Abstract
Nitrous oxide (N2O) contributes to greenhouse effect; however, little information on the consequences of different moisture levels on N2O/(N2O+N2) ratio is available. The aim of this work was to analyze the influence of different soil moisture values and thus of redox conditions on absolute and relative emissions of N2O and N2 at intact soil cores from a Vertic Argiudoll. For this reason, the effect of water-filled porosity space (WFPS) values of soil cores of 40, 80,100, and 120% (the last one with a 2-cm surface water layer) was investigated. The greatest N2O emission occurred at 80% WFPS treatment where conditions were not reductive enough to allow the complete reduction to N2. The N2O/(N2O+N2) ratio was lowest (0–0.051) under 120% WFPS and increased with decreasing soil moisture content. N2O/(N2O+N2) ratio values significantly correlated with soil Eh; redox conditions seemed to control the proportion of N gases emitted as N2O. N2O emissions did not correlate satisfactorily with N2O/(N2O+N2) ratio values, whereas they were significantly explained by the amount of total N2O+N2 emissions.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Dinitrogen (N2) gas is the end product of denitrification, and nitrous oxide (N2O) is the by-product with important harmful environmental consequences because of its contribution to greenhouse effect. In addition, N2O is the main source of stratospheric nitric oxide, which damages the ozone layer (Crutzen 1979). N2O is also produced by nitrification, but N2O emissions are considered to be more driven by reduction than by oxidation processes in soil (Bergsma et al. 2002).
Tiedje (1988) suggested that in aerobic systems, oxygen availability is the main limiting factor of denitrification, whereas in anaerobic systems, NO3 − availability may be limiting. Both soil redox status and oxygen diffusion are affected by soil moisture, but there are contradictory results in the literature. Dobbie and Smith (2003) found the greatest N2O fluxes from pasture soils at water-filled porosity space (WFPS) values higher than 60% when NO3 − concentration was nonlimiting. However, Bøckman and Olfs (1998) proposed that N2O formation is greater when soil WFPS ranges between 40 and 80%. Marinho et al. (2004) found that the maximum N2O fluxes occurred several days after strong rain events, with significant correlation coefficients (r = 0.84–0.94) between N2O emissions and precipitations during 1 week before measurements. Data of rice-based agricultural systems showed that N2O emissions were greatest at not continuously flooded fields (Xing and Zhu 1997). McSwiney et al. (2002) concluded that N2O production is greatest under conditions that are suboptimal for nitrifiers and denitrifiers.
Few information exist about the consequences of different moisture levels on N2O/(N2O+N2) ratio. Granli and Bøckman (1994) suggested that the N2O mole fraction produced by denitrification could increase by aeration. Entic soils from Canada displayed greater N2O/(N2O+N2) ratio values with WFPS lower than 30% with respect to soils at WFPS higher than 50% (Elmi et al. 2003); at the latter WFPS values, probably a greater reduction of N2O to N2 occurred. Weier et al. (1993) found smaller N2O/(N2O+N2) ratio values with the greatest moisture levels, although water saturation was not tested. Bandibas et al. (1994) found that N2O emissions were greater in saturated than in flooded soils, but they did not measure N2 emissions. Hofstra and Bouwman (2005) have suggested that wetland rice systems are more prone to denitrification than those in upland and grassland systems. It is important to underline that in Argentina, rice cultivation is mainly carried out in soils with Vertic properties. Despite the extensive research, the effect of either soil moisture or a superficial flooding water layer on both N2O and N2 emissions is not clear.
We have hypothesized that the presence of a superficial water layer would increase the reduction of N2O to N2, and therefore, the N2O/(N2O+N2) ratio under flooding conditions would be lower than nonflooding. The aim of this work was to analyze the influence of different moisture and thus of redox conditions on absolute and relative emissions of N2O and N2 with intact soil cores from a Vertic Argiudoll.
