively. For one set of the flasks, 20 mL gas sample were collected using a 25-mL gas-tight syringe with a stopcock from the headspace of each flask at the end of 6 h incubation and was injected into two pre-evacuated vials (18.5 mL) respectively, to determine the concentration of N2O and CO2. For another set of the flasks, 40-mL gas samples were also collected and transferred to a multilayer foil sampling bag (2 L) for NO analysis.
A sub-set of incubation flasks were destructively sampled on days 2, 5, 9, and 14 for analysis of NH4 +, NO3 −, and organic N. Specifically, three flasks were randomly selected from each labeling treatment, and the soil was extracted using 100 mL 2 M KCl solution to determine the concentration and isotopic composition of NH4 + and NO3 −. The extraction procedure was also performed 0.5 h following 15N addition to avoid disturbance from abiotic N immobilization. After KCl extraction, residual soil was washed with deionized water, oven-dried at 60 °C to a constant weight, and ground to pass through a 0.15-mm sieve for 15N analysis of insoluble organic N.
Soil analysis
Soil pH was measured in a slurry with a soil:water ratio of 1:2.5 (v/v) using a DMP-2 mV-pH detector (Quark Ltd., Nanjing, China). Soil texture was determined with a laser particle characterization analyzer (Beckman Coulter, Los Angeles, CA, USA). The soil organic C was determined by wet digestion with H2SO4-K2Cr2O7, while soil organic N was determined by semi-micro Kjeldahl digestion using Se, CuSO4 and K2SO4 as catalysts. NH4 + and NO3 − concentrations were determined with a continuous-flow analyzer (Skalar Analytical, Breda, The Netherlands). Klason lignin content including acid-soluble and acid-insoluble lignin was estimated according to TAPPI standards (T13wd-74 and T222om-88, respectively) (Tappi 2006).
The isotopic compositions of NH4 + and NO3 − were measured using an automated C/N analyzer isotope ratio mass spectrometer (Europa Scientific Integra, Sercon 20-22, UK). NH4 + and NO3 − were separated for 15N measurements by distillation with magnesium oxide and Devarda’s alloy (Bremner 1996). In detail, a portion of the extract was steam-distilled with MgO to separate NH4 + on a steam distillation system. The sample in the flask was distilled again after the addition of Devarda’s alloy to separate out the NO3 −. The liberated NH3 was trapped using boric acid solution. To prevent isotopic cross-contamination between samples, 25 mL of reagent-grade ethanol was added to the distillation flasks and steam-distilled for 3 min between each distillation (Hauck 1982). Trapped N was acidified and converted to (NH4)2SO4 using 0.005 mol L−1 H2SO4 solution. The H2SO4 solution (containing NH4 +) was then evaporated to dryness at 60 °C in an oven and analyzed for 15N abundance.
Prior to NO determination, gas samples drawn from flasks were diluted to 1 L with highly purified He. The NO concentration was measured using a NOx analyzer (ThermoFisher 42i, Chemiluminescence Detector, USA). N2O concentrations were determined with a gas chromatograph (Agilent 7890, Santa Clara, CA, USA) equipped with a 63Ni electron capture detector (ECD) operated at 300 °C. Separation was performed using a stainless-steel column packed with 80/100 mesh Porapak Q at 65 °C. The injection port was maintained at 100 °C. The carrier gas was argon (Ar) gas and contained 5 % CH4 at a flow rate of 40 mL min−1. Meanwhile, CO2 concentrations were determined with a gas chromatograph (Agilent 7890) equipped with a thermal conductivity detector using a column packed with Porapak Q (80/100 mesh). The temperatures of the column oven, injector, and detector were 40, 100, and 300 °C, respectively.
Calculations and statistical analysis
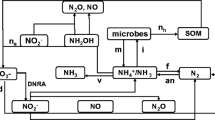
Gross N transformation rates were calculated using a 15N tracing model (Müller et al. 2007, Fig. 1). The model calculated gross N transformation rates by simultaneously optimizing the kinetic parameters for the various N transformations by minimizing the misfit between modeled and observed NH4 + and NO3 − concentrations (Fig. 4) and their respective 15N enrichments (Fig. 5) in the 15NH4NO3 and NH4 15NO3 labeled treatments. Parameter optimization was carried out with Markov chain Monte Carlo Metropolis algorithm. The MCMC-MA routine was programmed in MatLab (Version 7.2, MathWorks Inc.), which calls models that are separately set up in Simulink (version 6.4, MathWorks Inc.). The optimization procedure led to a probability density function (PDF) for each parameter, from which the parameter averages and standard deviations were calculated. Each analysis was conducted with three parallel sequences to identify adequate iteration numbers. According to the kinetic settings and the final parameters, the average N transformation rates were calculated over the entire period and expressed in units of mg N kg−1 soil day−1 (Table 2). The cumulative gross N mineralization was thus calculated as days of the whole incubation × the average gross N transformation rates. The proportion of N mineralized from added organic material N was calculated as the difference in the cumulative gross N mineralization between organic amended and CK treatments divided by added organic material N. Cumulative CO2, N2O, and NO emissions were calculated using linear interpolation across sampling intervals.
