Abstract
Three plant-growth-promoting isolates of endophytic bacteria from sugar beet roots produced indole-3-acetic acid (IAA) in vitro in a chemically defined medium. The three isolates were selected from 221 endophytic bacteria isolated from surface-disinfected beet roots and evaluated for potential to produce IAA and to promote beet growth under gnotobiotic and glasshouse conditions. The inoculation of roots of beet by three selected bacteria isolates significantly increased plant height fresh and dry weights and number of leaves per plant, as well as levels (p < 0.01) of phytormones compared with control plants. In the glasshouse test, the three selected bacterial isolates were recovered from inside roots in all samplings, up to 8 weeks after inoculation, indicating that the roots of healthy beet may be a habitat for these endophytic bacteria.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Endophytes have been defined as those organisms that reside at some phases of their life cycle within living plant tissues without causing them apparent damage (Akello et al. 2008) or which can be extracted from inner plant parts or isolated from surface-disinfected plant tissues (El-Tarabily et al. 2009). Currently, there is great interest in the introduction and manipulation of endophytes for consistent and effective increases in crop productivity (Aravind et al. 2009; Sapak et al. 2008). Although growth promotion by endophytic bacteria (Bacon and Hinton 2002) and endophytic fungi (Maria and Sridhar 2003) has been reported, there are no reports on endophytic bacteria promoting growth of sugar beet. To effectively use endophytic bacteria to increase plant production, many kinds of bacteria with this function need to be found, and their mechanisms for promoting plant growth should be clarified.
Some endophytes and bacteria provide beneficial effects on host plants by producing plant growth regulators (PGRs; Ting et al. 2008; Raja et al. 2008). Auxins are a class of PGRs known to stimulate both rapid (e.g. increases in cell elongation) and long-term (e.g. cell division and differentiation) responses in plants (Taghavi et al. 2009). In both higher plants and microorganisms, l-tryptophan (l-TRP) is considered as a physiological precursor of IAA biosynthesis (Sapak et al. 2008).
In the present study, from sugar beet, we screened endophytic bacteria isolates that produced IAA and examined the abilities of the isolates to promote beet growth under gnotobiotic conditions. The endophytic potential of the more promising auxin-producing isolates to colonise beet was assessed. After inoculation with endophytic bacteria under controlled glasshouse conditions, we evaluated plant growth and levels of endogenous auxins in roots and shoots.
Materials and methods
Isolation of endophytic bacteria from surface-disinfected beet plants
Twenty-two sugar beet plants were harvested by hand from border rows of a research plot in Xinjiang, China during the 2006 growing season (July–October). The number and species of endophytes present in the root tissue were recorded. Endophyte populations in sugar beet roots were first enumerated during the growth season. Bacterial populations were assessed for the next 7 weeks. Beets were harvested shortly after the seventh week of testing, when the plants were about 15 cm tall at the crown.
Freshly harvested sugar beet plants were washed thoroughly with tap water to remove adhering soil and debris, rinsed in 95% ethanol and then flamed. This minimised carry-over of external bacteria when cross-sectioning at various locations using a sterile knife. Samples were aseptically removed from the core, periphery and secondary root emergence zone (crease) areas. Each sample of root tissue was aseptically weighed, then added to 100 mL of sterile saline (0.85%), and blended for 2 min in a Waring blender. The blended samples were initially diluted to standardise all preparations. This was followed by additional serial decimal dilution in sterile saline. Endophytic bacteria were isolated using an aerobic spread-plate method. Dilution volumes of 0.1 mL were plated in triplicate on nutrient agar (NA) supplemented with 2.0 g/L sucrose. The spread plates were incubated at room temperature for 7 days and colony counts recorded by standard methods (Kodaka et al. 2005). Pure cultures were subsequently isolated, and bacterial isolates were stored on agar slants.
