Abstract
Humic substances [humic acid (HA), fulvic acid (FA), and insoluble humin], particulate organic matter (POM), and glomalin comprise the majority (ca 75%) of operationally defined extractable soil organic matter (SOM). The purpose of this work was to compare amounts of carbon (C) and nitrogen (N) in HA, FA, POM, and glomalin pools in six undisturbed soils. POM, glomalin, HA, and FA in POM, and glomalin, HA, and FA in POM-free soil were extracted in the following sequence: (1) POM fraction separation from the soil, (2) glomalin extraction from the POM fraction and POM-free soil, and (3) co-extraction of HA and FA from the POM fraction and POM-free soil. Only trace amounts of HA and FA were present in the POM fraction, while POM-associated glomalin (POM-glomalin) and POM alone contributed 2 and 12%, respectively, of the total C in the soil. Mean combined weights for chemically extracted pools from POM and from POM-free soil were 9.92 g glomalin, 1.12 g HA, and 0.88 g FA kg−1 soil. Total protein and C, N, and H concentrations showed that glomalin and HA were, for the most part, separate pools, although protein was detected in HA extracts. Even though percentage carbon was higher in HA than in glomalin, glomalin was a larger (almost nine times) operationally defined pool of soil organic C. Glomalin was also the largest pool of soil N of all the pools isolated, but all pools combined only contained 31% of the total N in the soil.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Soil organic matter (SOM) is important for soil stabilization, nutrient cycling, and carbon (C) storage. Identifying soil C and N pools will aid in developing agricultural practices to optimize terrestrial C storage while improving soil quality.
Humic substances (HS) are the largest operationally defined soil organic carbon (SOC) pool containing 70–80% of the total carbon in soils (Hayes and Graham 2000). These substances are defined as highly condensed plant material and the byproducts of microbial decomposition of plant residues (Burdon 2001; Hayes and Clapp 2001; MacCarthy 2001). Humic material is operationally defined by solubility characteristics as alkaline-soluble humic acid (HA), acid- and alkaline-soluble fulvic acid (FA), and mostly insoluble humin. All three of these pools are structurally heterogeneous (Burdon 2001; MacCarthy 2001). Amino acids, carbohydrates, lipids, and metals are frequently extracted with HA and treated as contaminants (Clapp and Hayes 1999; Hayes and Graham 2000; Schulten and Schnitzer 1997). These impurities and co-extracted inorganic ash material (Schnitzer and Schuppli 1989) inflate the size of the HA pool reported in most soils.
Particulate organic matter (POM) consists of relatively fresh plant debris primarily from roots. This material is the first soil C pool in the decomposition of plant residue and is an energy and nutrient source in soils (Gale and Cambardella 2000). Operationally, POM is defined as the pool of SOM that floats in concentrated sodium chloride or high-density sodium polytungstate (Gale and Cambardella 2000; Wolf et al. 1994).
Glomalin is a glycoprotein produced by arbuscular mycorrhizal (AM) fungi and is found in high concentrations (2 to 14 g kg−1 soil) in a variety of temperate soils (Wright and Upadhyaya 1998). SOM is expected to have a high concentration of glomalin because (1) glomalin coats the surface of AM fungal hyphae (Wright 1999; Wright et al. 1996; Wright and Upadhyaya 1999) and undisturbed soils may have hyphal lengths >100 m g−1 (Miller et al. 1995); (2) glomalin resists degradation by proteases, high heat, and low pH (Wright and Upadhyaya 1998); and (3) glomalin in undisturbed soils has a turnover time of at least 7 to 42 years (Rillig et al. 2001) and maybe up to 200 years (R.M. Miller, personal communication). Glomalin extracted from AM hyphae cultured in glomalin-free sand and from soil are chemically similar according to protein banding on sodium dodecyl sulfate-polyacrylamide gel electrophoresis, immunoassays, glycoprotein assays, C, N and H concentrations, and nuclear magnetic resonance (NMR) spectra (Wright and Upadhyaya 1996; Wright et al. 1998; Rillig et al. 2001; Nichols 2003). Operationally, glomalin is defined as proteinaceous material that is soluble at high temperature (121°C) in an alkaline citrate solution (Wright et al. 1996; Wright and Upadhyaya 1996; Wright and Upadhyaya 1998).
