Abstract
Consistent use of mulches over several years can provide significant N to avocado. Study of a 3-year-old Ventura, California, avocado orchard mulched annually for 3 years with 12–14 Mg ha−1 chipped eucalyptus showed that total N in the mulched soil was double that of the control. Mulched intact soil cores released 53 kg ha−1 more N annually than control treatments. A litterbag study showed that net mineralization of the applied mulch commenced approximately 8.5 months following application. Mulched soils tended to be warmer and moister than control soils and temperatures varied less. Laboratory incubations of mulch and soil layers showed that net mineralization rates (mg kg−1 day−1) were greatest in the lowest decomposed mulch layer, but that more N mineralized overall (g m2) in the soil due to its greater density.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In California, organic mulching is a practice that is becoming important in new avocado plantations (Faber et al. 1996) due to both the availability of large volumes of municipal organic wastes (Anonymous 2000) and to efforts to control Phytophthora root rot with mulch (Downer 1998; Menge et al. 1999). Past scientific studies of avocado have considered mulching for Phytophthora control (Downer 1998; Menge et al. 1999) as well as the effect of mulching on soil water (Bredell et al. 1970), soil temperatures (Gregoriou and Rajkumar 1984), weed control, and nutrients (Broadbent et al. 1989; Embleton and Jones 1956). No N mineralization studies on mulched avocado soils have been reported, however. Nitrogen is the most limiting nutrient for avocado growth and fruit production (Goodall et al. 1978). Chemical fertilizers are the most common source of N added by growers, but there are indicators that some organic mulches can supply part or all of the required N through the mineralization process (Menge et al. 1999). Many different yardwaste materials, including leaves and grasses, wood wastes, and prunings, are available for use as mulches in avocado and citrus in Southern California (Anonymous 2000). Where avocado is grown, eucalyptus is a prominent part of the yardwaste stream (Faber et al. 1996). The effects of this organic material applied as mulch on soil N mineralization is unknown. Studies with eucalyptus litter in forests and eucalyptus plantations have shown that N immobilization is a major process (Adams and Attiwill 1986; Aggangan et al. 1999) and that the N mineralization rates are usually low (O’Connell and Rance 1999; Polglase and Attiwill 1992; Wang et al. 1998). In Southern California, a 4-year study by Downer (1998) showed that eucalyptus yardwaste mulch significantly increased the total N contents in the leaves and soil of young avocado trees, indicating that significant N may be released as a result of mulching. However, the N mineralization rates needed to incorporate mulches into overall nutrient budgets remain unknown.
One of the long-term effects of mulching is the formation of a visible series of mulch layers with different degrees of decomposition. Forest ecosystem studies have found that forest floor litter layers may have different net N mineralization rates, some of which can be higher than those of soil layers (Federer 1983; Persson and Wiren 1995; Tietema et al. 1992). Fresh eucalyptus layers with high C/N ratios (Attiwill and Leeper 1987) tend to immobilize soil N (Aggangan et al. 1999), while litter layers in advanced states of degradation and much lower C/N ratios may release it (Ashton 1975; O’Connell 1987). The influence of mulch and soil layers on the overall N mineralization process in mulched avocado soils has not been quantified. The objectives of this study were: (1) to determine the long-term effects of an established eucalyptus yardwaste mulch system on net N mineralization rates under avocado orchard field conditions; (2) to determine under laboratory conditions the net N mineralization rates in the individual mulch and soil layers under this orchard; and (3) to describe the magnitude and timing of N immobilization and release from fresh eucalyptus yardwaste mulch applied to the above-mentioned system.
Materials and methods
Two experiments were conducted to explore net N mineralization in mulch and soil layers of a commercial avocado orchard. The first experiment considered mineralization from mulched and unmulched soils under field conditions while the second was conducted on the same soils under laboratory conditions to investigate the individual contributions of different mulch and soil layers. Net N mineralization includes the processes of N immobilization, N mineralization, and, in the field, plots the deposition of atmospheric N, and N in irrigation water. The NO3−-N content in the irrigation water was approximately 1.5 mg l−1. Differences between mulched and control treatments may be used to estimate mineralized mulch N.