Screening for indole-3-acetic acid production isolates
The indole-3-acetic acid (IAA) assay was performed using the method of Patten and Glick (2002) with some modifications. Flasks (500 mL), each containing 100 mL of sterile glucose peptone broth (GPB), were amended with 25 mL of 5% filter-sterilised l-TRP (Millipore membranes; pore size, 0.22 μm, Millipore Corporation, Bedford, MA, USA; Khalid et al. 2004). The flasks were inoculated with 10 mL of each of the isolated bacteria prepared from a 2-day-old shaken GPB culture of approximately 1 × 108 CFU mL−1, covered with aluminium foil and incubated on a shaker at 200 rpm at 30°C in the dark for 7 days. Non-inoculated flasks served as controls. After incubation for 96 h, the suspension from each flask was centrifuged for 30 min at 12,000×g. The supernatant was filtered through sterile Millipore membranes (pore size, 0.22 μm) and collected in sterile tubes. The culture supernatants (3 mL) were pipetted into test tubes, and treated with 2 mL of Salkowski reagent (2 mL of 0.5 M FeCl3 + 98 mL 35% HClO4) (Gordon and Weber 1951). The tubes containing the mixture were left for 30 min for red colour development. The colour intensity was determined by optical density at 530 nm using a scanning spectrophotometer (UV-2550 PC, Shimadzu Corporation, Analytical Instruments Division, Kyoto, Japan). Similarly, colour was also developed in standard solutions of IAA used to prepare a standard curve (Gordon and Weber 1951). Four independent replicates of each isolate were analysed.
Assessment of growth promotion under gnotobiotic conditions
Three isolates that produced detectable levels of IAA (isolates 2-1, 2-2 and 3-1) were further tested under gnotobiotic conditions to study their effectiveness on beet root and shoot growth. A non-IAA-producing endophytic bacteria isolates (1–6) was also included for comparison. The bacterial isolates were inoculated into seedlings grown in sterilised sand, which had been acid-washed overnight in 1:1 (w/v) sand/6 mol L−1 HCl rinsed with tap water for 30 min, rinsed ten times with deionised water for 40 min and autoclaved for 40 min. Glass tubes (300 × 35 mm diameter) were filled with autoclaved, acid-washed sand (250 g), moistened with distilled water and the tubes autoclaved again for 40 min. Filter-sterilised (pore size, 0.22 μm, Millipore) nutrient solution (65 mL) amended with or without l-TRP (3 mg kg−1 sand) was added to each tube as a single application after planting the seedlings as recommended by Frankenberger and Poth (1987).
Seedlings were inoculated with the bacterial suspensions. Healthy beet seeds were surface disinfected and pre-germinated on moist-sterile filter paper at 25°C in the dark for 2 days to obtain uniform seedlings. When roots were about 15 mm long, the root tips (3 mm) were trimmed using a sterilised scalpel to facilitate the uptake of the inoculum (Bressan and Borges 2004). The seedlings were placed in sterile plastic cups at 25°C for 3 h with only their roots in contact with the inoculum suspension of each isolate at 108 CFU mL−1. As controls, seedlings with severed root tips were treated with autoclaved inoculum. The beet seedlings with or without the living bacterial isolates were then planted into the tubes under aseptic conditions and watered as needed with sterile-distilled water. The tubes were incubated in a growth room with 200 μmol m−2 s−1 fluorescent light and light/dark cycle of 16:8 h at 25:20°C. Two weeks after transplantation, the plants were harvested, washed and separated into roots and shoots and the lengths and weights of shoots and roots recorded. Each treatment was replicated eight times with one seedling per replicate.
