Abstract
Animal manures may differ strongly in composition and as a result may differ in the emission of N2O following application to soil. An incubation study was carried out to assess the effects of type of mineral N fertilizer and manure, application technique and application rate on N2O emission from a sandy soil with low organic matter content. Fluxes of N2O were measured 30 times over a 98-day period. The total N2O emission from mineral N fertilizer ranged from 2.1 to 4.0% of the N applied. High emissions were associated with manures with high contents of inorganic N, easily mineralizable N and easily mineralizable C, such as liquid pig manure (7.3–13.9% of the N applied). The emission from cattle slurries ranged from 1.8 to 3.0% and that of poultry manures from 0.5 to 1.9%. The total N2O emission during the experimental period tended to increase linearly with increasing N application rate of NH4NO3 and liquid pig manure. The N2O emission from surface-applied NH4NO3 was significantly smaller than that following the incorporation of NH4NO3 in the soil. The N2O emission from pig manure placed in a row at 5 cm depth was significantly higher than from surface-application and other techniques in which manure was incorporated in the soil. The results show that modification of the composition and application technique may be tools to mitigate emission of N2O.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Fertilized soils are important sources of N2O. The global direct N2O emissions from fertilized agricultural soils are estimated at 0.9 (range: 0.18–1.6) Tg N year−1 for soils fertilized with mineral N fertilizers and 0.6 (range: 0.12–1.1) Tg N year−1 for soils fertilized with animal wastes (Mosier et al. 1998), but the uncertainties in these estimates are large. In soils, N2O is produced during the microbial processes of nitrification and denitrification and is controlled by many factors, including contents of mineral N, available C, O2, and the temperature (Granli and Bøckman 1994).
Fertilizer and manure type may affect N2O emission in several ways, i.e.: (1) the type of N (NO3 −, NH4 +, and organic N) which affects N2O production during nitrification and denitrification, (2) the presence of easily available C, which stimulates denitrification activity and O2 consumption in the soil following its application, and (3) effects on biological, chemical and physical soil processes because of changes in pH and the addition of other compounds (salt, water). Animal manures are a mixture of mineral N, easily mineralizable and resistant organic N and C compounds, salts and water. There are large differences in composition between animal manures, due to animal species and nutrition (Chadwick et al. 2000b; Van Faassen and Van Dijk 1987). In general, the degradability of organic C and N of cattle manure is smaller than that of pig and poultry manure (Chadwick et al. 2000b; Castellanos and Pratt 1981; Kirchmann 1991). We expect that animal manures with a relatively high content of mineralizable C result in higher N2O emissions after soil application than animal manures with more resistant C. In the current IPCC methodology the total amount of N applied is considered as the major factor controlling N2O emission from agricultural soils (Mosier et al. 1998). One single N2O emission factor of 1.25% of total N applied is used for all types of fertilizers and manures and application techniques. This suggests a linear relationship between the amount of N applied and the N2O emission. In daily agricultural practice, many different animal species are kept. These species have distinct feeding rations and are housed in specific stables. Many different manure storage facilities are in use and manure application techniques vary widely. Environmental concerns about NO3 − leaching and NH3 volatilizatioṅ led, and are still leading to, the adaptation of agricultural practices at the farm level including adoption of ecological farming principles, separate storage of urine and manure and changes in building designs for stables (Henkens and Van Keulen 2001). All these changes may impact on the composition of manures and the emissions of N2O. The formulation of hypotheses on effects of agricultural practices may eventually enable farmers to define adequate actions to reduce these emissions.