Field experiment
The field study was located in Somis, California, in a 3-year-old “Hass” avocado orchard growing on Duke 7 rootstock and irrigated with microsprinklers. The soil was a Rincon silty loam (fine, montmorillonitic, thermic Mollic Haploxeralfs) infested with Phytophthora root rot. Eucalyptus yardwaste is readily available in the region. At the time of the study, each tree had been mulched during each of the three previous years with 0.25–0.38 m3 per tree (12–14 Mg dry weight ha−1) of eucalyptus yardwaste (leaves and shredded branches) and very lightly fertilized (3.6 kg N ha−1 year−1). The mulch was located beneath the canopy, and was first applied early in the summer of 1997. The mulch contained 0.972% N, 49.49% C (for a C/N ratio of 51), and 23.6% lignin (technically the acid insoluble carbon fraction). A completely random design was used with ten mulched and ten unmulched trees as experimental units.
An intact soil core methodology was used to measure soil net N mineralization (DiStefano and Gholz 1986; Hubner et al. 1991). This technique includes the use of PVC tubes and resins; the tubes are used to prevent uptake of mineral N by roots (Raison et al. 1987), and resins to trap the leached N in the bottom of the tubes (Hubner et al. 1991). Mulched and unmulched plots were prepared similarly, but in the mulched plots the mulch layers were first carefully removed and soil samples were taken at 0- to 25-cm depth at the beginning of the experiment (September 1997) from each tree. A sharpened PVC tube (25×7.5 cm diameter) was inserted into the soil. The tube was drawn out immediately and a 1-cm-thick soil layer was scraped from the bottom of the soil core. This free space was then filled with a fine-mesh nylon bag containing 10.28 g dry weight anion-cation exchange resins plus 10 g glass beads (0.3 cm diameter). Of the total resin dry weight, 8.5 g was anionic (1-X8 BioRad Lab) and the rest cationic (50W-1X BioRad Lab). The glass beads were included to get a resin bed of sufficient thickness and to allow a homogeneous resin distribution in the bags (Hubner et al. 1991). To reduce microbial activity in the resin, 5% of the resin exchange capacity was saturated with a solution of CuSO4 (Bollag and Barabasz 1979; Giller et al. 1998). The nylon bag was fixed into the tube with a 0.4-cm-thick PVC ring. In the mulched plots, the original mulch layers were placed on top of the PVC tubes and they were left open to the atmosphere to catch the N leached from the mulch layers in the soil cores. On 27 January 1998 and 22 April 1998 additional sets of tubes were inserted in the soil, and the previous tubes were removed for chemical analysis. The third and final set of tubes was collected on 25 August 1998. Bulk 0- to 25-cm-depth soil samples were taken each time a new set of PVC tubes were installed into the soil.
After collection, the tubes were transported to the laboratory. The resin bags were washed with distilled water and dried at room temperature and the resins were separated from the glass beads. The soil cores were weighed and samples were taken to determine moisture content and bulk density. Soil inorganic N (NH4+-N and NO3−-N) was extracted by shaking 4 g soil with 40 ml 2 M KCl for 1 h and then filtering the suspension (Whatman no. 42). Resin NH4+-N and NO3−-N were removed by shaking 4 g dry weight with 150 ml 2 M KCl for 12 h. A second and third extraction with the same volume of 2 M KCl was made by shaking the samples for 3 h each time. Ammonium and NO3− concentrations were determined colorimetrically with a Technicon Auto Analyzer. Total N was measured with an automatic analyzer (NA 1500 Carlo-Erba, Milan, Italy).
Nitrogen mineralized (NH4+-N + NO3−-N) in the mulched plots was calculated as follows (Hubner et al. 1991)
where Nmin is the N mineralized in the mulched plot (kg ha−1), Nsc, mineral N in the soil core at the end of incubation period (kg ha−1), Nres, mineral N in the resin at the end of incubation period (kg ha−1), and Nin is the initial mineral N in bulk soil at the beginning of the incubation period (kg ha−1).
The same procedure was followed with the soil and resin samples of the unmulched plots. Rates of NO3−-N, NH4+-N and net N mineralization in kg ha−1 d−1 were calculated by dividing the estimated amount of N over the days of each incubation period according with the procedure reported elsewhere (Adams and Attiwill 1986; Goncalves and Carlyle 1994). Because the objective of the study was to estimate the available N for avocado trees no other N factors such as denitrification were included.