The protocols used to separate operationally defined SOM pools are not specific enough to isolate a single compound, but the resulting mixture is dominated by one specific pool. A previous study showed that the purity of HA is enhanced, as measured by the amount of co-extracted protein, by extracting glomalin from a sample before extracting HA (Nichols and Wright 2005). Also, POM is a heterogeneous pool that may contain glomalin on plant roots or humic substances attached to nondecomposed plant material. Therefore, to isolate these operationally defined soil C pools, SOM was extracted in the following sequence: (1) POM fraction separation from soil, (2) glomalin extraction from the POM fraction and POM-free soil, and (3) HA and FA extraction from the POM fraction and POM-free soil. The objectives of this study were to: (1) isolate eight soil organic C and N pools from each soil sample, (2) compare weights of C and N in each pool, and (3) compare pools using protein assay values and/or percentages of C, N, and H.
Materials and methods
Soils
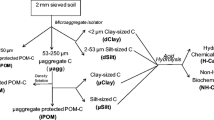
Bulk samples (0–10 cm depth) were collected with a shovel from two locations in each of three US states: Baltimore A site and Baltimore B site in Maryland (MD), Sampson and Haxtun in Colorado (CO), and Pacolet and Cecil in Georgia (GA). Soils were air-dried and sieved to remove material <2 mm. All six soils were acidic loams (pH 4.4 to 6.7), but they varied in textural (clay contents from 100 to 250 g kg−1) and chemical characteristics (CEC, P, and Fe) (Nichols and Wright 2005). The SOC content was ca 30 g kg−1 for all soils, except the Haxtun soil, which had 7 g kg−1. Organic matter pools were extracted sequentially from five 2-g subsamples from each soil (Fig. 1).
Soil organic matter extractions
The POM fraction was removed by floatation using a method modified from Wolf et al. (1994). Briefly, soil samples were covered with a 12% NaCl solution, vortexed, and allowed to settle for 30 min. After the mineral fraction (POM-free soil) had settled, the solution was carefully decanted. Floating organic matter (POM fraction) was collected on a 0.053-mm screen. This procedure was repeated five times. The POM fraction from all five 2-g samples was collected together, washed with distilled water to remove salt, rinsed from the screen into preweighed weigh boats, and dried at 70°C. POM-free soil was washed with distilled water, pelleted by centrifugation, and dried at 70°C.
Glomalin was extracted from the POM fraction and POM-free soil and treated as described by Nichols and Wright (2005). Briefly, soil was incubated in 50 mM sodium citrate, pH 8.0, at 121°C for 1 h (Wright and Upadhyaya 1999). The glomalin extract was separated from the soil by centrifugation, and the extraction procedure was repeated until the supernatant was straw-colored (up to three more times). Supernatants from each extraction cycle were combined and treated as follows: (1) a subsample was removed for protein assays (see below), and (2) glomalin was flocculated at pH 2.0–2.5, with HCl on ice, and collected by centrifugation. The pellet was dissolved in NaOH and dialyzed against deionized water (dH2O; up to five changes of dH2O) in dialysis tubing with molecular weight cut-off (MWCO) of 8,000 to 12,000 Da. Dialyzed material was centrifuged and the supernatant was collected and freeze dried. All centrifugations were at 6,850×g for 10 min.
The procedure described by Nichols and Wright (2005) was used to extract HA and FA at room temperature (RT). Briefly, POM and POM-free soil were preincubated in HCl followed by a multistep extraction procedure using NaOH under N2 and acidification with HCl to separate HA (precipitate) from FA (supernatant). This extraction was repeated until the solution was almost clear. Insoluble solids and inorganic ash material were removed from HA extracts with concentrated KCl and an HCl–HF solution, respectively. A subsample (0.5 ml) was removed for protein assays (see below) from HA redissolved in a minimal measured volume of NaOH. The remaining solution was acidified, collected, and washed with dH2O by repeated centrifugation at 10,844×g and freeze-dried. A sample was removed from the FA extracts for protein assays (see below) and the remaining solutions were dialyzed (MWCO of 8,000 to 12,000 Da) against dH2O until the pH was neutral. Insoluble solids were pelleted at 6,850×g for 10 min and the supernatant was freeze-dried.