15N tracing model developed by Müller et al. (2007) for calculating gross N transformation rates (NH4 + = ammonium; NH4 + ads = adsorbed NH4 +; Nlab = labile soil organic N; NO3 − = nitrate; Nrec = recalcitrant soil organic N). M Nrec, mineralization of recalcitrant organic N (Nrec) to NH4 +; M Nlab, mineralization of labile organic N (Nlab) to NH4 +; I NH4-Nrec, immobilization of NH4 + to Nrec; I NH4-Nlab, immobilization of NH4 + to Nlab; O NH4, oxidation of NH4 + to NO3 − (autotrophic nitrification); O Nrec, oxidation of recalcitrant organic N to NO3 − (heterotrophic nitrification); I NO3, immobilization of NO3 − to recalcitrant organic N; DNRA, dissimilatory NO3 − reduction to NH4 +; A NH4, adsorption of NH4 + on cation exchange sites; R NH4, release of adsorbed NH4 +
The difference in cumulative productions of CO2, N2O, NO, NO/ N2O ratio, the percentage of 15N recovery in the insoluble organic N pool, and the net N transformation rates over the 14-day incubation period was evaluated by one-way ANOVA followed by a least significant difference (LSD) test. The correlation between CO2 and N2O and NO emissions was assessed using Pearson’s correlation test. All statistical analyses were performed using SPSS 13.0. All results are reported as the mean ± standard deviation on a soil dry weight basis.
Results
Soil C mineralization
The CO2 emission rates gradually decreased during the whole incubation period, irrespective of organic material amendments (Fig. 2a). The CO2 emission rates were generally higher in the organic amended than in the CK treatments during the whole incubation period. As a result, the cumulative CO2 emissions over the 14-day incubation period (an index of soil respiration) were significantly stimulated by organic material amendments (P < 0.001; Fig. 3), with the stimulation much more pronounced in the CM1 than in the RM and CM2 treatments. The cumulative CO2-C emission as a percentage of the C added was 34.2, 56.7, and 14.2 % in the RM, CM1, and CM2 treatments, respectively.
Emission rates of CO2 (a), N2O (b), and NO (c) during the 14-day incubation period in a vineyard soil treated with various organic materials. The vertical bars indicate standard deviation. CK, no amendment control; RM, rapeseed meal amendment; CM1, chicken manure 1 amendment; CM2, chicken manure 2 amendment. Chicken manure 1 and chicken manure 2 are two kinds of chicken manure differing in quality
Cumulative emissions of CO2, N2O, and NO during the 14-day incubation period in a vineyard soil treated with various organic materials. The vertical bars indicate standard deviation. Different letters indicate a significant (P < 0.05) difference among treatments. CK, no amendment control; RM, rapeseed meal amendment; CM1, chicken manure 1 amendment; CM2, chicken manure 2 amendment. Chicken manure 1 and chicken manure 2 are two kinds of chicken manure differing in quality
Inorganic N concentration and 15N enrichments
The simulated concentrations and 15N enrichments were almost within the range of the observed averages ± SD (Figs. 4 and 5). In the CK treatment, NH4 + concentrations gradually decreased during the first 9 days of incubation and thereafter remained stable (Fig. 4a). In the RM treatment, NH4 + concentrations decreased during the first 2 days of incubation and increased during days 2–5, and subsequently declined again toward the end of incubation. In the CM1 treatment, NH4 + concentrations gradually decreased during the whole incubation, while they decreased during the first 9 days of incubation, and then increased in the CM2 treatment (Fig. 4a). NO3 − concentrations increased during the whole incubation, regardless of organic material amendments (Fig. 4b). At the end of the incubation (14 day), NO3 − concentrations were highest in CM1 treatment, intermediate in the other two organic material amended treatments, and lowest in the CK treatment.