Soil moisture and temperature were measured to consider their possible influence on N mineralization. Soil temperature was measured at 10-cm depth with Onset Stowaway Tidbit XT temperature loggers programmed to record hourly temperatures. Soil moisture was measured gravimetrically at 0- to 25-cm soil depth and then the volumetric water content (θv, g/m3) was calculated as the product of the gravimetric water content (g/g) and the soil bulk density (g/cm3). Bulk density was determined by drying 0- to 25-cm soil cores of known volume at 110°C. Statistically significant differences were evaluated with Student’s t test (Steel and Torrie 1980).
A litterbag method was used to describe the magnitude and timing of N immobilization and release from fresh eucalyptus yardwaste mulch (McClaugherty et al. 1985); 10 g samples of fresh mulch material were oven dried at 65°C for 48 h and placed inside 20×13-cm (0.026 m2) nylon mesh bags with 1.0-mm openings. Six bags were buried halfway through the mulch per tree on 9 October 1997. One bag per tree was collected on 19 December 1997, 3 March 1998, 28 April 1998, 18 June 1998, 8 October 1998 and 17 May 1999. Collected bags were transported in a cooler to the laboratory where they were air dried and cleaned with a fine brush to remove soil and debris. All mulch sample weights were determined after oven drying the samples at 65°C for 48 h and measurements were adjusted to report data on an ash-free basis. It was assumed that the ash content in the mulches remained constant over time. Samples were ground in a Wiley mill using a 60 mesh and then analyzed for total N and C with an automatic analyzer (Na 1500 Carlo-Erba, Milan, Italy). Initial mulch material was also analyzed for acid insoluble compounds (lignin and other recalcitrant compounds) as reported by Geng et al. (1993).
Laboratory experiment
Mulch and soil samples were collected from the field plots. Mulch material was collected from a 19×19 cm (361 cm2) area under the canopy of ten mulched trees. Each sample was collected at a point equal in distance and orientation to the bole of the mulched tree. Mulch samples were separated by hand into top (T), middle (M) and lower (L) layers. The T layer was 6 cm thick and was composed of fresh material that had been applied 1 month previously. The M layer was approximately 5.6 cm thick and corresponded to partially decomposed mulch from previous years. Finally, the L layer, which was in contact with the topsoil layer, was approximately 2.1 cm thick, more than 2 years old and significantly humified. Soil samples were collected from the same location as the mulch samples using a sharpened 7.62-cm-diameter PVC tube. Corresponding soil samples were also collected from unmulched trees. The samples were placed in plastic bags and transported on ice to the laboratory. Once in the laboratory, soil cores were divided in three sections (0–5, 5–10 and 10–25 cm). Soil and mulch sub-samples were air dried and analyzed for initial concentrations of inorganic N (NH4+-N + NO3−-N) using a Technicon AutoAnalyzer. Fresh mulch samples were passed through a 5-mm mesh by cutting the organic materials where required, while the mineral soil was sieved (<2 mm) to remove roots and stones (Tietema et al. 1992). Portions of 6 g dry weight of T and M mulch, 10 g L and 20 g mineral soil were placed in 120-ml covered plastic cups, each with a 5-mm diameter hole in its lid for gas exchange (Persson and Wiren 1995). Forty cups per layer were prepared and incubated in the dark at 25°C. The water content of the samples was kept at 60% of water-holding capacity by weighing the cups every day. One hundred percent water-holding capacity was defined as the water content of saturated samples allowed to drain for 12 h in the plastic cups (Persson and Wiren 1995). Every 12 days ten cups from each layer were removed from the incubator, extracted with 2 M KCl and analyzed for mineral N (NH4+-N + NO3−-N). The complete incubation period was 36 days.
Mineralized N (NH4+-N + NO3−-N) was estimated as the difference between the inorganic N (NH4+-N + NO3−-N) at the end of the incubation period minus the initial level. The net N mineralization rate (mg kg−1 day−1) was calculated by dividing the mineralized N by the number of days of incubation. The amount of N mineralized (g m−2) from each layer was estimated using its inorganic N concentration and its corresponding bulk density.