Materials and methods
Soil sampling and handling
Two hundred and four undisturbed soil cores were sampled within PVC cylinders of 10 cm length and 5 cm of internal diameter from the surface horizon of a Vertic Argiudoll, Martín Fierro series, located at the INTA Castelar experimental station (S 34°36′20″, W 58°40′20″). The study area was covered by a native grassland. The cylinders were randomly extracted from a 3 × 3-m area, sealed on the base, conducted to the laboratory, and put into plastic jars. The fresh soil had the following properties: clay 247 g kg−1, silt 587 g kg−1, sand 166 g kg−1, N–NO3 − 30.7 mg kg−1, N–NH4 + 6.1 mg kg−1, total organic C 25.9 g kg−1, total N (Nt) 2.4 g kg−1, pH H2O 6.07, and WFPS at field capacity 42.71%.
The soil of cylinders was air-dried for 20 days when the soil moisture was close to 24% WFPS in all cylinders. The behavior of soil moisture can be particularly important because denitrifying enzymes are differentially induced by wetting, and this can affect denitrification products (Bergsma et al. 2002).
Soil treatments and incubation
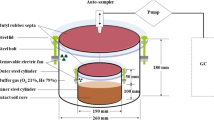
The experiment was conducted under laboratory conditions, with temperature ranging between 18 and 22°C. Treatments were randomly assigned to the soil cores in a completely randomized design, and each treatment was replicated three times. The following different soil moisture contents were reached by adding distilled water to soil cores: 40, 80, 100, and 120% WFPS, with the last treatment involving a 2-cm surface water layer. Water was added so as to avoid soil alteration and gas trampling. Cylinders containing soils at 120% WFPS treatment had in their superior part an open PVC cylinder (5 cm high and with an internal diameter a little bigger than the soil cylinder) so as to maintain a superficial water layer of 2 cm during the experiment. Soil moisture was maintained constant by adding water lost by evaporation.
Analyses
Denitrification losses (N2O+N2) were measured by the acetylene blockage technique (Yoshinari et al. 1977). In this study, we consider N2O emission the N2O which had left the soil–floodwater system, whereas the water-dissolved N2O was not taken into consideration. Gas emission was measured from the same soil cores throughout. Each replication included a pair of intact soil cores, one incubated with acetylene and the other one without acetylene. N2O emission was the value determined without acetylene, whereas the N2O emitted with acetylene represented N2O+N2 emission. Cylinders were incubated within plastic jars with hermetic covers with a rubber septum for the injection of acetylene and the collection of gaseous samples. Ten percent of the headspace air was replaced with a syringe by an equal amount of acetylene at the beginning of each measurement in the acetylene-treated soil cores. Three additional jars without soil were used as blanks. Both N2O and N2 were accumulated within the jars for 24 h before they were determined; jars were left open between measurements. Triplicate gas samples (2 ml) were taken from the headspace of the jars by using disposable syringes, and they were immediately analyzed by the Gaseous Chromatograph Agilent 6890 (Palo Alto, CA, USA) with ECD detector and capillary column Carboplot and using helium (He) gas as carrier; the oven, injector, and detector temperatures were 100, 100 and 250°C, respectively. Nitrogen emissions were measured at 0, 2, 7, 14, and 21 days. Cumulative emissions were calculated by averaging daily rates between two consecutive measurements and multiplying the average emission rate by the number of days elapsed between measurements.
In addition to the two soil cores for gas determinations, each replicate at each determination time involved three additional soil cores that were incubated so as to carry out chemical analysis. Before chemical analysis, the samples were air-dried, ground, and sieved (2-mm mesh size). The pH was measured in soil–water relationship 1:2.5 (Thomas 1996).
Soluble organic C (WSOC) was extracted by shaking soil suspensions (10 g in 20 ml 0.5 M K2SO4) for 30 min in horizontal shaker; then, the supernatant was vacuum-filtered through Whatman No. 42 filter previously rinsed with distilled water. Soluble organic C of the filtered solution was analyzed by the modified dichromate oxidation method (Nelson and Sommers 1982), which involved the oxidation of 3 ml of the filtered extract with 1.5 ml 0.06 N potassium dichromate and 3 ml of concentrated H2SO4. The residual dichromate was titrated with 0.03 N Mohr’s salt.