Identification of endophytic bacteria
Pure cultures of the bacterial isolates were prepared for identification purpose. The bacterial isolates 2-1, 2-2 and 3-1 were characterised based on their morphological characteristics and subsequently identified by using 16S rDNA sequences. The biochemical and physiological characterizations of endophytic bacteria were determined and further identified as reported above (Matthews et al. 1990; Garrity and Holt 2001). The 16S rRNA gene of genomic DNA isolated was extracted from pure bacterial colonies following standard protocols (Halda-Alija and Johnston 1999). The primers used for polymerase chain reaction (PCR) were pF (5 -AGA GTT TGATCC TGG CTC AG-3) and pR (5 -AAG GAG GTG ATC CAG CCG CA-3).The PCR products with the expected size (about 1,500 bp) were purified using a DNA Gel Extraction Kit and cloned into pMD18-T vector followed by sequencing. Sequence analysis was performed using the BLAST algorithm (http://www.ncbi.nlm.nih.gov). Bacterial identifications were based on 16S rRNA gene sequence similarity. For further characterization of isolates 2-1, 2-2 and 3-1, a neighbour-joining phylogenetic tree was constructed with the MEGA 4.0 program.
Quantitative determination of phytohormones of the selected bacteria isolates
For detection of phytohormones, endophytic bacterial strains were cultivated in liquid GPB medium for 48, 72, 96, 120, 144 and 168 h, centrifuged at 11,000×g for 15 min and the supernatant quantified by enzyme-linked immunosorbent assay (ELISA) method as described by von Aderkasa et al. (2001). ELISA results were confirmed by follow-up gas chromatograph analyses using a TRACE2000 gas chromatograph (Finnigan, USA). Five replicates for each measurement were performed. Confidence intervals of hormonal concentrations were determined at 95% confidence limits (P < 0.05).
Glasshouse trials
Soil, inoculum production and glasshouse in vivo trials
The effect of endophytes on beet growth was further tested in vivo in soil. Soil was collected from the same beet field and sieved. The soil characteristics were pH 7.85, electrical conductivity 1.32 dS m−1, organic C 2.41% and bicarbonate extractable K+ and P were 150 and 1,100 mg kg−1 soil dry weight, respectively. Free-draining pots (30 cm diameter) were filled with 8 kg of soil.
The isolates 2-1, 2-2 and 3-1 were grown in liquid GPB medium shaken at 200 rpm at 30°C for 48 h, after which bacterial cells were precipitated by centrifuging the culture at 5,000×g for 5 min. The cells were suspended in sterile distilled water and the concentration adjusted to 106 CFU mL−1 with a spectrophotometer (Mayak et al. 2004). Sugar beet seeds were surface-sterilised with 70% alcohol for 8 min followed by 5% hydrogen peroxide solution for 2 h and then rinsed in sterile water (Caitriona et al. 2004). The disinfected seeds were immersed in the bacterial suspension for 6 h and then sown at five seeds per pot. After germination, the pots were thinned to one plant per pot. There were ten replicates, with seeds immersed in distilled water instead of the bacterial suspension serving as controls (CK). The pots were kept in a growth chamber with 200 μmol m−2 s−1 light and light/dark cycle of 12:12 h at 30:25°C. Soil moisture was maintained at 60% of soil moisture-holding capacity. Germination percentage was recorded 7 days later with plant height, fresh and dry weights and number of leaves. Endogenous phytohormones were measured 30 days later and were measured every 5 days, until 30 days.
Estimation of internal root colonisation
Pot trials were designed to assess the internal colonisation of sugar beet roots after seedling inoculation through the soaking seed method described above. Rifampicin-resistant mutants of isolates 2-1, 2-2 and 3-1 were prepared as described by He et al. (2004). The obtained mutants were compared with their wild types in relation to their ability to produce auxins. None of these mutants differed morphologically from their parental strains, and all mutants had identical growth rates and auxin-production ability with their parental strains. Sugar beet seedlings were prepared as described above and inoculated with isolates 2-1, 2-2 and 3-1. Every week after planting (1–8), roots were sampled from the soil, washed thoroughly in tap water, surface disinfested as described above and the population densities of isolates 2-1, 2-2 and 3-1 (log10 CFU g−1 fresh root weight) were determined using NA amended with rifampicin (300 μg ml−1, Sigma). Each treatment was replicated five times with two plants in each replicate for each sampling.