Statistical differences between treatments in the different soil layers, as well as differences between mulch and soil layers in the mulched plots were calculated by one-way variance analysis. Means were separated by Fisher’s protected LSD after significant ANOVA (Garcia-Diaz and Phillips 1995).
Results
Field experiment
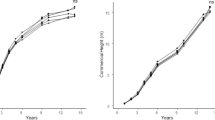
At the beginning of this study, total N concentrations in soil samples from the mulched experimental plots were twice as high as in the unmulched plots. Total N averaged 0.20% (0.04%) in the mulched plots and 0.10% (0.02%) in the control plots. Values in parentheses represent one standard deviation. Corresponding organic C values were 2.74% (0.78%) and 0.95% (0.28%), respectively. Values for both total N and organic C differed significantly between the treatment and control plots at the P <0.001 probability level (t test, n =10). The C content was close to three times greater in the mulched plots so that overall C/N ratios were also significantly greater in the mulched plots. In the mulched plots, the total N mass at 0- to 25-cm soil depth from the three previous years of mulching was 1.21 Mg ha−1 and it increased to 1.34 Mg ha−1 when the fresh layer applied within the year was taken into account (Table 1). The control plots contained only 0.65 Mg ha−1 total N indicating mulching more than doubled the soil N pool. Initial C/N ratios were also higher in mulched plot layers less than 10 cm deep. During the course of the experiment the soil moisture content was significantly affected by mulching (Fig. 1). An average water content of 28.8 m3/m3 was measured in mulched plots compared to 24.4 m3/m3 in the control plots. Weekly minimum and maximum soil temperatures measured at 10-cm soil depth were also significantly modified by mulching (Fig. 2). The most affected was the weekly minimum temperature, which had a mean during the entire study of 15.5°C in mulched soil and 12.4°C in the control soil. The maximum temperature was significantly greater in control soil plots with a mean of 18.8°C while in mulched soil plots the mean was 17.4°C. The mean temperature in the mulched plots, at 16.4°C, was also significantly greater than in the control plots where temperatures averaged 15.6°C.
N mineralization
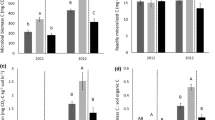
In soils of both mulched and control treatments, the lowest net N mineralization rates were measured during the first incubation period while the highest rates were observed in the last, which occurred during the summer. Relative to the control, mulching resulted in significantly greater N mineralization during each of the incubation periods particularly during the last sampling period. Nitrate was responsible for the majority of the overall increase in mineral N in both the mulched and control plots (Table 2), however, the relative increase of treatment over the control was greater for NH4+-N than NO3−-N. When considering rates totaled over all three incubation periods, net N mineralization rates in mulched plots were 0.42 kg ha−1 day−1, which was 56% higher than in the control plots. The total N mineralized in the mulched plots during the present study averaged 147 kg N ha−1 while for the control plots this value was 94 kg N ha−1, indicating that mulching released approximately 53 kg ha−1 additional N to the soil.
Fresh mulch N dynamics
The total N dynamics in the fresh mulch material are shown in Fig. 3 along with corresponding changes in the mulch C/N ratio. During the first 2 months the mulch N content did not change. However, by the fifth month (second sampling date) the N content of the mulch had increased by 21%. After this, the N content steadily declined. The N originally contained in the mulch began to be released after 8.5 months by which time 40% of original mulch weight had been lost. The total amount of the original N that was released during the 19 months of this study was 70 kg N ha−1 and the maximum amount of N that was immobilized in the mulch was 28 kg N ha−1. The C/N ratio followed a descendent trajectory during the first 8.5 months passing from 50 (initial value) to around 24, where it remained for the rest of the study. The C/N ratio at the time when N release commenced was near 30.
Laboratory experiment
Nitrogen mineralized at different rates in the mulch and soil layers of mulched plots (Fig. 4). At 12 days, the top mulch layer (T) presented a high immobilization rate. The humified mulch layer (L) also immobilized N, but at a much lower rate, while N changed little in the rest of layers. At 24 and 36 days, L showed the higher N mineralization rates followed by the 0- to 5-cm soil layer and M. The T layer started to mineralize N at 36 days. By the end of this study all layers mineralized N. Over the whole incubation period the L layer had the highest N mineralization rate with 6.96 mg N kg−1 day−1 while T had a negative mineralization rate of −0.864 mg N kg−1 day−1 (Table 3). However, when data were expressed on an area basis (g m−2), which adjusts for bulk density, significantly more mineralized N was observed in the soil layers, particularly in the lower layer relative to mulch layers. As in the field experiment, mulching increased net N mineralization rates significantly, although only in the 0- to 5-cm soil layer. The rate in this soil layer averaged 2.01 mg kg−1 day−1 which represented an increase of 50% over the control soil rate.