Redox potential (Eh) was measured with specific platinum combination electrode (Digital Ionalyzer/501, Orion Research, Boston, MA, USA) at a constant depth of 3 cm (Patrick et al. 1996).
Nitrate–N was determined by extracting a 20-g sample from each soil core with 100 ml 0.25% CuSO4 + 0.01 M BO3H3 solution; the soil solution was filtered, and the N–NO3 − content was determined colorimetrically by the hydrazine-reduction method (Carole and Scarigelli 1971) without drying or sieving.
Statistical analysis and calculations
N2 emissions were obtained by subtracting N2O emission without acetylene to N2O emission with acetylene (Ryden et al. 1979), and then the N2O/(N2O+N2) ratio was then calculated.
Data were analyzed by the Statistical Analysis System (SAS) package (SAS Institute Inc. 1985). Absolute and relative gas emissions and daily and cumulative emissions were log-transformed to obtain their normality due to the asymmetry. Simple and multiple regression analyses between the total emissions (N2O+N2), N2O emissions, and N2O/(N2O+N2) ratio and the different soil chemical measured variables were performed with PROC REG procedure of SAS. Variance analysis with PROC MIXED procedure of SAS package assessed differences in daily N2O+N2 and N2O emissions and N2O/(N2O+N2) ratio between different treatments. A repeated-measures model was chosen because the measurements were made in the same soil cores throughout the incubation, and sphericity and compound symmetry conditions were met. In this way, incubation day factor was straightforwardly analyzed. Differences in cumulative and daily N2O+N2 and N2O emissions between treatments were evaluated through conventional variance analysis with PROC GLM procedure of SAS package. Afterwards, a multiple comparison test (least significant difference) was made to see which treatments really differed.
Results
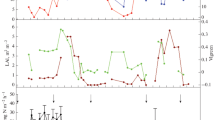
N2 emissions accounted in average for 78% of the total emissions and were significantly correlated with total (N2O+N2) emissions (p < 0.0001, R 2 = 0.86). Indeed, total N emissions followed a similar pattern to N2 emissions (Fig. 1a,b). Total emissions were high at the beginning of the incubation and decreased during the incubation (Fig. 1a), with the exception of the 40% WFPS treatment that displayed low and relatively constant values throughout the experiment. Total losses significantly increased (p < 0.05) by increasing WFPS values (Fig. 1a, Table 1), reaching the higher values under 120% WFPS. Interaction between moisture levels and incubation time was not detected. Average daily fluxes during the studied period were of 2.26, 12.42, 17.31, and 28.47 μg N kg−1 of soil for 40, 80, 100, and 120% WFPS treatments, respectively. Cumulative total N emissions at 40% WFPS were significantly lower (p = 0.02) than those at 80, 100, and 120% WFPS, and the latter values were statistically similar.
N2O emissions on the first day of incubation were statistically different from the rest of the incubation days (p < 0.005), with a high initial outburst in the N2O emission which differed depending on the moisture level (Fig. 1c). This effect was more relevant at the flooded situation (120% WFPS), with a marked reduction during incubation. After 10 days of incubation, N2O increased both under 80 and 100% WFPS (Fig. 1c). Under prevailing aerobic conditions (40% WFPS), N-N2O emissions were low and practically constant after the small initial outburst. N2O emissions presented daily averages of 0.53, 5.84, 2.58, and 1.18 μg N kg−1 of soil for the 40, 80, 100, and 120% WFPS treatments, respectively. Daily N2O emissions were significantly different (Table 1) between different moisture levels, with no existing interaction between this moisture levels and incubation time (p = 0.52). Overall daily emissions of this gas were statistically highest at 80% WFPS (p < 0.05). N2O emissions under 100% WFPS treatment were significantly greater than those under 40% WFPS (p = 0.06) and than those under 120% WFPS, although this last difference was statistically significant only after 14 days (p = 0.07). Cumulative N2O emissions along the incubation were statistically similar between treatments (p = 0.31, Table 1).