The N2O emission factor of the IPCC is based on a compilation of long-term (>1 year) monitoring experiments of N2O emissions from fertilized soils (Bouwman 1996). No effect of fertilizer type on N2O emission could be derived from this compilation which only included <25 long-term experiments. However, there are several studies which indicate that fertilizer type significantly affects the N2O emission. The N2O emission from NO3 −-containing fertilizers was much higher than that from NH4 +-containing fertilizers during wet conditions in grassland studies of Clayton et al. (1997) and Velthof et al. (1997). This was attributed to higher denitrification rates in the NO3 −-amended soil. During dry conditions, N2O emission was higher from NH4 + fertilizers and urea than from NO3 − fertilizers (Clayton et al. 1997). Relatively low N2O emissions (<1% of the N applied) have been found for animal manures applied to grassland (Chadwick et al. 2000a; Egginton and Smith 1986; Velthof et al. 1997). Combined application of manure and NO3 − fertilizer may enhance N2O production, because the addition of available C with manure may increase denitrification of the NO3 − applied with the fertilizer (Clayton et al. 1997; Stevens and Laughlin 2001). The effects of application technique of animal slurries on N2O emission are still unclear. In some studies no effects of the application technique are found (Velthof et al. 1997) and in other studies higher emissions are found for injected slurries than from surface-applied slurries (Flessa and Beese 2000).
Here we report on experiments that were set up to screen for the effects and identify possible measures to abate emissions of N2O. In incubations under controlled conditions we assessed the effects of the mineral fertilizers NH4NO3 and (NH4)2SO4 and a range of manures from pigs, cattle and poultry with strongly different chemical compositions on N2O emission from soil. The effects of application rate and the method of fertilizer and liquid manure application were also assessed. We hypothesize that the N2O emission factor (in percent of the N applied) increases with increasing N application rate, because the mole fraction N2O/N2 of the denitrification products increases with increasing NO3 − contents (Firestone et al. 1980). We also hypothesize that incorporation of fertilizer and manure will increase the N concentration in soil and lead to higher N2O emissions. This may to some extent be counterbalanced by higher consumption of N2O following production in the soil rather than on the surface of the soil.
Materials and methods
Treatments
Incubation experiments were carried out with 500 g moist soil in 1-l jars at 15°C. The sandy soil was taken from the 20- to 40-cm layer of an arable soil (Umbric Gleysol; FAO classification) in Wageningen in the Netherlands with potato as the previous crop. The ploughing depth of this soil was 30 cm. Thus, the used soil was a mixture of the nutrient-rich top soil layer and nutrient-poor sub soil layer. This soil sample strategy was chosen to avoid high background soil emissions of N2O when using only topsoil. Selected properties of this soil are: 4% of particles <2 μm, 8% of particles <16 μm, 0.60 g total N kg−1, 13.6 g total C kg−1, 46 mg water-soluble C kg−1, pHKCl 4.83, 1.4 mg NO3 −-N kg−1 and 1.3 mg NH4 +-N kg−1.
Nine manures were selected and include liquid and solid manures with both low and high C and N contents: (1) cattle slurry (traditional farming), (2) cattle slurry (organic farming), (3) slurry of young cattle, (4) liquid manure of fattening pigs (traditional farming), (5) liquid manure of fattening pigs (organic farming), (6) liquid manure of sows (including piglets), (7) layer manure (manure belt), (8) manure of broiler-breeder chickens (kept on fully slatted floor), and (9) manure of ducks (including straw). Cattle manure represents about 60% and pig manure about 20% of the total amount of produced manure N in the Netherlands. The manures were collected from farms in the Netherlands and analysed for dry matter, pH, total and mineral N content, and total C contents (Table 1). The pH of the manures was determined directly using a pH electrode, except for the relatively dry duck manure. The pH of the duck manure was determined in an extract of 5 g fresh manure and 25 ml demineralized water. Contents of C and N were analysed using the Dumas combustion method (Vario EL analyser; Elementar Analysensysteme, Hanau).
The effects of fertilizer and manure type, N application rate and application technique on N2O emission were examined in three experiments. In the first experiment, the effect of type of fertilizer and manure on the N2O emission was assessed. This experiment consisted of 12 treatments in two replicates: a control (unfertilized soil), and NH4NO3, (NH4)2SO4, and the nine animal manures at one application rate (100 mg N kg−1 soil). The fertilizers and manures were homogeneously mixed through the soil. In the second experiment, the effect of application rate on N2O emission was assessed. This experiment consisted of nine treatments in two replicates: a control (unfertilized soil), NH4NO3 applied at four rates (25, 50, 100, and 200 mg N kg−1 soil) and liquid manure of fattening pigs (traditional farming) applied at four rates (25, 50, 100, and 200 mg N kg−1 soil). These N application rates reflect the range of soil mineral N concentrations in the topsoil after application of fertilizer N and manure additions as practised in the Netherlands. The fertilizers and manures were homogeneously mixed through the soil. In the third experiment, the effect of application method on N2O emission was assessed. This experiment consisted of 11 treatments in two replicates: a control (unfertilized soil) and two fertilizers, i.e. NH4NO3 and liquid manure of fattening pigs (traditional farming) applied in five different ways, i.e.: (1) surface application, (2) placed at 5 cm depth, (3) placed at 10 cm depth, (4) placed in one row at 5 cm depth, and (5) homogeneously mixed through the soil.