Extraction and ELISA analysis of endogenous phytohormones in beet roots and shoots
Endogenous phytohormones were extracted from tissues of the terminal part of the root and shoot systems using the method described by Guinn et al. (1986). Briefly, the tissues were quickly frozen at −85°C and ground in cold 80% extracting solvent. The macerate was then transferred to a flask with fresh extracting solvent, and the volume adjusted to 20 mL and filtered. The filtrate was evaporated at 35°C in a rotary flash evaporator, leaving the aqueous phase. The aqueous phase was then adjusted to pH 8 with K2HPO4, the sample was partitioned three times with equal volumes of washed ethyl acetate-butylated hydroxytoluene and the aqueous phase was adjusted to pH 2.8 with H3PO4 (Sigma). The acidified solution was passed through a C18 Sep-Pak cartridge (Waters Corporation, Milford, MA, USA) to trap auxins. Auxins were then eluted with NH4OH (Sigma) and the pH quickly adjusted to 2.8 with H3PO4. The aqueous eluted phase was partitioned three times, each with 10 mL of washed diethyl ether-butylated hydroxytoluene. The ether was evaporated by rotary flash evaporation at 40°C, the residue was immediately dissolved in methanol and the supernatant quantified by ELISA method as described by von Aderkasa et al. (2001). ELISA results were confirmed by follow-up gas chromatograph analyses using a Vista/6000 gas chromatograph. There were five replicates for each measurement. Confidence intervals of hormonal concentrations were determined at 95% confidence limits (P < 0.05).
Statistical analysis
All experiments were arranged in completely randomised block designs. Population data were transformed into log10 CFU g−1 fresh weight. Data were subjected to analysis of variance, and treatment means were compared using Fisher’s protected least significant difference test at P < 0.05.
Results
Isolation of endophytic bacteria and in vitro screening for IAA production
The populations of endophytic bacteria in beet roots were in the range of 3.7 × 103 to 3.6 × 109 CFU g−1 fresh root weight. There was no contamination in sterility checks, indicating successful surface disinfection. A total of 221 bacteria isolates were obtained from the beet root triturate. IAA was detected only in liquid cultures of 221 isolates, of which three isolates were selected. Bacterial isolates varied greatly in their efficiency of IAA production in GPB medium in the presence of l-TRP (Table 1); with six isolates producing a large amount of IAA. The remaining isolates did not produce IAA and did not show any colour change after reagent addition to their culture filtrates in the presence of l-TRP. However, IAA-producing isolates formed a dark red colour on reagent addition.
Assessment of growth promotion under gnotobiotic conditions
Sugar beet seedling vigour as measured by root and/or shoot lengths and weights in l-TRP-amended or non-amended soil was significantly (P < 0.05) increased by the inoculation with IAA-producing bacteria isolates compared to controls or seedlings inoculated with non-IAA-producing bacteria isolates (1–6) (Table 2). The addition of l-TRP supported significantly (P < 0.05) better root and shoot development than l-TRP-non-amended soil in the presence of bacterial isolates (Table 2). Different isolates of endophytic bacteria had variable effects on root and shoot growth (Table 2). Of the six IAA-producing isolates, the isolates 2-1, 2-2 and 3-1 provided the best growth promotion in the presence or absence of l-TRP (Table 2) and were chosen for further glasshouse studies.
Identification of the selected bacteria isolates
On the basis of in vitro IAA production and based upon the performance of the bacterial isolates on the growth of beet seedlings under gnotobiotic conditions, only three isolates (isolates 2-1, 2-2 and 3-1) were tested in pot trials of their endophytic colonisation of roots and their effects on beet growth and development under glasshouse conditions.