In spite of a highly variable pattern, 40% WFPS treatment generally presented the greatest N2O/(N2O+N2) ratio values (p = 0.0008). The N2O/(N2O+N2) ratio values increased with time, after a small initial decrease, under 80 and 100% WFPS (Fig. 1d). The ratio was lowest (0–0.051) under 120% WFPS. Average N2O/(N2O+N2) ratio values for the entire incubation were 0.32, 0.27, 019, and 0.13, for 40, 80, 100, and 120% WFPS, respectively, being statistically different only under 40 and 120% WFPS (p = 0.04).
N2O emissions showed a linear and positive relationship with total denitrification emissions (p = 0.04, R 2 = 0.481), (Fig. 2a), whereas the relationship between N2O emissions and N2O/(N2O+N2) ratio values was not consistent (p = 0.96). The N2O/(N2O+N2) ratio displayed a negative relationship with total N emissions (p = 0.09, R 2 = 0.487) (Fig. 2b).
N2O emissions were not significantly related to any of the measured soil variables (Table 2). The averages of daily emissions for each moisture level showed a quadratic relationship with WFPS (Fig. 3). The N2O/(N2O+N2) ratio values were significantly and positively correlated with soil potential redox levels (p = 0.02, R 2 = 0.4) (Table 2).
Multiple explanatory models of total emissions by denitrification and of N2O/(N2O+N2) ratio including variables such as WFPS, NO3 −, WSOC, pH, and redox potential were significant (p < 0.05), but with low explaining power (R 2 = 0.41 and 0.45, respectively), and for this reason, they are not presented in this work. Multiple regression model trying to explain N2O emissions variability, including all the above-mentioned soil variables, was not significant (p = 0.4). Moreover, no model of multiple linear regression satisfactorily explained the changes in the N2O emissions.
Discussion
Total N2O+N2 emissions presented in this work (2.26–28.47 μg N kg−1 of soil day−1) are in the same range of those reported by Sainz Rozas et al. (2001), who found denitrification losses of 14.64 μg N kg−1 day−1 at intact cores taken from a Typic Argiudoll from Argentina. Weier et al. (1993) found denitrification values as low as 2.9 μg N kg−1 day−1 at repacked soil cores taken from a Typic Argiudoll soil at 75% WFPS, but these emissions reached a value of 251 μg N kg−1 day−1 at 90% WFPS. Olde Venterink et al. (2002) have found that denitrification rates increased markedly when WFPS exceeded 60–80% and were the highest on the first day. We have found that the highest denitrification losses occurred at the highest moisture contents, thus confirming the close relationship between these emissions and redox conditions. However, the first day of the experiment presented both relatively high redox potential values (132–265 mV, data not shown), indicative of aerobic conditions, and the highest denitrification losses; this apparent contradiction may depend on the fact that NO3 − is reduced to NO2 − under Eh values as high as 300 mV (Rowell 1981); in addition, Eh values gave an average value of the bulk soil, and the presence of high potential redox values does not exclude the presence of hot spots where denitrification losses can occur (Parkin 1987) because these active microsites can have a reduction potential greater than the rest of the soil matrix.
N2O emissions presented daily averages of 0.53, 5.84, 2.58, and 1.18 μg N kg−1 of soil for the 40, 80, 100, and 120% WFPS treatments, respectively. Carran et al. (1995) found N2O emission values ranging from 6 to 9.05 μg N kg−1 day−1 at intact cores from silty loam soils with 70% WFPS, with soils having carbon and nitrogen contents at least twofold higher than those of the present work.
N2O emissions displayed a high initial peak after the addition of water probably because the transition between aerobic and anaerobic conditions increased the formation of N2O (Kester et al. 1997) due to differences in the synthesis and regulation of the enzymes involved in the denitrifying sequence (Zumft 1997). An increase in the N2O production after the moistening of a dry soil has also been reported by Hao et al. (1988).