Measured (point) and modeled (line) concentrations of NH4 +-N (a) and NO3 −-N (b) in a vineyard soil treated with various organic materials. Because NH4 +-N and NO3 −-N concentrations were not different between the 15NH4NO3 and NH4 15NO3 labeled treatments, results for the two labeling types were merged. Error bars represent the standard deviation of the mean. CK, no amendment control; RM, rapeseed meal amendment; CM1, chicken manure 1 amendment; CM2, chicken manure 2 amendment. Chicken manure 1 and chicken manure 2 are two kinds of chicken manure differing in quality
Change of 15N enrichment of NH4 +-N (a), NO3 −-N (b) in the 15NH4NO3 labeled treatment, and NH4 +-N (c), NO3 −-N (d) in the NH4 15NO3 labeled treatment in a vineyard soil treated with various organic materials over a 14-day incubation period. The line indicates modeled results of 15N enrichment of NH4 +-N and NO3 −-N. Error bars represent the standard deviation of the mean. CK, no amendment control; RM, rapeseed meal amendment; CM1, chicken manure 1 amendment; CM2, chicken manure 2 amendment. Chicken manure 1 and chicken manure 2 are two kinds of chicken manure differing in quality
In the 15NH4 + labeled treatments, the 15N enrichment of NH4 + gradually declined (Fig. 5a), and the 15N enrichments of NO3 − increased in all treatments (Fig. 5b). Such results indicated that a continuous input of NH4 + at natural abundance or low 15N enrichment into the 15N-labeled NH4 + pool and ammonia oxidation of 15N enriched NH4 + to NO3 −. An increase in the 15N enrichment of NH4 + over time in the 15NO3 − labeled treatments indicated the occurrence of reduction of labeled NO3 − to NH4 + or re-mineralization of recently immobilized 15NO3 − (Fig. 5c). In all treatments, the 15N enrichment of NO3 − in the 15NO3 − labeled treatments declined over time except for the CK treatment, in which the 15N enrichment of NO3 − declined during the first 5 days of incubation, and then kept almost constant (Fig. 5d).
Nitrogen transformations
The organic material amendments resulted in increasing total gross N mineralization (M Nrec + M Nlab) rates, following the order of CM2 < CM1 < RM treatments (Table 2). The cumulative total gross N mineralization was responsible for 64.2, 32.2, and 18.5 % of the N added in the RM, CM1, and CM2 treatments. The total gross NH4 + immobilization (I NH4-Nrec + I NH4-Nlab) rates were significantly enhanced by RM amendment and to a lesser extent by CM1 and CM2 amendments (Table 2), which was supported by the results of 15N recovery in the insoluble organic N pool in the 15NH4 + labeled treatments. At the end of the incubation, the percentage of 15N recovery in the insoluble organic N pool increased from 10.3 % in the CK treatment to 29.2 % in the RM treatment and 18.7–21.7 % in the CM1 and CM2 treatments (Table 3). The total gross NH4 + immobilization rates were consistently lower than total gross N mineralization rates, regardless of organic material amendments.
In all treatments, heterotrophic nitrification (oxidation of recalcitrant organic N) did not occur, and thus NO3 − production was solely from autotrophic nitrification (oxidation of NH4 + to NO3 −) (Table 2). The gross autotrophic nitrification rates were stimulated by CM1 and to a lesser extent by other two organic material amended treatments. The ratio of autotrophic nitrification and NH4 + immobilization (N/I) was 1.6, 1.1, 5.8, and 2.4 in the CK, RM, CM1, and CM2 treatments, respectively. Compared with substantial NH4 + immobilization, NO3 − immobilization was negligible in all treatments, indicating a preferential uptake of NH4 + by soil microorganisms (Table 2). The percentage of 15N recovery in the insoluble organic N pool in the 15NO3 − labeled treatments remained stable during the whole incubation period and was not affected by any kind of organic material amendment (Table 3), also demonstrating the absence of NO3 − immobilization in all treatments. The rates of dissimilatory NO3 − reduction to NH4 + (DNRA), another NO3 − consumption process, were enhanced by organic material amendments, but a significance difference was only found in the CM1 treatment.
In general, total NO3 − production (heterotrophic + autotrophic nitrification) increased to a greater extent than NO3 − consumption (NO3 − immobilization + dissimilatory NO3 − reduction to NH4 +) following organic material amendments, leading to higher net nitrification rate in the organic material amended treatments compared with the CK treatment (Table 2). Similarly, organic material amendments increased more total inorganic N production (mineralization + heterotrophic nitrification) than total inorganic N consumption (immobilization of NH4 + and NO3 −), and thus increasing net N mineralization rates due to organic material amendments (Table 2).
N2O and NO emissions
In the CK and CM2 treatments, the N2O emission rates gradually decreased during the whole incubation period, while the N2O emission rates peaked at day 2 in the RM and CM1 treatments (Fig. 2b). In contrast, the NO emission rates decreased during the whole incubation period in all treatments (Fig. 2c). The cumulative N2O emissions over the 14-day incubation were 29.9, 42.0, and 4.1-fold higher in the RM, CM1, and CM2 treatments than in the CK treatment, respectively (Fig. 3). In the RM, CM1, and CM2 treatments, the cumulative NO emissions were 0.9, 3.3, and 0.8 times higher than in the CK treatment, respectively (Fig. 3). The ratio of NO to N2O emission was 0.35, 0.02, 0.03, and 0.12 in the CK, RM, CM1, and CM2 treatments, indicating that organic material amendments decrease this ratio. Both N2O and NO emissions were positively correlated with CO2 emission (P < 0.01).