Field moist soil was directly used for the incubation without further physical treatment, except that the initial soil moisture content was adjusted to 200 g kg-1 (about field capacity with an estimated WFPS of approximately 65%) at the start of all incubations. The amount of water added with the manures was treated as a manure effect and therefore, the soil moisture contents after application of the manures varied from 201 g kg−1 for broiler-breeder manure to 252 g kg−1 for liquid sow manure. The incubation jars were left open between successive N2O flux measurements and because of evaporation, the soil moisture content gradually decreased with time (see below). After 57 days, 170 ml water kg−1 soil was added to the control treatment to adjust the soil moisture content again to about field capacity. The same amount of water was added to the treatments with fertilizers and manures. This drying-wetting cycle was included in the experiments to include a period favouring mineralization and nitrification, followed by a period more favourable for denitrification.
Measurement of N2O emission
Fluxes of N2O were measured 30 times over a 98-day period. Changes in the concentrations of N2O in the headspace of the jars after closing the lid were determined with a photo-acoustic infra-red gas analyser (Innova Air Tech Instruments, Ballerup, Denmark) which was directly attached to the jars by two teflon tubes and needles through septa. The gas analyser was fitted with optical filters to measure selectively concentrations of N2O, CO2 and water vapour. To prevent a strong accumulation of CO2 in the headspace of the jars, which may interfere with the N2O measurement, a soda lime trap was installed in the tube at the inlet to the gas monitor. The soda lime adsorbed CO2, so that the CO2 concentration in the sampled air was always <200 μl l−1. The photo-acoustic analyser was calibrated for interference of CO2 and water vapour and the concentrations of N2O were automatically compensated for this interference. The concentration of N2O in the headspace was measured 1 h after closing the jar. The N2O flux was calculated assuming a linear relationship between the N2O concentration in the headspace and time which was checked several times (Velthof and Oenema 1995). The smallest detectable flux with the used incubation technique and the photo acoustic analyser was about 0.15 μg N2O-N kg−1 h−1.
The N2O emission factor, expressed in percentage of the N applied as fertilizer or manure was calculated as
where N2O-Ntreatment is the total N2O emission of the fertilizer/manure treatment (mg N kg−1), N2O-Ncontrol is the total N2O emission from the control treatment (mg N kg−1) and applied N is the amount of N applied via fertilizer or manure (mg N kg−1).
Calculations and statistical analyses
The total N2O emission for each jar during the experimental period was calculated by linear interpolation of the measured fluxes in time. Statistically significant differences in total N2O between the treatments of each experiment were analysed using ANOVA and least significant difference at 5% significance level. All statistical analyses were carried out using Genstat 5 (Genstat 5 Committee 1993).
Results
Composition of manure
The dry matter contents ranged from 19 g kg−1 for liquid manure of sows to 699 kg kg−1 for broiler-breeder manure (Table 1). The NH4 + contents ranged from 0.98 g N kg−1 product for liquid sow manure to 3.98 g kg−1 for broiler-breeder manure. There was no NO3 − in the manures. The NH4 + content expressed as percentage of total N ranged from 8% for broiler-breeder manure to 60% for the liquid manure of fattening pigs (traditional farming). Total C-to-N-ratio of the organic matter ranged from 5 for broiler-breeder manure to 18 for cattle slurry from organic farming. The amount of manure applied differed strongly when 100 mg manure-N kg−1 soil was applied; from 2 g kg−1 soil for broiler-breeder manure to 53 g kg−1 soil for liquid manure of sows.