Soil organic matter analysis
Soil organic matter pools were analyzed for total and immunoreactive protein concentrations, gravimetric weight, and C, N, and H concentrations as appropriate. Total and immunoreactive protein concentrations were measured on glomalin, HA, and FA extracts using the Bradford total protein assay (Wright et al. 1996) and enzyme-linked immunosorbent assay (ELISA) (Nichols and Wright 2005). Percent immunoreactivity was calculated as the amount of immunoreactive protein divided by the amount of total protein times 100. POM (dried at 70°C), freeze-dried glomalin, HA and FA, and residual soil (soil after all extractions) were weighed to the nearest 0.1 mg (corrected for amounts removed for protein assays as necessary). Carbon, N, and H concentrations were measured by combustion with a Perkin-Elmer Series II C, H, N, S/O 2400 Analyzer (Shelton, CT) on soil samples before extraction, POM, POM-associated glomalin (POM-glomalin), glomalin, HA, and FA from POM-free soil, and residual soil. Replicate samples of glomalin, HA, and FA extracted from POM-free soil were combined. All samples were stored under vacuum before combustion. Gravimetric weights were multiplied by percentage C and N to give C and N weights (grams per kilogram soil) in each pool. Nonextracted organic C in the POM-free soil was classified as humin.
Statistical analysis
Means and SEs were calculated for all soils combined. Mean comparisons for these values were made at α≤0.05 by Analysis of Variance with PROC MIXED using Restricted Maximum Likelihood after meeting the assumptions for normality and homogeneity of variance for the residuals using SAS software, ver. 8 (SAS Institute 1999). Log transformations were made to meet the assumptions as needed.
Results
Sequential extraction from POM and POM-free soil resulted in the isolation of six major OM pools: two from POM—(1) POM remaining after chemical extractions (POM), (2) glomalin from POM (POM-glomalin)—and four from POM-free soil—(3) glomalin, (4) HA, (5) FA, and (6) insoluble humin (carbon remaining in the residual soil). Although HA and FA were extracted from POM, amounts were below the threshold of the assays used for quantification or were much less than the six major pools (gravimetric weight <0.4 g kg−1) and were not examined further.
Total and immunoreactive protein assays showed that proteinaceous material was present in POM-glomalin, glomalin, and HA. The total protein values for FA were below the assay detection limits (<1.25 μg/200 μl). There was significantly more total protein in glomalin than in all other pools for all six soils combined (Table 1). In the Sampson soil, POM-glomalin and glomalin had similar total protein concentrations, but these values were greater than in HA. Immunoreactivity of proteinaceous material averaged 14% in POM-glomalin, 25% in glomalin, and 18% in HA (data not shown). The weight proportion of proteinaceous material in POM-glomalin was ca 100%, whereas only 25% of the glomalin was proteinaceous (Table 1).
POM and glomalin had the highest weights of material (Table 1) and the highest proportions of extracted C (Fig. 2), even though HA had significantly higher percentages of C, N, and H than any other pool (Table 2). The proportion of C in glomalin was highest in the Baltimore A and Haxtun soils and lowest in the Georgia soils (Pacolet and Cecil) (Fig. 2). Combined glomalin and POM-glomalin accounted for 15% of the total soil organic carbon (SOC). The ranking for C contributed by other pools was 12% for POM, 2% for HA, and 1% for FA. On average, 42% of the SOC in the soils was not extractable and was classified as humin. The total C from the extractable pools and humin averaged 72.4% of the total organic C measured in the initial soil, indicating an average loss of 27.6% C during extraction and purification of these pools.