The 2-1 strain had the same phenotypic properties. Cells were Gram-negative rods measuring 1.0–1.2 × 1.5–5.0 μm and could grow at a broad range of pH (6–9) in Luria broth medium with optimum growth at pH 7.5 and 37°C. Its NaCl tolerance was up to 15%. They were motile with a growth temperature of 4–41°C. The cytochrome oxidase test was positive. Nitrate reduction was negative.
The 2-2 strain had the same phenotypic properties. Cells were Gram-negative rods measuring 0.5–1.0 × 2.5–4.5 μm. They were motile at a growth temperature of 4–41°C. The cytochrome oxidase test was positive. Nitrate reduction was positive, but denitrication was negative. Fluorescent pigment was produced weakly on King medium B.
The 3-1 strain had the same phenotypic properties. Cells were Gram-negative rods measuring 0.5–1.0 × 0.9–5.8 μm. They were motile at a growth temperature of 4–41°C. The cytochrome oxidase test was positive. Nitrate reduction was negative.
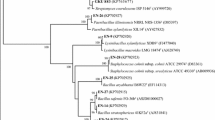
Phenotypic data of morphological, physiological, and biochemical characteristics combined with 16S rRNA gene (GenBank accession numbers EU594552, EU594563 and EU594557) analysis (Fig. 1) allowed the identification of the strains 2-1, 2-2 and 3-1 as Bacillus pumilus, Chryseobacterium indologene and Acinetobacter johnsonii, respectively. Strains 2-1, 2-2 and 3-1 presented high similarity values with B. pumilus, C. indologene and A. johnsonii (99%) in the BLASTn search, respectively, and were recovered in a tight cluster with B. pumilus, C. indologene and A. johnsonii in the phylogenetic tree, respectively (Fig. 1).
Phylogenetic analysis of partial 16S rDNA sequence of isolates B. pumilus, C. indologenes, A. johnsonii strains (strains 2-1, 2-2 and 3-1) obtained from sugar beet. Numbers in parentheses represent the sequences accession number in GenBank. The number at each branch points is the percentage supported by bootstrap
Phytohormone production of three selected bacterial isolates
Phytohormone production by the three strains was confirmed by ELISA. Of the three strains, the highest production was by strain 2-1 (Tables 3, 4, 5 and 6). IAA was not detected in either Entercococcus faecalis 1-1 or the sterile broth (control).
Estimation of internal root colonisation
The rifampicin-resistant mutants of isolates 2-1, 2-2 and 3-1 were isolated from the surface-disinfected beet roots, indicating that these strains were endophytic. Isolates 2-1, 2-2 and 3-1 maintained their endophytic colonising abilities and were isolated from healthy beet roots in all samplings until week 8 (Table 7). An initial increase in colonies of three endophytic bacteria after 1, 2 and 3 weeks was followed by a decrease at the fourth week. However, at and after week 5 and up to week 8, the populations were relatively stable (Table 7).
Glasshouse in vivo trials
There was significantly (P < 0.05) enhanced growth of beet plants inoculated with isolates 2-1 and 3-1 in l-TRP-amended soil as shown by the increased height, fresh and dry weights and number of leaves compared with control plants grown in l-TRP-amended soil (Table 8). There were no significant differences between fresh and dry weights of beet plants inoculated with 2-2 in l-TRP-amended soil and controls (Table 8).
Measurement of endogenous hormones from roots and shoots
Plants inoculated with three endophytic bacteria and grown in soil had significantly (P < 0.05) higher endogenous hormones compared to the control plants (Fig. 2a–d). There were no significant differences between the endogenous gibberellic acid (GA) contents of roots or shoots of inoculated beet plants compared to controls (Fig. 2c). There were no significant differences between the endogenous zeatin riboside (ZR) and IAA contents of roots or shoots of beet plants inoculated with isolate 3-1 compared to 2-1 (Fig. 2a, d). There were significant differences between the endogenous abscisic acid (ABA) contents of roots or shoots of beet plants inoculated with isolate 3-1 compared to 2-1 and 2-2 (Fig. 2b).