Effects of type of fertilizer and manure on N2O emission
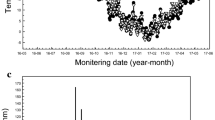
Fluxes of N2O during the first day after soil application were up to 5 times higher for the slurries and manures than for (NH4)2SO4 and NH4NO3 (Fig. 1). Fluxes of N2O from the slurries and manures decreased from day 1 and onwards and the flux from (NH4)2SO4 and NH4NO3 increased from day 1 and onwards (Figs. 1, 2). There were no differences in N2O flux between (NH4)2SO4 and NH4NO3 in the first week after N application. Fluxes of N2O were low in all treatments in the relatively dry period between about day 30 and day 57, and sharply increased for all treatments after the addition of water at day 57 (Fig. 2). At this moment, manures responded stronger than fertilizers, despite similar soil moisture contents, and these higher fluxes lasted for a longer period after the addition of water at day 57 than at the start of the experiment.
Time course of N2O emission from unfertilized soil and NH4NO3, (NH4)2SO4, cattle slurry (traditional farming), liquid fattening pig manure (traditional farming) and layer manure (upper figure) applied at 100 mg N kg−1 soil and the soil moisture contents in the control (lower figure). At day 57 water was added
Total N2O emission strongly varied between the fertilizers and manures (Table 2). The N2O emission were much higher from the three pig manures (ranging from 7.3 to 13.9% of the N applied) than from the cattle slurries (1.8–3.0% of the applied N) and poultry manures (0.5–1.9% of the applied N). The emission was higher from treatments with (NH4)2SO4 than with NH4NO3, though not statistically significant. Regression analysis indicated that both mineral N and total C contents of the applied manures were major factors controlling N2O emission (not shown). A multiple linear regression model with the mineral N and total C application as independent variables explained 54% of the variance in N2O emission from the manures (based on log-transformed values).
Effect of application rate on N2O emission
Fluxes of N2O increased after application of NH4NO3 and peaked in the period between 5 and 10 days (Fig. 3). Both flux magnitude and duration of the flux increased in the order 0<25<50<100<200 mg N kg−1. Fluxes increased after addition of water at day 57. Fluxes of N2O strongly increased for 1–3 days after application of liquid pig manure, but there was no clear effect of application rate on the initial N2O flux. Thereafter, the flux of N2O decreased to similar levels as the control (Fig. 3). The flux of the highest pig manure application (200 mg N kg−1) was much higher than that of the other application rates in the period between 20 and 45 days. The flux of N2O increased in the order 0<25<50<100<200 mg N kg−1 after the addition of water at day 57. The total N2O emission during the experimental period tended to increase linearly with increasing N application rate with an N2O emission factor for NH4NO3 of 2.1–2.8% and of liquid pig manure of 7.2–9.6% (Table 3).
Time course of the N2O fluxes from the control (0N) and (NH4)2SO4 (upper figure) and liquid fattening pig manure (traditional farming; lower figure) applied to soil at four application rates: 25 mg N kg-1 (25N), 50 mg N kg-1 (50N), 100 mg N kg-1 (100N), 200 mg N kg-1 (200N). At day 57 water was added (see Fig. 2)
Effect of application technique on N2O emission
The flux of N2O was much smaller after surface application of NH4NO3 than after incorporation of NH4NO3 in the soil (Fig. 4). Similar but less pronounced effects were found after the addition of water at day 57. The total N2O emission from NH4NO3 increased in the order surface applied<homogeneously mixed<placed at 5 cm depth≤placed at 10 cm depth≤placed in a row at 5 cm depth, but differences between the three placement treatments were not statistically significant (Table 4).
Effect of application technique on N2O emission from soil fertilized with NH4NO3 (upper figure) and liquid pig manure (traditional farming; lower figure). Note differences in scale of y-axes. At day 57 water was added (see Fig. 2)
In the first 1–3 days after application of liquid pig manure, there were no clear differences in N2O flux between the application techniques. In the period between day 3 and 11, the flux of surface-applied pig manure was higher than for the other application techniques. After addition of water at day 57, the flux was highest for row application of liquid pig manure at 5 cm. The total N2O emission was highest for row placement of the manure and lowest for placement of manure at 10 cm depth; the total emission of the other application techniques were intermediate (Table 4).