Discussion
Mineralization in both the mulched and control soils was more rapid in the laboratory than in the field, presumably due to the increase in substrate bioavailability after sieving and to the maintenance of favorable moisture and temperature conditions (Hassink 1992; Piccolo et al. 1994). In both the field and laboratory studies, significant increases in net N mineralization rates were found in soils mulched with eucalyptus yardwaste relative to the control soils. In the field study, the N mineralized in the mulched plots was 56% greater than the amount measured in the control plots while in the laboratory study this difference was 23%. The smaller difference between the treatment and the control in the laboratory study may be attributable to N assimilation by decomposer microorganisms exploiting C liberated during sample preparations. Microbial N demand would be greater in the treatment plots relative to the control plots due to the higher C/N ratios associated with the mulch.
The amounts of N mineralized in the mulched plots were higher than those reported in N mineralization studies of eucalyptus litter in forest and plantations (O’Connell and Rance 1999; Polglase and Attiwill 1992; Wang et al. 1998) This was due primarily to the higher amount of organic material applied in the present study. Even though eucalyptus yardwaste was applied to the soil surface, it affected the total N content in the 0- to 25-cm soil profile. This was likely due to the downward movement of particulated organic matter by earthworms which were observed in great numbers in the mulched soils (Edwards and Bohlen 1996). Tian et al. (1997) also reported significant increases in earthworm populations following organic mulch applications. Fine root proliferation may also have increased total N under the mulches. Fine root growth and decomposition was accelerated in the mulched plots especially in the top soil layers (Downer et al. 2001; Valenzuela-Solano 2003).
A total of 147 kg ha−1 mineral N accumulated in the mulched plots during the study, an amount exceeding the 126 kg ha−1 fertilizer rate recommended for 5-year-old avocado trees growing in Southern California (Goodall et al. 1978). The corresponding amount in the control plots was 94 kg ha−1, which would meet the needs of trees up to 4 years old (63 kg ha−1). These results show that in addition to the effects eucalyptus yardwaste mulch may have on the control of avocado root rot (Downer 1998; Menge et al. 1999), it may also significantly increase the N available for tree growth and should be considered in a comprehensive N fertilization program. However, it is important to point out that the supply of N through mulch application does not occur immediately. The soil used in the present study was mulched during the three previous years with eucalyptus yardwaste at rates that varied from 12 to 14 Mg ha−1, which approximately doubled the total (organic and inorganic) N pool relative to the control (Table 1). In the fresh eucalyptus mulch study, the original N contained in the tissues began to be released between 5 and 8.5 months after the beginning of the study (Fig. 3). After 8.5 months, 20% of the N in the fresh mulch had disappeared, a value that increased to 30% after 12 months, and 50% after 19 months. It is not likely that mulching with fresh eucalyptus yardwaste will contribute significantly to soil available N until the material has decomposed for more than 1 year under field conditions. The period of time required in the present study for the onset of N release is shorter than some reported for eucalyptus litter from Australia and New Zealand (Guo and Sims 1999; O’Connell 1988; Wedderburn and Carter 1999). O’Connell (1988) and Wedderburn and Carter (1999) found that N release was limited by the initial lignin/N ratio of eucalyptus litter. Initial lignin/N ratios in those studies varied between 63 and 228. In this study the initial mulch lignin/N ratio was 24, which probably did not affect the decay rate and subsequent N release.
Moisture and, to a lesser degree, temperature may have influenced N mineralization. Indeed, water content was always significantly higher in mulched plots relative to the control (Fig. 1), and both mean and especially minimum soil temperatures remained significantly higher during the fall and winter months in mulched plots (Fig. 2), which might have enhanced microbial decomposition and N mineralization.