N2O emissions were influenced by moisture treatments, and the greatest emissions occurred at 80 and 100% WFPS, coincident with other authors who showed that the greatest N2O emission potential occurs at WFPS values close to 80% (Inubushi et al. 1996; Dobbie and Smith 2001, 2003; Khalil and Baggs 2005). The general relationship found between N2O emissions and WFPS with a quadratic function shape (Fig. 3) enhances this concept, which is in agreement with the data presented by Dalal et al. (2003). However, other authors found a linear (Dobbie and Smith 2001) or exponential relationship (Dobbie and Smith 2003), where the maximum WFPS values were 90 and 100%, respectively.
Although denitrification is the main process generating N2O, nitrification can also produce low amounts of this gas (Ritchie and Nicholas 1972). Acetylene inhibits at low concentrations the first step of autotrophic nitrification (Klemedtsson et al. 1988); thus, N2O+N2 emissions may be underestimated under well-aerated situations, as the 40% WFPS treatment in this report. However, the observed low N2O emissions at 40% WFPS support the hypothesis that denitrification is more important than nitrification in producing N2O.
Bandibas et al. (1994) proposed that the N2O emission is affected by the relative emission N2O/(N2O+N2) ratio rather than by the denitrification activity. However, in our work, N2O emissions were not significantly correlated with the N2O/(N2O+N2) ratio values, but they were significantly correlated (p = 0.04) with the total N emissions.
N2O emissions were poorly related to the analyzed soil chemical properties probably because they depend on the complex combination of temperature, soil structure, NO3 − concentration, aeration, and moisture content, and each factor can affect denitrification and vary in space and time (Liang and Mackenzie 1997).
The low N2O/(N2O+N2) ratio values under 120% WFPS should probably be due to strong anaerobic conditions created by the presence of a superficial water layer, which promoted N2O reduction to N2, as suggested by Xu et al. (2004). Weier et al. (1993) also found lower N2O/(N2O+N2) ratio values under increasing moisture levels, although the greatest moisture contents analyzed did not reach soil saturation (90% WFPS). The important effect of reductive conditions were supported by the significant and positive relationship between N2O/(N2O+N2) ratio values and soil Eh values. In addition, the surface water layer probably limited N2O upward diffusion, as suggested by Yan et al. (2000), and this probably stimulated N2O reduction to N2. Another possible explanation would be the greater sensitivity of the N2O reductase than the other denitrifying enzymes to oxygen (Knowles 1982).
It is known that high NO3 − concentrations inhibit N2O reduction to N2 (Gaskell et al. 1981; Bandibas et al. 1994). Schlegel (1992) explained this phenomenon by stating that NO3 − is preferred as an electron acceptor with respect to N2O. However, neither N2O nor the N2O/(N2O+N2) ratio was related to soil NO3 − levels probably because of the strong control of moisture exerted on these variables.
The N2O/(N2O+N2) ratio, as N2O or total N emissions data, was not satisfactorily fitted by any of the used multiple regression model. Using multiple linear regression, Clayton et al. (1997) could only explain 28% of N2O emissions. Probably, any variable controlling the N2O emission can be a rate-limiting one at different moments depending on the particular conditions (Dobbie and Smith 2003).
In conclusion, maximum N2O emissions were found when 80 to 100% of pores were occupied by water, where conditions were not reductive enough to carry out completely the denitrifying sequence. This led to higher N2O/(N2O+N2) ratio values under these situations than at 120% WFPS, confirming the proposed hypothesis. Soil Eh values significantly correlated with N2O/(N2O+N2) ratio, suggesting that this soil parameter regulates the proportion of N gases emitted as N2O. N2O emissions did not correlate satisfactorily with N2O/(N2O+N2) ratio values, whereas these emissions were significantly explained by total N emissions.