Discussion
Application of manure and fertilizer increases the amount of mineral N in soil and leads to higher emission of N2O. Most research so far provides emissions for animal manure as such without discriminating between a range of manure qualities that are found in agricultural practice. The results reported here suggest that N2O emission may be quite different depending on manure species and related quality and on manure management and handling. Most of these effects can be attributed to specific manure or fertilizer characteristics. Even though our results are from laboratory incubations using a soil with relatively low organic matter content and low pH, they may form the basis for designed testing and verification methods in field conditions and eventually lead to the formulation of agricultural practices with lower emissions of N2O. Such practices include the choice of mineral fertilizer (NH4 + or NO3 − based), choice and timing of manure application (arable or grassland and alone or in combination with mineral fertilizers) and manure storage and manure handling at the farm (separation of liquid from solid fractions in manure). The effects of type of manure and fertilizer on N2O emission may be affected by soil properties such as content of organic matter, texture and pH. For example, the effect of addition of available C with manures on denitrification and N2O emission is probably larger in soils with a low organic matter content than in soils with a high organic matter content. Possible interactions between manure/fertilizer composition and soil composition on N2O emission should be further tested.
The sharp peak in N2O emission during the first days after manure application (Fig. 1) is likely due to enhanced denitrification of soil NO3 − by the addition of easily degradable organic substrates. Chadwick et al. (2000a) also reported a peak in N2O emission in the first days after manure application. Stevens and Laughlin (2001) showed that the mole fraction of N2O produced by denitrification increased after addition of liquid manure of beef cattle from 0.5 to 0.85 in the first 12 h after application and that >94% of this N2O was caused by a reduction of soil NO3 −. Volatile fatty acids are rapidly degradable C compounds in manures and are produced during the digestion in the animal and excreted with faeces (Canh et al. 1998) and during anaerobic storage of animal slurries (Cooper and Cornforth 1978; Guenzi and Beard 1981). In soil, volatile fatty acids are metabolized within a few days by soil bacteria, increasing denitrification and/or immobilization of N (Kirchmann and Lundvall 1993; Paul and Beauchamp 1989). Generally, the volatile fatty acids content is higher in pig slurries than in cattle slurry. Up to >30% of total C in pig slurries may be present as volatile fatty acids (Cooper and Cornforth 1978; Kirchmann and Lundvall 1993; Paul and Beauchamp 1989). The higher N2O emissions from the liquid pig manures than from the cattle slurries during the first days after application are likely related to the amount of easily degradable C compounds (and especially volatile fatty acids) in the pig manures. Also, these manures contain more water and this may distribute the C and N better through the soil matrix leading to higher rates of loss from partially anaerobic micro sites. It would also explain the relatively slow start of N2O emission from the NO3 −- and NH4 +-containing fertilizers.
The relatively high N2O emission from (NH4)2SO4 relative to NH4NO3 suggests that nitrification was a major N2O source. The higher N2O emissions from NH4NO3 and (NH4)2SO4 than from the manures from day 2 and onwards are probably related to a continued high availability of mineral N during the first days. All of the N applied via mineral fertilizer is mineral and can be directly transformed into N2O by nitrifiers and/or denitrifiers provided substrate is available. A (large) part of the N of the manures has to be mineralized before it can be transformed into N2O. This requires some time and may increase N2O emission after some time, as shown for example for the highest application rate of pig slurry (Fig. 3). Chadwick et al. (2000a) found similar patterns of N2O emission from animal manures and attributed this to succeeding periods of mineralization, nitrification, and denitrification.