Discussion
Soil C mineralization
Our results showed that the application of organic materials with low C/N ratio stimulated CO2 emission in a vineyard soil, in accordance with previous studies (Chantigny et al. 2002; Khalil et al. 2005). The stimulation may be attributed to mineralization of added organic materials C, or mineralization of native soil organic C (priming effect) due to organic materials input (Garnier et al. 2003; Major et al. 2010). However, the effects of various organic materials on CO2 emission appeared to be different, and emission depended on the quality of organic materials. The C/N ratio has been proposed as an indicator of organic material decomposition rate (Melillo et al. 1982; Trinsoutrot et al. 2000). Residues with a high C/N ratio posed a nutrient limitation on decomposers during the early stage of decomposition (Eiland et al. 2001), and thus there was a significant negative relationship between residue C/N ratio and the rate of mineralization of residues (Vigil and Kissel 1991; Huang et al. 2004). Similarly, our results also demonstrated that CO2 emissions increased with decreasing residue C/N ratio. In addition to C/N ratio, initial N, lignin, and polyphenol contents were linked to residue quality (Palm et al. 2001). Nitrogen availability limits microbial degradation, lignin is one of the most recalcitrant C compounds, and polyphenols can bind with proteins thereby immobilizing N (Palm and Rowland 1997; Gentile et al. 2008). Low-quality residue is associated with low N and high lignin contents (<2.5 % N; >15 % lignin) (Palm et al. 2001). Therefore, low quality of CM2 (2.1 % N; 21.3 % lignin) contributed to low CO2 emission in this study. Alternatively, lignin/N ratio has been found to be related to decomposition rate of plant residues (Melillo et al. 1982; Chantigny et al. 2002; Wang et al. 2004). Our study also demonstrated that CO2 emission and the percentage of the C added as CO2-C emission increased with a decline in lignin/N ratio. In general, organic materials characterized by lower C/N ratio and lignin/N ratio were involved with higher C mineralization.
Gross N mineralization-immobilization turnover
Generally, long-term inputs of exogenous C, such as crop straw and animal manure, could promote gross N mineralization and immobilization turnover (MIT) rates simultaneously, irrespective of whether C/N ratio was narrow or wide (Luxhøi et al. 2007; Zhang et al. 2012). Similarly, our study also found that short-term addition of organic materials with low C/N ratio (≤16) into a vineyard soil increased MIT rates. The increase in gross N mineralization following the application of organic materials could be due to the mineralization of organic materials or stimulation of soil organic N mineralization (Shindo and Nishio 2005), while increasing gross N immobilization was a consequence of additional inorganic N need for microbial growth under enhanced C availability (Burger and Jackson 2003). Finally, organic materials and their induced N immobilization will re-mineralize, potentially causing soil mineralization to increase in the long term.
Since soil C and N cycles are closely coupled, the factors regulating C mineralization may govern N mineralization (Murphy et al. 2003). However, our results found that both lower C/N ratio and lignin/N ratio were not responsible for higher gross N mineralization rate, despite the fact that soil C mineralization increased with decreasing C/N ratio and lignin/N ratio. Such results may indicate that other factors may be associated with N mineralization. Our study showed that gross N mineralization increased with increasing substrate N content of organic materials in a vineyard soil, in agreement with previous reports that the low substrate N content of organic materials likely limited its microbial degradation (Recous et al. 1995; Trinsoutrot et al. 2000). Therefore, substrate N content of organic materials probably played a vital role in gross N mineralization and should be taken into account when predicting gross N mineralization using C mineralization.
It is generally believed that C/N ratio of substrate governs the balance between net N mineralization and immobilization, and the break-even point of substrate was at a C/N ratio of around 20–40 (Whitmore 1996; Van Kessel et al. 2000). Our results also found that the input of organic materials with low C/N ratio (≤16) into a vineyard soil cause net N mineralization (Table 2). Net N mineralization took place because gross N mineralization was stimulated to a greater degree than gross N immobilization by input of organic materials with low C/N ratio in this study. In contrast, the input of organic materials with high C/N ratio led to a lesser stimulation of gross N mineralization than immobilization and thus net N immobilization, as microbial needed additional inorganic N to complete the decomposition process under abundant C availability (Shindo and Nishio 2005).
Gross nitrification and NO3 − immobilization
There is a general agreement that the application of organic materials with low C/N ratio can promote nitrification (Shi et al. 2004; Habteselassie et al. 2006; He et al. 2007). Our results further demonstrated that autotrophic nitrification was the sole pathway producing NO3 − in a vineyard soil, and organic material amendments enhanced nitrification rate also resulted from autotrophic nitrification. These results were in line with results by Müller et al. (2003) who found that the application of cattle slurry to a grassland soil stimulated the nitrification of NH4 + to NO3 − and had no effect on the oxidation of organic N. For acid soil, an increase in pH could be responsible for the stimulation of activity of autotrophic nitrifiers following organic material amendments (Comfort et al. 1988; Paul and Beauchamp 1989). Not only because most of the organic material itself contain ash alkalinity but also because organic material addition can increase local pH by enhancing mineralization (de Boer et al. 1988; Cai et al. 2015). Furthermore, input of organic materials with low C/N ratio resulted in a greater stimulation of gross N mineralization than gross N immobilization, and thus inevitably provided more available NH4 + for nitrifiers. Likewise, long-term application of organic manure with NPK fertilizers can buffer the soil from pH changes and providing not only substrates and nutrients but also a suitable habitat for the ammonia-oxidizing bacteria (AOB) and ammonia-oxidizing archaea (AOA) (He et al. 2007). It has been reported that the AOB-specific nitrification potential and AOB numbers were significantly higher in organic amended soil than in mineral-fertilized soil (Innerebner et al. 2006; Chu et al. 2008). Thus, the stimulation of autotrophic nitrification in the organic amended treatments was probably driven by the increase in AOB population size and activity.