References
Bandibas J, Vermoesen A, De Groot CJ, Van Cleemput O (1994) The effect of different moisture regimes and soil characteristics on nitrous oxide emission and consumption by different soils. Soil Sci 158:106–114
Bergsma TT, Robertson GP, Ostrom NE (2002) Influence of soil moisture and land use history on denitrification end-products. J Environ Qual 31:711–717
Bøckman OC, Olfs HW (1998) Fertilizers, agronomy and N2O. Nutr Cycl Agroecosyst 52:165–170
Carole RS, Scarigelli FP (1971) Colorimetric determination of nitrate after hydrazine reduction to nitrite. Microchem J 16:657–672
Carran RA, Theobald PW, Evans JP (1995) Emission of nitrous oxide from some grazed pasture soils in New Zealand. Aust J Soil Res 33:341–352
Clayton H, McTaggart IP, Parker J (1997) Nitrous oxide emission from fertilized grassland: a 2-year study of the effects of N fertilizer form and environmental conditions. Biol Fertil Soils 25:252–260
Crutzen PJ (1979) The role of NO and NO2 in the chemistry of the troposphere and stratosphere. Annu Rev Earth Planet Sci 7:443–472
Dalal RC, Wang W, Robertson GP, Parton WJ (2003) Nitrous oxide emission from Australian agricultural lands and mitigation options: a review. Aust J Soil Res 41:165–195
Dobbie KE, Smith KA (2001) The effects of temperature, water-filled pore space and land use on N2O emissions from an imperfectly drained gleysol. Eur J Soil Sci 52:667–673
Dobbie KE, Smith KA (2003) Nitrous oxide emission factors for agricultural soils in Great Britain: the impact of soil water-filled pore space and other controlling variables. Glob Change Biol 9:204–218
Elmi AA, Madramootoo C, Hamel C, Liu A (2003) Denitrification and nitrous oxide to nitrous oxide plus dinitrogen ratios in the soil profile under three tillage systems. Biol Fertil Soils 38:340–348
Gaskell JF, Blackmer AM, Bremner JM (1981) Comparison of effects of nitrate, and nitric oxide on reduction of nitrous oxide to dinitrogen by soil microorganisms. Soil Sci Soc Am J 45:1124–1127
Granli T, Bøckman O (1994) Nitrous oxide from agriculture. Nor J Agric Sci Suppl 12:7–128
Hao WM, Scharffe D, Crutzen PJ, Sanhueza E (1988) Production of N2O, CH4 and CO2 from soils in the typical savanna during the dry season. J Atmos Chem 7:93–105
Hofstra N, Bouwman AF (2005) Denitrification in agricultural soils: summarizing published data and estimating global annual rates. Nutr Cycl Agroecosyst 72:267–278
Inubushi K, Naganuma H, Kitahara S (1996) Contribution of denitrification and autotrophic and heterotrophic nitrification to nitrous oxide production in andosols. Biol Fertil Soils 23:292–298
Kester RA, Deboer W, Laanbroek HJ (1997) Production of NO and N2O by pure cultures of nitrifying and denitrifying bacteria during changes in aeration. Appl Environ Microbiol 63:3872–3877
Khalil MI, Baggs EM (2005) CH4 oxidation and N2O emissions at varied soil water-filled pore spaces and headspace CH4 concentrations. Soil Biol Biochem 37:1785–1794
Klemedtsson L, Svensson BH, Roswall T (1988) A method of selective inhibition to distinguish between nitrification and denitrification as sources of nitrous oxide in soil. Biol Fertil Soils 6:112–119
Knowles R (1982) Denitrification. Microbiol Rev 46:43–70
Liang BC, Mackenzie AF (1997) Seasonal denitrification rates under corn (Zea mays L.) in two Quebec soils. Can J Soil Sci 77:21–25
Marinho EVA, DeLaune RD, Lindau CW (2004) Nitrous oxide flux from soybeans grown on Mississippi alluvial soil. Commun Soil Sci Plant Anal 35:1–8