The tested soil was a subsoil with a relatively low organic matter content so as to initiate a low emission in the control treatment (Table 1). The large difference in N2O emission between the mineral N fertilizers and slurries after the application of water at day 57 is probably related to the continued or renewed presence of available C in treatments with manures. The peak of N2O emission from the manures after water addition at day 57 was much higher and lasted longer than that of the peak just after application of the manures to the soil. It is suggested that the initial short peak is due to the denitrification of soil NO3 − as a consequence of the addition of easily available C (volatile fatty acids) and the second peak is caused by denitrification of mineralized and nitrified manure N. The higher total N2O emission from pig manures compared to cattle and poultry manure may be related to its lower C/N ratio, the higher mineral N fraction, and the higher water content in pig manures (Table 1). Manures with a C/N ratio of higher than about 15 may result in an initial immobilization of N. If this is true for all soils, then applying cattle manure would initially lead to the immobilization of N and a low N2O emission from nitrification or dinitrification, and postponed emission would be more likely (Chadwick et al. 2000b; Van Faassen and Van Dijk 1987). In our incubations there was no removal of N by plant uptake or leaching, so that the mineral N content in the soil was relatively high during the whole experimental period. This may have resulted in higher N2O emissions than under field conditions, as shown by Williams et al. (1998) for grassland. However, in arable farming systems, manures are often applied several weeks or months before the crop is grown. In this period, relatively high soil mineral N contents and N2O emissions may occur. We suggest that the risk of N2O emission is higher from animal manures than from mineral N fertilizers when they are applied to soils with a relatively low organic matter content, such as arable soils, than from soils with high organic matter contents, such as permanent grassland. In arable soils the applied N can be mineralized and nitrified before it is taken up by the crop or leached from the profile. Grassland soils have a high N uptake capacity, a high organic matter content and high denitrification potentials (Bijay-Singh et al. 1988). In these soils, N2O emission from NO3 −-containing mineral fertilizers are (much) higher than from animal manures (Egginton and Smith 1986; Velthof et al. 1997).
Our results do not support the hypothesis that increasing the application rate increases the N2O emission factor (in percent of the N applied), because the mole fraction of N2O/N2 of the denitrification products increases when the NO3 − content in the soil increases (Firestone et al. 1980). There was no clear effect of N application rate on the N2O emission factor for both NH4NO3 and pig manure in the present incubation study (Table 3). This supports the use of emission factors for N2O in percentage of the N applied, assuming a linear relation between application rate and N2O emission. However, the N uptake by the crop and N leaching losses may also affect the relation between N application rate and N2O emission in the field. Velthof et al. (1997) showed that for grassland the N2O emission factor increased from 0.6 to 3.1% when the N application rate increased from 50 to 300 kg N ha−1. It was concluded that the N uptake capacity of grassland was exceeded at high N application rates, so that the soil mineral N contents and N2O emission remained high during a relatively long period.
Legal procedures and agricultural guidelines promote incorporation of animal slurries into the soil to decrease NH3 losses. There are several incorporation techniques, including surface application directly followed by ploughing or harrowing, narrow band spreading, deep injection (band placed at 10 cm depth) and shallow injection (band placed at 5 cm depth) (Huijsmans et al. 1997). Some studies indicate no clear or no effect of application technique on N2O emission and denitrification from animal slurries applied to soil (Sommer et al. 1996; Velthof et al. 1997; Dendooven et al. 1998), but other studies indicate that injection of slurry enhances N2O emission and denitrification (Flessa and Beese 2000; Thompson et al. 1987). In the present study, the highest N2O emission was found when the slurry was placed in a row at 5 cm depth and lowest when the slurry was placed at 10 cm depth. Placing the slurry in a row at 5 cm depth may be considered as shallow injection of slurry which is a common application technique in parts of western Europe (Huijsmans et al. 1997). The shift from surface-application of slurry to shallow injection in the 1990s may thus have led to an increase in the direct slurry-derived N2O emission. This increase is partly counterbalanced by a decrease in indirect N2O emissions with shallow injection (i.e. the N2O derived when the emitted NH3 is deposited on the soil). There are no clear explanations for the differences between the effects of the application techniques in the present study, but they are probably related to O2 effects on N2O production in the soil, local N concentrations in the soil and the length of the diffusion path of N2O towards the atmosphere. The longer this diffusion path, the larger the chance that the produced N2O is reduced to give N2. Both the results in the literature and of the present study indicate that the slurry application technique may affect N2O emission. However, there is no constant and overall effect of application technique on N2O emission, probably because factors such as moisture content and organic matter in the soil, soil texture and rooting (crop type) interact. Verification in further field studies is required and may improve insights into the effects of manure application techniques on N2O emission in order to quantify more accurately N2O emissions from manure-amended soil and to set up mitigation strategies to reduce N2O emission from animal excreta.