Our results showed that microbial NO3 − immobilization did not occur in the vineyard soil studied, which was consistent with previous general understanding that microbial NO3 − immobilization was negligible in agricultural soil (Jansson et al. 1955; Rice and Tiedje 1989; Recous et al 1990; Shi and Norton 2000; Shi et al. 2004; Zhang et al. 2013). Generally, microbial assimilation NO3 − need higher energy costs in comparison with NH4 + (Lindell and Post 2001). In addition, high NH4 + concentration can repress NO3 − transport or synthesis of NO3 − reductase (Van’t Riet et al. 1968; Sias and Ingraham 1979; Cresswell and Syrett 1979). Alternatively, the absence of microbial immobilization of NO3 − could be ascribed to heterotrophic microorganisms C limitation in the agricultural soil (Shi and Norton 2000). Recous et al. (1990) found that microbial assimilation of NO3 − in cultivated soil was negligible when KNO3 was added without the addition of glucose-C, whereas assimilation of NO3 − occurred when glucose-C at 500 μg g−1 was added along with the same amount of KNO3. In the forest ecosystems, NO3 − assimilation has been observed to increase at glucose-C concentrations above 1,000 μg g−1 (Bradley 2001). The addition of sufficient glucose-C might have resulted in a rapid depletion of NH4 +, and thus microbe turned to assimilate NO3 − for maintaining self-growth, finally causing enhanced NO3 − immobilization rate. In contrast, the application rate of organic material was as high as 752–1,618 μg C g−1, but NO3 − immobilization did not occur in the vineyard soil studied, in line with the results by Shi and Norton (2000) and Shi et al. (2004), showing that NO3 − immobilization did not exist in the compost treated soil with C/N ratio of compost less than 12. Therefore, it is likely that organic material amendments with low C/N ratio could not provide sufficient readily available C for supporting microbial growth, while input of organic materials with high C/N ratio supported more activity of heterotrophs with greater indigenous soil N demand, and thus enhanced NO3 − immobilization. The input of wheat straw has been demonstrated to enhance NO3 − immobilization in agricultural soil (Nishio et al. 2001; Cheng et al. 2012). In addition, significant NO3 − immobilization has been observed in forest soil, grassland soil, and organic farming systems due to microbial demand for N as a result of higher C availability (Davidson et al. 1990; Bradley 2001; Burger and Jackson 2003),
Nitrogen losses
Our study showed that N2O emissions were positively correlated with CO2 emissions following organic material addition (R 2 = 0.76, P < 0.01), in line with previous studies (Miller et al. 2008; Chen et al. 2013). Thus, it was possible to predicate soil N2O emissions through soil respiration after organic material addition. The stimulation of N2O emission following organic material addition was therefore due to stimulating microbial respiration and increasing oxygen depletion, creating temporary anaerobic microsites for denitrification and N2O production (Goek and Ottow 1988). Another possibility was that organic material addition can provide available C as the energy source for denitrification. The NO/N2O ratio was <1, and decreased by the organic material addition, further demonstrating the increasing importance of denitrification to N2O production following organic material addition (Skiba et al. 1992; Hayakawa et al. 2009). In contrast, Begum et al. (2014) found that residue decomposition linked to N immobilization reduced N2O emissions. The ratio of autotrophic nitrification and NH4 + immobilization (N/I) was generally greater than 1 for all treatments, indicating that autotrophic nitrification was the main fate of NH4 + and potential NO3 − losses probably occurred through leaching, runoff, and gaseous N emission (Stockdale et al. 2002; Murphy et al. 2003). Thus, the higher rate of application of organic material with low C/N ratio into agricultural soil should be approached with caution when considering the increasing risk of N losses.
Conclusions
The application of organic material with low C/N ratio to a vineyard soil increased gross N mineralization, NH4 + immobilization, autotrophic nitrification rates, and CO2, N2O, and NO emissions, and had no effect on heterotrophic nitrification and NO3 − immobilization rates. Carbon mineralization of organic material depended on C/N ratio and lignin/N ratio of organic material, while N mineralization was associated with substrate N concentration. The ratio of autotrophic nitrification and NH4 + immobilization was generally greater than 1 for all treatments, indicating that autotrophic nitrification was the main fate of NH4 +. In addition, total NO3 − production (heterotrophic + autotrophic nitrification) increased to a greater extent than NO3 − consumption (NO3 − immobilization + dissimilatory NO3 − reduction to NH4 +) following organic material amendments, leading to more rapid accumulation of NO3 − in soils. Although the application of organic material with low C/N ratio increased N availability for microorganisms and plants, the negative environmental consequences should be carefully considered. Further studies are needed to clarify the long-term application of organic material with low C/N ratio on soil N cycle, and related soil biodiversity and functional genes using molecular and proteomic techniques.