McSwiney CP, McDowell WH, Keller M (2002) Distribution of nitrous oxide and regulators of its production across a tropical rainforest catena in the Luquillo Experimental Forest, Puerto Rico. Eur J Soil Sci 53:265–286
Nelson DW, Sommers LE (1982) Total carbon, organic carbon and organic matter. In: Page AL (ed) Methods of soil analysis. Part 2. American Society of Agronomy, Madison, WI, pp 539–579
Olde Venterink H, Davidsson TE, Kiehl K, Leonardson L (2002) Impact of drying and re-wetting on N, P and K dynamics in a wetland soil. Plant Soil 243:119–130
Parkin TB (1987) Soil microsites as a source of denitrification variability. Soil Sci Soc Am J 51:1194–1199
Patrick WH, Gambrell RP, Faulkner SP (1996) Redox measurement of Soils. In: Sparks DL (ed) Methods of soil analysis. Part 3. American Society of Agronomy, Madison, WI, pp 1255–1273
Ritchie GAF, Nicholas DJD (1972) Identification of nitrous oxide produced by oxidative and reductive processes in Nitrosomonas europaea. Biochem J 126:1181–1191
Rowell DL (1981) Oxidation and reduction. In: Greenland DJ, Hayes MHB (eds) The chemistry of soil processes. Wiley, New York, pp 401–462
Ryden JC, Lund J, Focht DD (1979) Direct measurement of denitrification loss from soils: I. Laboratory evaluation of acetylene inhibition of nitrous oxide reduction. Soil Sci Soc Am J 43:104–110
Sainz Rozas HR, Echeverría HE, Picone LI (2001) Denitrification in maize under no-tillage: effect of nitrogen rate and application time. Soil Sci Soc Am J 65:1314–1323
SAS Institute Inc (1985) User’s guide: statistics. 5th edn. SAS Institute, Cary, NC
Schlegel HG (1992) Allgemeine Mikrobiologie. 7th edn. Thieme, Stuttgart, New York
Thomas GW (1996) Soil pH and soil acidity. In: Sparks DL (ed) Methods of soil analysis. Part 3. Soil Science Society of America, Madison, WI, pp 475–490
Tiedje JM (1988) Ecology of denitrification and dissimilatory nitrate reduction to ammonium. In: Zehnder AJB (ed) Biology of anaerobic microorganisms. Wiley, New York, pp 179–244
Weier KL, Doran JW, Power JF, Walters DT (1993) Denitrification and the dinitrogen/nitrous oxide ratio as affected by soil water, available carbon, and nitrate. Soil Sci Soc Am J 57:66–72
Xing GX, Zhu ZL (1997) Preliminary studies on N2O emission fluxes from upland soil and paddy soils in China. Nutr Cycl Agroecosyst 49:17–22
Xu YC, Shen QR, Li ML, Dittert K, Sattelmacher B (2004) Effect of soil water status and mulching on N2O and CH4 emission from lowland rice field in China. Biol Fertil Soils 39:215–217
Yan X, Du L, Shi S, Xing G (2000) Nitrous oxide emission from wetland rice soil as affected by the application of controlled-availability fertilizers and mid-season aeration. Biol Fertil Soils 32:60–66
Yoshinari T, Hynes R, Knowles R (1977) Acetylene inhibition of nitrous oxide reduction and measurement of denitrification and nitrogen fixation in soil. Soil Biol Biochem 9:177–183
Zumft WG (1997) Cell biology and molecular basis of denitrification. Microbiol Mol Biol Rev 61:553–568
Acknowledgments
This work was supported by the UBACyT G-038 grant from the Buenos Aires University. Marta Conti and Gerado Rubio are members of the National Council for Scientific Research (CONICET).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ciarlo, E., Conti, M., Bartoloni, N. et al. The effect of moisture on nitrous oxide emissions from soil and the N2O/(N2O+N2) ratio under laboratory conditions. Biol Fertil Soils 43, 675–681 (2007). https://doi.org/10.1007/s00374-006-0147-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00374-006-0147-9