The total N2O emission of surface-applied NH4NO3 of 0.9% of the N applied (Table 4) corresponds well with the IPCC emission factor of 1.25%. The IPCC emission factor is mainly based on surface-applied mineral N fertilizers (Bouwman 1996). Our incubation study indicates that all techniques of placement and incorporation of NH4NO3 into an uncropped soil increased N2O emission by more than a factor of 2 (Table 4). Placement of mineral fertilizers near roots may increase the N uptake and the N efficiency of the N fertilizer. This may result in a lower requirement of N fertilizer and, thus, in lower N2O emission. Thus, part of the increase in the N2O emission due to N placement in the soil may be counterbalanced by a reduction in N2O emission because of a lower requirement of N fertilizer. The net effect of fertilizer placement on N2O emission should be tested in studies with cropped soils.
In conclusion, we showed that both the composition and application technique of animal manure may strongly affect the emission of N2O after application to uncropped soil. Large emissions were associated with manures with high contents of inorganic N, easily mineralizable N and easily mineralizable C, such as liquid pig manure. Animal nutrition affects the composition of the manure and, thereby, also the N and C transformations and losses during storage and after soil application (Misselbrook et al. 1998). Adjustment of animal nutrition and proper manure application techniques are possible tools with which to decrease N2O emissions following application of manure to soils. In the present study, it was also shown that the N2O emission from surface-applied NH4NO3 was smaller than that from NH4NO3 incorporated into soil. There may be large differences between types of fertilizer and manure and between different application techniques. It is worthwhile refining the IPCC methodology so that mitigation options based on changes in fertilizer type, manure composition and application techniques can be quantified and reported. Also, more accurate estimates of actual N2O emissions may be obtained from the application of more specific emission factors for different manures. Such a refinement requires field experiments and a campaign of N2O measurement with cropped soils to verify the results from the laboratory incubations.
References
Bijay-Singh, Ryden JC, Whitehead DC (1988) Some relationships between denitrification potential and fractions of organic carbon in air-dried and field-moist soils. Soil Biol Biochem 20:737–741
Bouwman AF (1996) Direct emission of nitrous oxide from agricultural soils. Nutr Cycl Agroecosyst 46:53–70
Canh TT, Sutton AL, Aarnink AJA, Verstegen MWA, Schrama JW, Bakker GCM (1998) Dietary carbohydrates alter the faecal composition and pH and the ammonia emission from slurry of growing pigs. J Anim Sci 76:1887–1895
Castellanos JZ, Pratt PF (1981) Mineralisation of manure nitrogen correlation with laboratory indexes. Soil Sci Soc Am J 45:354–357
Chadwick DR, Pain BF, Brookman SKE (2000a) Nitrous oxide and methane emissions following application of animal manures to grassland. J Environ Qual 29:277–287
Chadwick DR, John F, Pain BF, Chambers BJ, Williams J (2000b) Plant uptake of nitrogen form the organic nitrogen fraction of animal manures: a laboratory experiment. J Agric Sci Camb 134:159–168
Clayton H, McTaggart IP, Parker J, Swan L, Smith KA (1997) Nitrous oxide emissions from fertilised grassland: a 2-year study of the effects of N fertiliser form and environmental conditions. Biol Fertil Soils 25:252–260
Cooper P, Cornforth IS (1978) Volatile fatty acids in stored animal slurry. J Sci Food Agric 29:19–27
Dendooven L, Bonhomme E, Merckx R, Vlassak K (1998) Injection of pig slurry and its effects on dynamics of nitrogen and carbon in a loamy soil under laboratory conditions. Biol Fertil Soils 27:5–8