References
Begum N, Guppy C, Herridge D, Schwenke G (2014) Influence of source and quality of plant residues on emissions of N2O and CO2 from a fertile, acidic Black Vertisol. Biol Fertil Soils 50:499–506
Bradley RL (2001) An alternative explanation for the post-disturbance NO3 − flush in some forest ecosystems. Ecol Lett 4:412–416
Bremner JM (1996) Nitrogen-total. In: Sparks DL (ed) Methods of soil analysis. Part 3. Chemical methods. Soil Science Society of America, Madison, pp 1085–1121
Burger M, Jackson LE (2003) Microbial immobilization of ammonium and nitrate in relation to ammonification and nitrification rates in organic and conventional cropping systems. Soil Biol Biochem 35:29–36
Cai ZJ, Wang BR, Xu MG, Zhang HM, He XH, Zhang L, Gao SD (2015) Intensified soil acidification from chemical N fertilization and prevention by manure in an 18-year field experiment in the red soil of southern China. J Soils Sediments 15:260–270
Chantigny MH, Angers DA, Rochette P (2002) Fate of carbon and nitrogen from animal manure and crop residues in wet and cold soils. Soil Biol Biochem 34:509–517
Chen HH, Li XC, Hu F, Shi W (2013) Soil nitrous oxide emissions following crop residue addition: a meta-analysis. Glob Chang Biol 19:2956–2964
Cheng Y, Cai ZC, Chang SX, Wang J, Zhang JB (2012) Wheat straw and its biochar have contrasting effects on inorganic N retention and N2O production in a cultivated Black Chernozem. Biol Fertil Soils 48:941–946
Cheng Y, Wang J, Mary B, Zhang JB, Cai ZC, Chang SX (2013) Soil pH has contrasting effects on gross and net nitrogen mineralizations in adjacent forest and grassland soils in central Alberta, Canada. Soil Biol Biochem 57:848–857
Cheng Y, Jia YS, Wang Y, Zhao X, Yang LZ, Wang SQ (2014) Nutrient inputs and soil fertility status in Orchards of Zhushan Bay in Taihu Lake Watershed (in Chinese with English abstract). J Agro-Environ Sci 33:1940–1947
Choi WJ, Ro HM, Chang SX (2004) Recovery of fertilizer-derived inorganic-15N in a vegetable field soil as affected by application of an organic amendment. Plant Soil 263:191–201
Chu HY, Fujii T, Morimoto S, Lin XG, Yagi K (2008) Population size and specific nitrification potential of soil ammonia-oxidizing bacteria under long-term fertilizer management. Soil Biol Biochem 40:1960–1963
Comfort SD, Kelling KA, Keeney DR, Converse JC (1988) The fate of nitrogen from injected liquid manure in a silt loam soil. J Environ Qual 17:317–322
Cresswell RC, Syrett PJ (1979) Ammonium inhibition of nitrate uptake by the diatom, Phaeodactylum tricornutum. Plant Sci Lett 14:321–325
Davidson EA, Stark JM, Firestone MK (1990) Microbial production and consumption of nitrate in an annual grassland. Ecology 71:1968–1975
de Boer W, Duyts H, Laanbroek HJ (1988) Autotrophic nitrification in a fertilized heath soil. Soil Biol Biochem 20:845–850
Eiland F, Klamer M, Lind AM, Leth M, Baath E (2001) Influence of initial C/N ratio on chemical and microbial composition during long term composting of straw. Microb Ecol 41:272–280
Garnier P, Néel C, Aita C, Recous S, Lafolie F, Mary B (2003) Modelling carbon and nitrogen dynamics in a bare soil with and without straw incorporation. Eur J Soil Sci 54:555–568
Gentile R, Vanlauwe B, Chivenge P, Six J (2008) Interactive effects from combining fertilizer and organic residue inputs on nitrogen transformations. Soil Biol Biochem 40:2375–2384
Goek M, Ottow JCG (1988) Effect of cellulose and straw incorporation in soil on total denitrification and nitrogen immobilization at initially aerobic and permanent anaerobic conditions. Biol Fertil Soils 5:317–322
Guo HY, Wang XR, Zhu JG (2004) Quantification and index of non-point source pollution in Taihu Lake region with GIS. Environ Geochem Health 26:147–156
Habteselassie MY, Miller BE, Thacker SG, Stark JM, Norton JM (2006) Soil nitrogen and nutrient dynamics after repeated application of treated dairy-waste. Soil Sci Soc Am J 70:1328–1337
Hauck RD (1982) Nitrogen-isotope ration analysis. In: Page AL (ed) Methods of soil analysis part 2 chemical and microbiological properties. Soil Sci Soc Am, Madison, pp 735–779
Hayakawa A, Akiyama H, Sudo S, Yagi K (2009) N2O and NO emissions from Andisol field as influenced by pelleted poultry manure. Soil Biol Biochem 41:21–529
