Abstract
Gender differences in lifespan and aging are known across species. Sex differences in longevity within a species can be useful to understand sex-specific aging. Drosophila melanogaster is a good model to study the problem of sex differences in longevity since females are longer lived than males. There is evidence that stress resistance influences longevity. The objective of this study was to investigate if there is a relationship between sex differences in longevity and oxidative stress resistance in D. melanogaster. We observed a progressive age-dependent decrease in the activity of SOD and catalase, major antioxidant enzymes involved in defense mechanisms against oxidative stress in parallel to the increased ROS levels over time. Longer-lived females showed lower ROS levels and higher antioxidant enzymes than males as a function of age. Using ethanol as a stressor, we have shown differential susceptibility of the sexes to ethanol wherein females exhibited higher resistance to ethanol-induced mortality and locomotor behavior compared to males. Our results show strong correlation between sex differences in oxidative stress resistance, antioxidant defenses and longevity. The study suggests that higher antioxidant defenses in females may confer resistance to oxidative stress, which could be a factor that influences sex-specific aging in D. melanogaster.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Aging is a progressive functional decline in an organism that leads to mortality (Finch 1990; Charlesworth 1994). Although several theories have been postulated to explain the evolutionary selection involving fitness and reproduction that influence the longevity of a species (Hughes and Reynolds 2005), our understanding of the actual aging process remains unclear. A model of conceptual link between free radicals and aging was first proposed by Harman (1956), wherein oxidative stress leads to accumulation of damage that causes functional decline in aging. Several studies have provided correlative evidence that free radicals contribute to the aging process or senescence which support the free radical hypothesis (Beckman and Ames 1998; Sohal 2002). The complex relationship between oxidative stress and lifespan has been examined from the perspective of life history theory, which predicts that involvement of reproductive effort impacts oxidative stress management and there is a trade-off between reproductive effort and lifespan (Monaghan et al. 2009; Isaksson et al. 2011). Increased reproductive effort elevates metabolic rate which leads to oxidative stress as a consequence of the increased production of reactive oxygen species (ROS) by the mitochondria (Kavanagh 1987; Ernsting and Isaaks 1991). Although metabolic rate is inversely correlated with lifespan in many species, it may not always be associated with raise in ROS (Okada et al. 2011). Recently, Liochev (2015) has proposed that synergistic action of two or more causes increase over time that explains acceleration of aging and mortality with age, as evident from studies on yeast and Drosophila. Therefore, aging is not explainable by a single major cause and, elimination or attenuation of one of the causes including free radicals, will affect lifespan.
Antioxidant defense mechanisms that protect cells from the attack of free radicals and associated oxidative damage include enzymes such as superoxide dismutase (SOD), catalase, glutathione peroxidase (GPx) and heat shock proteins. Stress resistance in organisms has been implicated in longevity (Rose et al. 1992; Johnson et al. 2001; Murakami et al. 2003). Several positive correlations have been drawn between oxidative stress resistance and longevity by experimental induction of oxidative stress using chemicals such as paraquat (Kurepa et al. 1998; Migliaccio et al. 1999; Finkel and Holbrook 2000; Vermeulen et al. 2005). However, the role of antioxidant mechanism in the aging process has not been proven conclusively because of conflicting results in several aging models (Perez et al. 2009; Speakman and Selman 2011). Although, studies have reported that overexpression of antioxidant enzymes resulted in greater protection against oxidative stress, other reports did not find a correlative link between such protection and lifespan. Also other criticism have claimed that extremely long-lived naked mole rats have unexceptional antioxidant defenses and exhibits relatively high, steady state of oxidative damage compared to mice (Andziak et al. 2006). Studies in this long-lived rodent suggests that factors other than oxidative stress play major role in aging process and provide only equivocal support for the role of oxidative stress in aging (Lewis et al. 2013). However, it is also suggested that more studies are needed to estimate the oxidative state of this rodent and to support definite conclusions (Kirkwood and Kowald 2012; Barja 2013).
Sex differences in longevity occur across many species and often females live longer than males (Lints et al. 1983; Austad and Fischer 2016). Reported differences in longevity of the sexes are often deduced from wild populations, attributed to risk-taking behavior, foraging pattern and sexual competition, which could be unrelated to sex differences in intrinsic rate of aging (Austad and Fischer 2016). According to sexual selection theory (Trivers 1972), there is a trade-off between investment in reproductive effort and lifespan that generates sex differences in ROS production and antioxidant defenses. However, the role of oxidative stress in sexual selection is yet to be understood. Longevity differences between the sexes within a species can be useful to understand sex-specific aging (Austad and Fischer 2016). Recent studies in the cricket, Gryllodes sigillatus suggest a link between oxidative stress and the evolution of differences in lifespan and aging (Archer et al. 2013). Gender differences in longevity can provide greater insights into mechanisms of aging including the possible role of oxidative stress resistance in longevity, yet remain little studied and current support for these mechanisms is weak (Austad and Fischer 2016). In aging brains, there is a decline in the antioxidant defense mechanisms which increases the vulnerability of the brain to oxidative damage (Finkel and Holbrook 2000). Mitochondrial DNA (mtDNA) is believed to be one of the major targets by free radicals in aged brain. In agreement with the free radical theory of aging, an inverse correlation has been shown between the extent of oxidative damage to mtDNA and maximum lifespan in the heart and brain of mammals (Barja and Herrero 2000). Interestingly, sex differences in mtDNA damage have also been reported in Wistar rats wherein longer-lived females exhibited lower DNA damage compared to males (Borras et al. 2003).