Egginton GM, Smith KA (1986) Nitrous oxide emission from a grassland soil fertilized with slurry and calcium nitrate. J Soil Sci 37:59–67
Firestone MK, Firestone RB, Tiedje JM (1980) Nitrous oxide from soil denitrification: factors controlling its biological production. Science 208:749–751
Flessa H, Beese F (2000) Laboratory estimates of trace gas emissions following surface application and injection of cattle slurry. J Environ Qual 29:262–268
Genstat 5 Committee (1993) Genstat 5 Release 3 reference manual. Oxford University Press, Oxford
Granli T, Bøckman OC (1994) Nitrous oxide from agriculture. Norw J Agri Sci [Suppl] 12
Guenzi WD, Beard WE (1981) Volatile fatty acids in a redox-controlled cattle manure slurry. J Environ Qual 10:479–482
Henkens PLCM, Van Keulen H (2001) Mineral policy in the Netherlands and nitrate policy within the European Community. Neth J Agric Sci 49:117–134
Huijsmans JFM, Hol JMG, Bussink DW (1997) Reduction of ammonia emission by new slurry application techniques on grassland. In: Jarvis SC, Pain BF (eds) Gaseous nitrogen emissions from grasslands. CAB International, Wallingford, pp 281–285
Kirchmann H (1991) Carbon and nitrogen mineralisation of fresh, aerobic and anaerobic animal manures during incubation with soil. Swed J Agric Sci 21:165–173
Kirchmann H, Lundvall A (1993) Relationship between N immobilization and volatile fatty acids in soil after application of pig and cattle slurry. Biol Fertil Soils 15:161–164
Misselbrook TH, Chadwick DR, Pain BF, Headon DM (1998) Dietary manipulation as a means of decreasing N losses and methane emissions and improving herbage N uptake following application of pig slurry to grassland. J Agric Sci 130:183–191
Mosier A, Kroeze C, Nevison C, Oenema O, Seitzinger S, Van Cleemput O (1998) Closing the global N2O budget: nitrous oxide emissions through the agricultural nitrogen cycle. Nutr Cycl Agroecosyst 52, 225–248
Paul JW, Beauchamp EG (1989) Effect of carbon constituents in manure on denitrification in soil. Can J Soil Sci 69:49–61
Sommer SG, Sherlock RR, Khan RZ (1996) Nitrous oxide and methane emissions from pig slurry amended soils. Soil Biol Biochem 28:1541–1544
Stevens RJ, Laughlin RJ (2001) Effect of liquid manure on the mole fraction of nitrous oxide evolved from soil containing nitrate. Chemosphere 42:105–111
Thompson RB, Ryden JC, Lockyer DR (1987) Fate of nitrogen in cattle slurry following surface application or injection to grassland. J Soil Sci 38:689–700
Van Faassen HG, Van Dijk H (1987) Manure as a source of nitrogen and phosphorus in soils. In: Van der Meer HG, Unwin RJ, Van Dijk TA, Ennik GC (eds) Animal manure on grassland and fodder crops. Fertiliser or waste. Nijhoff, Dordrecht, pp 27–45
Velthof GL, Oenema O (1995) Nitrous oxide fluxes from grassland in the Netherlands. I. Statistical analysis of flux chamber measurements. Eur J Soil Sci 46:533–540
Velthof GL, Oenema O, Postma R, Van Beusichem ML (1997) Effects of type and amount of applied nitrogen fertilizer on nitrous oxide fluxes from intensively managed grassland. Nutr Cycl Agroecosyst 46, 257–267
Williams PH, Jarvis SC, Dixon E (1998) Emission of nitric oxide and nitrous oxide from soil under field and laboratory conditions. Soil Biol Biochem 30:1885–1893
Acknowledgements
We greatly acknowledge Eduard Hummelink for the technical assistance in measuring N2O fluxes. This study was financed by the Dutch programme ROB (Reduction Programme for Non-CO2 Greenhouse Gases) under the NOVEM contract 374299/0021 and by the Climate Change Programme (344) of the Ministry of Agriculture, Nature Management and Fisheries.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Velthof, G.L., Kuikman, P.J. & Oenema, O. Nitrous oxide emission from animal manures applied to soil under controlled conditions. Biol Fertil Soils 37, 221–230 (2003). https://doi.org/10.1007/s00374-003-0589-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00374-003-0589-2