He JZ, Shen JP, Zhang LM, Zhu YG, Zheng YM, Xu MG, Di HJ (2007) Quantitative analyses of the abundance and composition of ammonia-oxidizing bacteria and ammonia-oxidizing archaea of a Chinese upland red soil under long-term fertilization practices. Environ Microbiol 9:2364–2374
Huang Y, Zou J, Zheng X, Wang Y, Xu X (2004) Nitrous oxide emissions as influenced by amendment of plant residues with different C:N ratio. Soil Biol Biochem 36:973–981
Innerebner G, Knapp B, Vasara T, Romantschuk M, Insam H (2006) Traceability of ammonia-oxidizing bacteria in compost-treated soils. Soil Biol Biochem 38:1092–1100
Jansson SL, Hallam MJ, Bartholomew WV (1955) Preferential utilization of ammonium over nitrate by micro-organisms in the decomposition of oat straw. Plant Soil 6:382–390
Ji HJ, Zhang RL, Wu SX, Zhang HZ, Zhang WL (2008) Analysis of fertilizer input and nutrient balance of farmland in Taihu watershed (in Chinese with English abstract). Soil Fertil Sci China 5:70–75
Jin XC, Xu QJ, Huang CZ (2005) Current status and future tendency of lake eutrophication in China. Sci China C Life Sci 48:948–954
Ju XT, Xing GX, Chen XP, Zhang SL, Zhang LJ, Liu XJ, Cui ZL, Yin B, Christie P, Zhu ZL, Zhang FS (2009) Reducing environmental risk by improving N management in intensive Chinese agricultural systems. Proc Natl Acad Sci 106:3041–3046
Khalil MI, Hossain MB, Schmidhalter U (2005) Carbon and nitrogen mineralization in different upland soils of the subtropics treated with organic materials. Soil Biol Biochem 37:1507–1518
Li P, Lang M (2014) Gross nitrogen transformations and related N2O emissions in uncultivated and cultivated black soi. Biol Fertil Soils 50:197–206
Lindell AF, Post D (2001) Ecological aspects of ntcA gene expression and its use as an indicator of the nitrogen status of marine Synechococcus spp. Appl Environ Microbiol 67:3340–3349
Luxhøi J, Elsgaard L, Thomsen IK, Jensen LS (2007) Effects of long-term annual inputs of straw and organic manure on plant N uptake and soil N fluxes. Soil Use Manag 23:368–373
Major J, Lehmann J, Rondon M, Goodale C (2010) Fate of soil-applied black carbon: downward migration, leaching and soil respiration. Glob Chang Biol 16:1366–1379
Melillo JM, Aber JD, Muratore JF (1982) Nitrogen and lignin control of hardwood leaf litter decomposition dynamics. Ecology 63:621–626
Miller MN, Zebarth BJ, Dandie CE, Burton DL, Goyer C, Trevors JT (2008) Crop residue influence on denitrification, N2O emissions and denitrifier community abundance in soil. Soil Biol Biochem 40:2553–2562
Müller C, Stevens RJ, Laughlin RJ (2003) Evidence of carbon stimulated N transformations in grassland soil after slurry application. Soil Biol Biochem 35:285–293
Müller C, Rütting T, Kattge J, Laughlin RJ, Stevens RJ (2007) Estimation of parameters in complex 15N tracing models via Monte Carlo sampling. Soil Biol Biochem 39:715–726
Murphy DV, Recous S, Stockdale EA, Fillery IRP, Jensen LS, Hatch DJ, Goulding KWT (2003) Gross nitrogen fluxes in soil: theory, measurement and application of 15N pool dilution techniques. Adv Agron 79:69–118
Nannipieri P, Paul EA (2009) The chemical and functional characterization of soil N and its biotic components. Soil Biol Biochem 41:2357–2369
Nishio T, Komada M, Arao T, Kanamori T (2001) Simultaneous determination of transformation rates of nitrate in soil. Jpn Agr Res Q 35:11–17
Palm CA, Rowland AP (1997) A minimum dataset for characterization of plant quality for decomposition. In: Cadisch G, Giller KE (eds) Driven by nature: plant litter quality and decomposition. CAB International, Wallingford, pp 379–392
Palm CA, Gachengo CN, Delve RJ, Cadisch G, Giller KE (2001) Organic inputs for soil fertility management in tropical agroecosystems: application of an organic resource database. Agric Ecosyst Environ 83:27–42
Paul JW, Beauchamp EG (1989) Biochemical changes in soil beneath dairy cattle slurry layer: the effect of volatile fatty acid oxidation on denitrification and soil pH. In: Hansen JA, Henriksen K (eds) Nitrogen in organic wastes applied to soils. Academic, San Diego, pp 261–270
Recous S, Mary B, Faurie G (1990) Microbial immobilization of ammonium and nitrate in cultivated soils. Soil Biol Biochem 22:913–922