The application of organic mulches has an important long-term effect on the distribution of organic matter and N in the field, which modifies the soil N mineralization pattern. In the plots used for the field study, different layers with contrasting degrees of decomposition were observed in the mulch above the soil surface as a result of the three previous years of annual mulch applications. The incubation under laboratory conditions of these mulch layers, along with soil layers, showed the possible role that each of these layers might have had on the N mineralization process in the field. The humified mulch layer (L), with its relatively high N content and low C/N ratio (Table 1), mineralized N at significantly higher rates (Table 3) than the other layers. These results are consistent with reports from forest studies where humified forest floor layers commonly have higher rates of net N mineralization than soil layers (Federer 1983; Persson and Wiren 1995; Tietema et al. 1992). However, due to its low density and shallow depth, the relative contribution of L to the total amount of N mineralized (g m−2) in the mulched plots was low (about 9%) compared to the soil layers. Contributions from the M layer, while positive, only amounted to about 5%. Table 3 shows that the N contributions from the mulched and control soil layers were not significantly different and that differences in the total mass of mineralized N may be attributed to losses from the L and M layers. Mulched and control soil N mineralization rates appeared to differ significantly in the field experiment, however (Table 2). In the field experiment, the PVC tubes used to incubate the soil cores were left open to the atmosphere and N released from M and L mulch layers may have been leached by irrigation water into the soil. Eucalyptus mulches appear to contribute significant mineral N after a year of decomposition and should be considered as a part of comprehensive nitrogen management programs.
References
Adams MA, Attiwill PM (1986) Nutrient cycling and nitrogen mineralization in eucalyptus forests of south-eastern Australia. II. Indices of nitrogen mineralization. Plant Soil 92:341–362
Aggangan RT, O’Connell AM, McGrath JF, Dell B (1999) The effects of Eucalyptus globules Labill. leaf litter on C and N mineralization in soils from pasture and native forest. Soil Biol Biochem 31:1481–1487
Anonymous (2000) California waste diversion status. Publication number 530-99-007. California Integrated Waste Management Board, Sacramento
Ashton DH (1975) Studies of litter on Eucalyptus regnans forests. Aust J Bot 23:413–433
Attiwill PM, Leeper GW (1987) Forest soils and nutrient cycles. Melbourne University Press, Carlton
Bollag JM, Barabasz W (1979) Effect of heavy metals on the denitrification process in soil. J Environ Qual 8:196–201
Bredell GS, Green GC, Smart G, Koen TJ (1970) Mulching of avocados. S Afr Citrus J 439:7–11
Broadbent P, Trochoulias T, Baigent DR, Abbot TS, Dettmann EB (1989) Effect of soil management on avocados in a Krasnozem soil. Sci Hortic 38:87–104
DiStefano JF, Gholz HL (1986) A proposed use of ion exchange resins to measure nitrogen mineralization and nitrification in intact soil cores. Commun Soil Sci Plant Anal 17:989–998
Downer AJ (1998) Control of avocado root rot and Phytophthora cinnamomi Rands in mulched soils. PhD dissertation, University of California, Riverside
Downer AJ, Menge JA, Pond E (2001) Association of cellulytic enzyme activities in eucalyptus mulches with biological control of Phytophthora cinnamomi. Phytopathology 91:847–855
Edwards CA, Bohlen PJ (1996) Biology and ecology of earthworms, 3rd edn. Chapman & Hall, London
Embleton TW, Jones WW (1956) Manure as source of nitrogen. Studies of tilled and nontilled citrus and avocado orchards show manure to be efficient in supplying nitrogen to tilled soil. Calif Agric 10:14–15
Faber BA, Downer AJ, Sakovich NJ, McGiffen ME, Cudney D, Mills SA (1996) Yardwaste mulches in avocado and citrus orchards. Technical report. University of California Cooperative Extension, Riverside, Calif.