The proportion of C measured in each of the six SOM pools [POM (POM separated from soil and extracted for glomalin, HA, and FA), POM-glomalin (glomalin extracted from POM), and glomalin, HA, FA, and humin (in POM-free soil)] collected from six soils [Baltimore A and Baltimore B (MD), Sampson and Haxtun (CO), and Pacolet and Cecil (GA)]. Values in the center of each bar are the proportion of C in the six pools combined compared to the total C in each soil
Glomalin and POM also had the highest proportions of extracted N (Fig. 3). Similar to C values, the N proportion was highest in glomalin extracted from the Baltimore A site and Haxtun soils. The seven extracted pools only accounted for 12% of the total organic N in the original sample (POM, 3.3%; POM-glomalin, 0.8%; glomalin, 6.5%; HA, 0.9%; FA, 0.4%). The humin portion was only 19% of the total N, leaving 69% that was lost during extraction and purification.
The proportion of N measured in the five major SOM pools [POM (POM separated from soil and extracted for glomalin, HA, and FA), POM-glomalin (glomalin extracted from POM), and glomalin, HA, and FA] collected from six soils [Baltimore A and Baltimore B (MD), Sampson and Haxtun (CO), and Pacolet and Cecil (GA)]. Values in the center of each bar are the proportion of N in the five pools combined compared to the total N in each soil
Discussion
Organic matter fractionation by the physical and chemical methods employed in this study were generally specific in isolating eight SOC pools (POM, POM-glomalin, POM-HA, POM-FA, glomalin, HA, FA, and humin). All of these pools, except POM-HA and POM-FA, contained a measurable amount of material. The largest pools were humin, POM, and glomalin.
In examining these pools, distinct material was isolated that goes beyond an operational definition for each pool. POM was operationally defined as an insoluble fraction of partially decomposed plant debris separated by physical fractionation, but the definition of POM in this study was extended to include POM and POM-glomalin. FA was a distinct pool defined as light-colored acid- and alkaline-soluble material with no proteinaceous residues. HA was a dark-colored, alkaline-soluble, and acid-insoluble SOC pool that was soluble at RT, contained proteinaceous residues, and was ca 50% C. Glomalin was similar to HA (proteinaceous, dark-colored, alkaline-soluble, and acid-insoluble material) but was minimally soluble at RT and was 35 to 40% C.
Extraction of glomalin before HA extraction did not yield protein-free HA (across all soils, 55% of the weight in HA was proteinaceous and 8% was immunoreactive). Immunoreactivity indicates the presence of glomalin in the protein fraction extracted from soils using alkaline citrate at high heat, but previous work shows that values for total and immunoreactive protein may not be equivalent (Nichols and Wright 2004). Our values for percentage immunoreactive protein in glomalin, HA, and POM-glomalin were within the lower range of values obtained from other studies, where multiple 1-h extractions were required to remove glomalin from soils. Samples from Georgia pasture soils (n=192; similar to the Cecil and Pacolet soils included in this study) range from 19 to 76% immunoreactive glomalin (Franzluebbers et al. 2000). Glomalin extracted from a Weld silt loam soil (Akron, CO) under perennial grass (n=9) is 27 to 87% immunoreactive (Wright and Anderson 2000). Extraction conditions such as citrate concentration and length of exposure to heat at least partially controlled immunoreactivity (Wright and Upadhyaya 1996). In addition, subsequent treatment of citrate-extracted soil with NaOH to extract HA results in co-extraction of a “recalcitrant” glomalin pool, as indicated by ELISA values for HA (Nichols and Wright 2005), and a small amount of glomalin is soluble in an alkaline solution at RT (Wright and Upadhyaya 1996). Therefore, glomalin may be a proteinaceous contaminant in HA despite the low concentrations of total and immunoreactive protein.
The contamination of HA with glomalin was expected, but Nichols and Wright (2005) show that contamination, as reflected by total protein values, is greater when HA is extracted before glomalin. Also, the percentages of C, N, and H indicate that HA and glomalin pools are dominated by different compounds (Nichols and Wright 2005). The percentages of C, N, and H (Table 2) in this study also indicate that POM-glomalin and glomalin have a similar structure, but that this structure differs from HA.