Recous S, Robin D, Darwis D, Mary B (1995) Soil inorganic N availability: effect on maize residue decomposition. Soil Biol Biochem 27:1529–1538
Rice CW, Tiedje JM (1989) Regulation of nitrate assimilation by ammonium in soils and in isolated soil microorganisms. Soil Biol Biochem 21:597–602
Shi W, Norton JM (2000) Microbial control of nitrate concentrations in an agricultural soil treated with dairy waste compost or ammonium fertilizer. Soil Biol Biochem 32:1453–1457
Shi W, Miller BE, Stark JM, Norton JM (2004) Microbial nitrogen transformations in response to treated dairy waste in agricultural soils. Soil Sci Soc Am J 68:1867–1874
Shindo H, Nishio T (2005) Immobilization and remineralization of N following addition of wheat straw into soil: determination of gross N transformation rates by 15N-ammonium isotope dilution technique. Soil Biol Biochem 37:425–432
Sias SR, Ingraham JL (1979) Isolation and analysis of mutants of Pseudomonas aeruginosa unable to assimilate nitrate. Arch Microbiol 122:263–270
Skiba U, Hargreaves KJ, Fowler D, Smith KA (1992) Fluxes of nitric and nitrous oxides from agricultural soils in a cool temperate climate. Atmos Environ Part A 26:2477–2488
Sørensen P (2004) Immobilisation, remineralisation and residual effects in subsequent crops of dairy cattle slurry nitrogen compared to mineral fertiliser nitrogen. Plant Soil 267:285–296
Stark JM, Hart SC (1997) High rates of nitrification and nitrate turnover in undisturbed coniferous forests. Nature 385:61–64
Ste-Marie C, Pare D (1999) Soil, pH and N availability effects on net nitrification in the forest floors of a range of boreal forest stands. Soil Biol Biochem 31:1579–1589
Stockdale EA, Hatch DJ, Murphy DV, Ledgard SF, Watson CJ (2002) Verifying the nitrification to immobilisation ratio (N/I) as a key determinant of potential nitrate loss in grassland and arable soils. Agronomie 22:831–838
Tappi (2006) 2006-2007 TAPPI test methods. TAPPI Press, Norcoss, GA 30092, USA
Trinsoutrot I, Recous S, Bentz B, Linères M, Chèneby D, Nicolardot B (2000) Biochemical quality of crop residues and carbon and nitrogen mineralization kinetics under nonlimiting nitrogen conditions. Soil Sci Soc Am J 64:918–926
Van Kessel JS, Reeves JB, Meisinger JJ (2000) Nitrogen and carbon mineralization of potential manure components. J Environ Qual 29:1669–1677
Van’t Riet J, Stouthammer AH, Planta RJ (1968) Regulation of nitrate assimilation and nitrate respiration in Aerobacter aerogenes. J Bacteriol 96:1455–1464
Vigil MF, Kissel DE (1991) Equations for estimating the amount of nitrogen mineralized from crop residues. Soil Sci Soc Am J 55:757–761
Wang WJ, Baldock JA, Dalal RC, Moody PW (2004) Decomposition dynamics of plant materials in relation to nitrogen availability and biochemistry determined by NMR and wet-chemical analysis. Soil Biol Biochem 36:2045–2058
Whitmore AP (1996) Modeling the release and loss of nitrogen after vegetable crops. Neth J Agric Sci 44:73–86
Zhang JB, Zhu TB, Cai ZC, Qin SW, Müller C (2012) Effects of long-term repeated mineral and organic fertilizer applications on soil nitrogen transformations. Eur J Soil Sci 63:75–85
Zhang JB, Zhu TB, Meng TZ, Zhang YC, Yang JJ, Yang WY, Müller C, Cai ZC (2013) Agricultural land use affects nitrate production and conservation in humid subtropical soils in China. Soil Biol Biochem 62:107–114
Zhu ZL, Chen DL (2002) Nitrogen fertilizer use in China—contributions to food production, impacts on the environment and best management strategies. Nutr Cycl Agroecosyst 63:117–127
Acknowledgments
This study was financially supported by the National Science & Technology Pillar Program (2012BAD15B03), the National Water Pollution Control and Management Technology Major Projects of China (2011ZX07101-004), the Jiangsu Agriculture Science and Technology Innovation Fund (CX(14)2050), the National Natural Science Foundation of China (41301238, 41222005), and the Natural Science Foundation of Jiangsu Province (BK20131045).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cheng, Y., Zhang, JB., Müller, C. et al. 15N tracing study to understand the N supply associated with organic amendments in a vineyard soil. Biol Fertil Soils 51, 983–993 (2015). https://doi.org/10.1007/s00374-015-1044-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00374-015-1044-x