Discussion
In the present study, three endophytic bacterial isolates (3-1, 2-1 and 2-2) capable of producing relatively high levels of IAA, GA, ABA and ZR in the growth medium amended with l-TRP also significantly promoted growth of beet under gnotobiotic and glasshouse conditions. In our study, these three isolates were selected from 221 endophytic bacteria isolates based upon in vitro IAA production and growth-promoting activity under gnotobiotic conditions. This is the first record of plant growth promotion by auxin-producing endophytic bacteria in beet roots.
All media used for isolation as well as for testing phytohormone production have very high nutrient contents, which are unrealistic even for endophytes. It might be possible that there are much more interesting endophytes, which do simply not grow on the used medium. Furthermore, phytohormone production might differ depending on the quality and quantity of the substrates. In this study, phytohormone production of isolation was higher in media than in host plant (Tables 3, 4, 5 and 6, Fig. 1). The ability of beet-associated bacteria to produce phytohormones and the effect of such microorganisms on plant development under natural and artificial conditions of cultivation might be different. The auxin production by endophytic bacteria in beet is a key factor promoting plant growth. Auxins are a class of PGRs known to stimulate both rapid and long-term responses in plants (Cleland 1990).
The promotion effects are mainly due to the activity of auxins. Plant growth promotion by other microorganisms has been recorded wherein the effective isolates were capable of producing only auxins (Bhuvaneswari et al. 2006; Dai et al. 2008; Sapak et al. 2008). It is, however, possible that growth-promoting factors other than those tested may also have had a role in the growth promotion of the plant. The 1-aminocyclopropane-1-carboxylate deaminase-containing plant growth-promoting bacteria can lower plant ethylene levels, and thus they can stimulate plant growth (Seyring and Vogt 2000). The exogenous application of l-TRP, combined with the auxin-producing isolates of the three strains, enhanced plant growth compared to non-amended soil. This could be explained by additional production of auxins by indigenous soil microflora following the l-TRP amendment with these produced auxins taken up by roots; however, plants have the ability to take up l-TRP and convert it to auxins using their own enzymes.
Although the growth of both roots and shoots were promoted by the three strains, there were no significant differences between growth responses of roots and shoots of beet plants inoculated with the three strains in l-TRP-amended soil and the respective control. This emphasises the importance of presence of a precursor in soil for the stimulation of plant growth.
The observed increase in plant growth by an endophyte is supported by other observations in which endophytic bacteria (Barretti et al. 2008; Nejad and Johnson 2000) and filamentous fungi (Dai et al. 2003; Lu et al. 2000; Rahman and Saiga 2005) enhanced plant growth. The three strains used in the present study were shown to produce IAA, GA and ZR, which can affect plant growth. Several endophytic bacteria (Harish et al. 2008; Sapak et al. 2008; Shin et al. 2007) and filamentous fungi (Lu et al. 2000) can produce auxins in vitro.
This study also indicates that there is a clear need to indicate that the plant growth-promoting endophytic yeasts can also be used to enhance plant productivity at a field scale.