Total protein values for glomalin from these six soils averaged 2.53 g kg−1 soil (Table 1) and are within the range of previously reported values for glomalin from temperate soils (Wright and Upadhyaya 1998). However, only about one-fourth of the gravimetric weight of glomalin from soil was proteinaceous, whereas almost all of the POM-glomalin from the Baltimore B site, Sampson, and Haxtun soils was proteinaceous (Table 1). This indicated that the extraction of glomalin from soil either co-extracted some other substance with similar solubility characteristics as glomalin (soluble at alkaline pH, not denatured at high temperature, and acid-insoluble after extraction), such as HA, or the extracted glomalin contained tightly bound, inorganic material. The lower percentage C values for glomalin (35% C) compared to POM-glomalin (40% C) (Table 2) suggested that it is unlikely that the co-extracted substances were organic, especially HA (53% C). In addition, NMR spectroscopy shows that glomalin does not have co-extracted or attached tannins (Rillig et al. 2001) and is composed of carbohydrates and hydrophobic amino acids (Nichols 2003, unpublished data).
Iron possibly contributes to high gravimetric weight and correspondingly low C values for glomalin from soil. Previous analysis of glomalin indicates a wide range of iron in glomalin from soils (0.8 to 8.8%) (Wright and Upadhyaya 1998). This is in contrast to lower values for iron in glomalin on hyphae collected from sterile pot cultures (ca 0.2%) (Nichols 2003). Iron content of glomalin is significantly and positively correlated with soil iron content and inversely related to percentage carbon in glomalin (Nichols and Wright 2005). Glomalin may accumulate iron over time in soil, and this characteristic could account for the resistance to decomposition and formation of stable complexes within soil aggregates (Nichols and Wright 2005).
In these six soils, the amount of C and N in all five of the extracted pools plus the nonextractable humin pool accounted for an average of 73% and 31% of the total C and N, respectively, across all soils (Figs. 2 and 3). The humin pool contributed about half of the C and N measured in these soils. Each of the extraction protocols contained steps where POM, carbohydrates, other proteins, and other C and N containing compounds may have been lost (Fig. 1). The dialysis procedure specifically used for FA and glomalin were not optimized to retain low molecular weight compounds. Also, frequently following centrifugation (i.e., the centrifugations following acidification and flocculation of glomalin, dialysis of both glomalin and FA, and the KCl and HCl–HF treatments of HA), either the supernatant or the pellet (both of which may have contained some organic compounds and inorganic N) was discarded. In addition, losses of POM, glomalin, HA, and FA may occur during decanting of extract solutions. Finally, some organic matter pools, such as microbial biomass or hot-water extractable carbohydrate-C, were not measured. Therefore, the mass balance of C and N in the original soil was not expected to balance with the SOM pools collected here. However, these pools contained about 75% of the total SOC.
Conclusions
Eight operationally defined SOM pools (POM, POM-glomalin, POM-HA, POM-FA, glomalin, HA, FA, and humin) were isolated in this study. Two of these pools (POM-HA and POM-FA) were immeasurable or very small compared to the other six pools. One pool (humin) was defined only as the insoluble carbon remaining in the soil after all extractions. In the remaining five pools:
-
(a)
Solubility characteristics, protein concentrations, and percentages of C, N, and H showed that POM, POM-glomalin, HA, and FA represent distinct pools.
-
(b)
Glomalin accounted for 13% of the total SOC and was approximately nine times larger than HA.
-
(c)
POM contributed 12% of the total SOC and a pool of glomalin was isolated from the POM fraction (POM-glomalin) that represented 2% of the total SOC.
-
(d)
Glomalin, POM, and HA were the largest pools of N extracted in this study and contained 7, 3, and 1% of the total soil N, respectively.