Federer CA (1983) Nitrogen mineralization and nitrification: depth and variation in four New Zealand forest soils. Soil Sci Soc Am J 47:1008–1014
Garcia-Diaz A, Phillips DT (1995) Principles of experimental design and analysis. Chapman & Hall, London
Geng X, Pastor J, Dewey B (1993) Decay and nitrogen dynamics of litter from disjunct, congeneric tree species in old-growth stands in northeastern China and Wisconsin. Can J Bot 71:693–699
Giller KE, Witter E, McGrath S (1998) Toxicity of heavy metals to microorganisms and microbial processes in agricultural soils: a review. Soil Biol Biochem 30:1389–1414
Goncalves JLM, Carlyle JC (1994) Modelling the influence of moisture and temperature on net nitrogen mineralization in a forested sandy soil. Soil Biol Biochem 26:1557–1564
Goodall GE, Embleton TW, Platt RG (1978) Avocado fertilization. Leaflet 2024. Division of Agricultural Science University of California, Riverside, Calif., pp 1–8
Gregoriou C, Rajkumar D (1984) Effects of irrigation and mulching on shoot and root growth of avocado (Persea americana Mill.) and mango (Mangifera indica L.). J Hortic Sci 59:109–117
Guo LB, Sims REH (1999) Litter decomposition and nutrient release via litter decomposition in New Zealand eucalypt short rotation forests. Agric Ecosyst Environ 75:133–140
Hassink J (1992) Effects of soil texture and structure on carbon and nitrogen mineralization in grassland soils. Biol Fertil Soils 14:126–134
Hubner C, Redl G, Wurst F (1991) In situ methodology for studying N-mineralization in soils using anion exchange resins. Soil Biol Biochem 23:701–702
McClaugherty CA, Pastor J, Aber JD, Melillo JM (1985) Forest litter decomposition in relation to soil nitrogen dynamics and litter quality. Ecology 66:266–275
Menge JA, Faber B, Downer J, Crohn D (1999) Compost demonstration project Southern California. Use of yard trimmings and compost on citrus and avocado. California Integrated Waste Management Board, Sacramento, Calif.
O’Connell AM (1987) Litter dynamics in karri (Eucalyptus diversicolor) forest of south-western Australia. J Ecol 75:781–796
O’Connell AM (1988) Nutrient dynamics in decomposing litter in karri (Eucalyptus diversicolor F. Muell.) forest of south-western Australia. J Ecol 76:1186–1203
O’Connell AM, Rance SJ (1999) Predicting nitrogen supply in plantation eucalypt forest. Soil Biol Biochem 31:1943–1951
Persson T, Wiren A (1995) Nitrogen mineralization and potential nitrification at different depths in acid forest soils. Plant Soil 168–169:55–65
Piccolo MC, Neill C, Cerri CC (1994) Net nitrogen mineralization and net nitrification along a tropical forest-to-pasture chronosequence. Plant Soil 162:61–70
Polglase PJ, Attiwill PM (1992) Nitrogen and phosphorus cycling in relation to stand age of Eucalyptus regnans F. Muell. I. Return from plant to soil in litterfall. Plant Soil 142:157–166
Raison RJ, Conell MJ, Khanna PK (1987) Methodology for studying fluxes of soil mineral-N in situ. Soil Biol Biochem 19:521–530
Steel GD, Torrie JH (1980) Principles and procedures of statistics. McGraw-Hill, New York
Tian G, Kang BT, Brussard L (1997) Effect of mulch quality on earthworm activity and nutrient supply in the humid tropics. Soil Biol Biochem 29:369–373
Tietema A, De Boer W, Riemer L, Verstraten JM (1992) Nitrate production in nitrogen-saturated acid forest soils: vertical distribution and characteristics. Soil Biol Biochem 24:235–240
Valenzuela-Solano C (2003) Organic mulches. I. Decomposition and nitrogen mineralization in mulch and soil layers. II. Effects of eucalyptus mulch on avocado tree fine root growth. PhD dissertation, University of California, Riverside
Wang XJ, Smethurst PJ, Holz GK (1998) Nitrogen in surface soils of 1–2-year-old eucalypt plantations in Tasmania. Aust J Soil Res 36:17–29
Wedderburn ME, Carter J (1999) Litter decomposition by functional tree types for use in silvopastoral systems. Soil Biol Biochem 31:455–461
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Valenzuela-Solano, C., Crohn, D.M. & Downer, J.A. Nitrogen mineralization from eucalyptus yardwaste mulch applied to young avocado trees. Biol Fertil Soils 41, 38–45 (2005). https://doi.org/10.1007/s00374-004-0798-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00374-004-0798-3