References
Akello J, Dubois T, Coyne D, Kyamanywa S (2008) Effect of endophytic Beauveria bassiana on populations of the banana weevil, Cosmopolites sordidus, and their damage in tissue-cultured banana plants. Entomol Exp Appl 129:157–165. doi:10.1111/j.1570-7458.2008.00759.x
Aravind R, Kumar A, Eapen SJ, Ramana KV (2009) Endophytic bacterial flora in root and stem tissues of black pepper (Piper nigrum L.) genotype: isolation, identification and evaluation against Phytophthora capsici. Lett Appl Microbiol 48:58–64. doi:10.1111/j.1472-765X.2008.02486.x
Bacon CW, Hinton DM (2002) Endophytic and biological control potential of Bacillus mojavensis and related species. Biol Control 23:274–284. doi:10.1006/bcon.2001.1016
Barretti PB, De Souza RM, Pozza AAA, Pozza EA, De Carvalho JG, De Souza JT (2008) Increased nutritional efficiency of tomato plants inoculated with growth-promoting endophytic bacteria. Rev Bras Cienc Do Solo 32:1541–1548. doi:10.1590/S0100-06832008000400018
Bhuvaneswari V, Kathiravan G, Gangadevi V, Muthumary J (2006) Extraction and estimation of indo, E-3-acetic acid (IAA) from some endophytic and pathogenic coelomycetes. Asian J Microbiol Biotechnol Environ Sci 8:243–248
Bressan W, Borges MT (2004) Delivery methods for introducing endophytic bacteria into maize. BioControl 49:315–322. doi:10.1023/B:BICO.0000025372.51658.93
Caitriona D, Wilson LW, McFadden H (2004) Gene expression profile changes in cotton root and hypocotyl tissues in response to infection with Fusarium oxysporum f. sp. vasinfectum. Mol Plant Microbe Interact 17:654–667. doi:10.1094/MPMI.2004.17.6.654
Cleland RE (1990) Auxin and cell elongation. In: Davies PJ (ed) Plant hormones and their role in plant growth and development. Kluwer, Dordrecht, pp 132–148
Dai C, Yu B, Xu Z, Yuan S (2003) Effect of environmental factors on the growth and fatty acid composition of five endophytic fungi from Sapium sebiferum. Chin J Appl Ecol 14:1525–1528
Dai CC, Yu BY, Li X (2008) Screening of endophytic fungi that promote the growth of Euphorbia pekinensis. Afr J Biotechnol 7:3505–3510
El-Tarabily KA, Nassar AH, Hardy GESJ, Sivasithamparam K (2009) Plant growth promotion and biological control of Pythium aphanidermatum, a pathogen of cucumber, by endophytic actinomycetes. J Appl Microbiol 106:13–26. doi:10.1111/j.1365-2672.2008.03926.x
Frankenberger WT Jr, Poth M (1987) Biosynthesis of indole-3-acetic acid by the pine ectomycorrhizal fungus, Pisolithus tinctorius. Appl Environ Microbiol 53:2908–2913
Garrity GM, Holt JG (2001) The road map to the manual. In: Boone DR, Castenholz RW, Garrity GM (eds) Bergey's manual of systematic bacteriology, vol 1, Secondth edn. Springer, New York, pp 119–166
Gordon SA, Weber RP (1951) Colorimetric estimation of indole acetic acid. Plant Physiol 26:192–195. doi:10.1104/pp. 26.1.192
Guinn G, Brummett DL, Beier RC (1986) Purification and measurement of abscisic acid and indole-acetic acid by high performance liquid chromatography. Plant Physiol 81:997–1002. doi:10.1104/pp. 81.4.997
Halda-Alija L, Johnston TC (1999) Diversity of culturable heterotrophic aerobic bacteria in pristine stream bed sediments. Can J Microbiol 45:879–884. doi:10.1139/cjm-45-10-879
Harish S, Kavino M, Kumar N, Saravanakumar D, Soorianathasundaram K, Samiyappan R (2008) Biohardening with plant growth promoting rhizosphere and endophytic bacteria induces systemic resistance against banana bunchy top virus. Appl Soil Ecol 39:187–200. doi:10.1016/j.apsoil.2007.12.006
He H, Qiu SX, Cai XQ, Guan X, Hu FP (2004) Colonization in plants and identification of endophytic bacteria BS-1 and BS-2 from Capsicum annuum. Acta Microbiol Sin 44:13–18