References
Burdon J (2001) Are the traditional concepts of the structures of humic substances realistic? Soil Sci 166:752–769
Clapp CE, Hayes MHB (1999) Characterization of humic substances isolated from clay- and silt-sized fractions of a corn residue-amended agricultural soil. Soil Sci 164:899–913
Franzluebbers AJ, Wright SF, Stuedemann A (2000) Soil aggregation and glomalin under pastures in the southern Piedmont USA. Soil Sci Soc Am J 64:1018–1026
Gale WJ, Cambardella CA (2000) Carbon dynamics of surface residue- and root-derived organic matter under simulated no-till. Soil Sci Soc Am J 64:190–195
Hayes MHB, Clapp CE (2001) Humic substances: considerations of compositions, aspects of structure, and environmental influences. Soil Sci 166:723–737
Hayes MHB, Graham CL (2000) Procedures for the isolation and fractionation of humic substances. In: Davies G, Ghabbour EA (eds) Humic substances seminar IV. The Royal Society of Chemistry, Cambridge, pp 91–109
MacCarthy P (2001) The principles of humic substances. Soil Sci 166:738–751
Miller RM, Reinhardt DR, Jastrow JD (1995) External hyphal production of vesicular–arbuscular mycorrhizal fungi in pasture and tallgrass prairie communities. Oecologia 103:17–23
Nichols KA (2003) Characterization of glomalin—a glycoprotein produced by arbuscular mycorrhizal fungi. Dissertation, University of Maryland, College Park, MD
Nichols KA, Wright SF (2004) Contributions of soil fungi to organic matter in agricultural soils. In: Magdoff F, Weil R (eds) Functions and management of soil organic matter in agroecosystems. CRC Press, Boca Raton, FL, pp 179–198
Nichols KA, Wright SF (2005) Comparison of glomalin and humic acid in eight native US soils. Soil Sci 170(12):985–997
Rillig MC, Wright SF, Nichols KA, Schmidt WF, Torn MS (2001) Large contribution of arbuscular mycorrhizal fungi to soil carbon pools in tropical forest soils. Plant Soil 233:167–177
SAS Institute (1999) SAS user’s guide: Statistics. Release 8.0. SAS Inst., Cary, NC
Schnitzer M, Schuppli P (1989) The extraction of organic matter from selected soils and particle size fractions with 0.5M NaOH and 0.1M Na4P2O7 solutions. Can J Soil Sci 69:253–262
Schulten H-R, Schnitzer M (1997) Chemical model structures for soil organic matter and soils. Soil Sci 162:115–130
Wolf DC, Legg JO, Boutton TW (1994) Isotopic methods for organic matter dynamics. In: Weaver RW et al (eds) Methods of soil analysis, part 2. Microbiological and biochemical properties. SSSA Book Series, vol 5. Madison, WI, pp 865–906
Wright SF (1999) A fluorescent antibody assay for hyphae and glomalin from arbuscular mycorrhizal fungi. Plant Soil 226:171–177
Wright SF, Anderson RL (2000) Aggregate stability and glomalin in alternative crop rotations for the central Great Plains. Biol Fertil Soils 31:249–253
Wright SF, Franke-Snyder M, Morton JB, Upadhyaya A (1996) Time-course study and partial characterization of a protein on hyphae of arbuscular mycorrhizal fungi during active colonization of roots. Plant Soil 181:193–203
Wright SF, Upadhyaya A (1996) Extraction of an abundant and unusual protein from soil and comparison with hyphal protein of arbuscular mycorrhizal fungi. Soil Sci 161:575–585
Wright SF, Upadhyaya A (1998) A survey of soils for aggregate stability and glomalin, a glycoprotein produced by hyphae of arbuscular mycorrhizal fungi. Plant Soil 198:97–107
Wright SF, Upadhyaya A (1999) Quantification of arbuscular mycorrhizal fungi activity by the glomalin concentration on hyphal traps. Mycorrhiza 8:283–285
Wright SF, Upadhyaya A, Buyer JS (1998) Comparison of N-linked oligosaccharides of glomalin from arbuscular mycorrhizal fungi and soils by capillary electrophoresis. Soil Biol Biochem 30:1853–1857
Acknowledgements
This study is part of a dissertation submitted by K.A.N. to satisfy requirements for a Doctorate of Philosophy at the University of Maryland. The US Department of Energy, Office of Science (DE-AI02-99ER20352) is acknowledged for partial support of this study. We thank C. Johnson and A. Franzluebbers for soils, L. McKenna and L. Jawson for technical assistance, and K. Dzantor, M. Liebig, J. Pikul, R. Weil, and S. Wuest for comments on the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nichols, K.A., Wright, S.F. Carbon and nitrogen in operationally defined soil organic matter pools. Biol Fertil Soils 43, 215–220 (2006). https://doi.org/10.1007/s00374-006-0097-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00374-006-0097-2