Khalid A, Arshad M, Zahir ZA (2004) Screening plant growth-promoting rhizobacteria for improving growth and yield of wheat. J Appl Microbiol 96:473–480. doi:10.1046/j.1365-2672.2003.02161.x
Kodaka H, Mizuochi S, Teramura H, Nirazuka T (2005) Comparison of the compact dry IC method with the standard pour plate method (AOAC Official Method 966.23) for determining aerobic colony counts in food samples. J AOAC INtern 88:1702–1713
Lu H, Zou WX, Meng JC, Hu J, Tan RX (2000) New bioactive metabolites produced by Colletotrichum sp., an endophytic fungus in Artemisia annua. Plant Sci 151:67–73. doi:10.1016/S0168-9452(99)00199-5
Maria GL, Sridhar KR (2003) Endophytic fungal assemblage of two halophytes from west coast mangrove habitats, India. Czech Mycol 55:241–251
Matthews KR, Oliver SP, King SH (1990) Comparison of Vitek Gram-positive identification system with API Staph Trac system for species identification of staphylococci of bovine origin. J Clin Microbiol 28:1649–1651
Mayak S, Tirosh T, Glick BR (2004) Plant growth-promoting bacteria confer resistance in tomato plants to salt stress. Plant Physiol Biochem 42:565–572. doi:10.1016/j.plaphy.2004.05.009
Nejad P, Johnson PA (2000) Endophytic bacteria induce growth promotion and wilt disease suppression in oilseed rape and tomato. Biol Control 18:208–215. doi:10.1006/bcon.2000.0837
Patten CL, Glick BR (2002) Role of Pseudomonas putida indoleacetic acid in development of the host plant root system. Appl Environ Microbiol 68:3795–3801. doi:10.1128/AEM.68.8.3795-3801.2002
Rahman MH, Saiga S (2005) Endophytic fungi (Neotyphodium coenophialum) affect the growth and mineral uptake, transport and efficiency ratios in tall fescue (Festuca arundinacea). Plant Soil 272:163–171. doi:10.1007/s11104-004-4682-6
Raja P, Balachandar D, Sundaram SP (2008) Genetic diversity and phylogeny of punk-pigmented facultative methylotrophic bacterai isolated from the pjyllosphere of tropical plants. Biol Fertil Soils 45:45–53. doi:10.1007/s00374-008-0306-2
Sapak Z, Meon S, Ahmad ZAM (2008) Effect of endophytic bacteria on growth and suppression of Ganoderma infection in oil palm. Int J Agric Biol 10:127–132
Seyring M, Vogt G (2000) Influencing growth of endophytic bacteria and quality of shoots in plant tissue cultures of Argyranthemum frutescens. Gartenbauwissenschaf 65:115–120
Shin DS, Park MS, Jung S, Lee MS, Lee KH, Bae KS, Kim SB (2007) Plant growth-promoting potential of endophytic bacteria isolated from roots of coastal sand dune plants. J Microbiol Biotechnol 17:1361–1368
Taghavi S, Garafola C, Monchy S, Newman L, Hoffman A, Weyens N, Barac T, Vangronsveld J, Van Der Lelie DD (2009) Genome survey and characterization of endophytic bacteria exhibiting a beneficial effect on growth and development of poplar trees. Appl Environ Microbiol 75:748–757. doi:10.1128/AEM.02239-08
Ting ASY, Meon S, Kadir J, Radu S, Singh G (2008) Endophytic microorganisms as potential growth promoters of banana. BioControl 53:541–553. doi:10.1007/s10526-007-9093-1
Von Aderkasa P, Lelub MA, Labelb P (2001) Plant growth regulator levels during maturation of larch somatic embryos. Plant Physiol Biochem 39:495–502. doi:10.1016/S0981-9428(01)01271-2
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shi, Y., Lou, K. & Li, C. Promotion of plant growth by phytohormone-producing endophytic microbes of sugar beet. Biol Fertil Soils 45, 645–653 (2009). https://doi.org/10.1007/s00374-009-0376-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00374-009-0376-9