Drosophila as a model is widely used in free radical research and aging (Le Bourg 2001). The laboratory populations of D. melanogaster exhibits sex differences in longevity wherein female is longer-lived sex than the male (Tower and Arbeitman 2009). In agreement with free radical theory of aging, depletion of SOD enzymes in muscle and nervous system resulted in higher ROS accumulation, impaired locomotor activity and shortened lifespan in Drosophila (Oka et al. 2015). Studies in wild-type strains of Drosophila such as Oregon R, Canton S, Dahomey showed significant correlation between mitochondrial ROS and lifespan including gender differences in redox homeostasis wherein longer-lived females exhibited lower ROS level than males (Sanz et al. 2010). In D. simulans, short-lived females showed higher levels of mitochondrial ROS levels compared to the males, which support the role of free-radical homeostasis in longevity (Ballard et al. 2007). Despite several studies on aging in Drosophila, relationship between oxidative stress resistance and sex differences in longevity have not been examined. Ethanol is considered as an environmental stressor for Drosophila (Service et al. 1985). Although Drosophila is naturally exposed to low concentration of ethanol in the fermented fruit, it is an oxidative stress inducer when exposed at higher concentration (Logan-Garbisch et al. 2014; Chauhan and Chauhan 2016). Upon exposure to acute doses of ethanol, Drosophila display behaviors that are remarkably similar to those observed in mammalian models and humans (Wolf et al. 2002). Exposure to ethanol results in hyperactivity, locomotor dysfunctions and eventually leading to sedation in flies (Bainton et al. 2000; Moore et al. 1998). Sexual dimorphism in ethanol toxicity has been observed in Drosophila (Malherbe et al. 2005; Devineni and Heberlein 2012), the biological basis of which is not clear. In this study, we have investigated the differential susceptibility of the sexes to ethanol-induced oxidative stress and examined whether the gender differences in longevity is correlated with oxidative stress resistance of the sexes.
Materials and methods
Drosophila culture
Wild-type D. melanogaster (Oregon K) adults (5–10 days old) were obtained from the Drosophila Stock centre, Department of Zoology, Manasagangotri, University of Mysore, Karnataka, India. Flies were maintained in a 150 ml culture bottle containing 30 ml of standard wheat cream–agar medium seeded with yeast. Both larval and adult numbers were maintained to moderate densities with 150–200 larvae per bottle. Flies were reared at 22 ± 1 °C and 70–80% relative humidity for all the experiments.
Lifespan assay
For lifespan study, flies were obtained from the culture bottles setup at a density of about 100 eggs per bottle. As with all our assays, females were exposed to males for 72 h following eclosion and then separated according to their sex. A total of 100 mated males and 100 mated females were used in this experiment. These flies were housed separately in vials at defined density supplemented with fresh standard wheat cream–agar medium (5 vials with 20 flies each per sex). All flies were transferred to new vials with fresh diet every 2–3 days depending on media condition to eliminate the emerging larval stages in the vials containing female flies and replenish nutrients which may have exhausted. Mortality was recorded every 24 h until all flies had died.
Age-related changes in the sexes
To explore the association between oxidative stress status and gender-specific aging, mated flies of the different age groups, i.e., newly eclosed flies (0 week; 1–2 days old), mid age (3 weeks; 20–22 days old) and old age (6 weeks; 40–42 days old and 9 weeks; 60–62 days old) were employed. We also investigated the possible sex difference in ethanol-induced oxidative stress response using flies of different age groups by conducting ethanol toxicity, behavioral and biochemical assays.
Ethanol toxicity and oxidative stress resistance
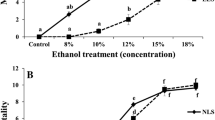
Briefly, 1-week-old flies (mated) were transferred to the parafilm-sealed vials (ten flies per vial) containing Whatman filter paper discs (diameter 2 cm) soaked with 5% sucrose solution and various concentrations of ethanol (8, 10, 12, 15 and 18%), whereas the control group received only 5% sucrose solution (Montooth et al. 2006). For each ethanol concentration, 60 flies (six replicates of ten flies each) per sex were tested. Mortality was scored after 24 h and expressed as percentage mortality. A dose–response curve was plotted from which LC50 was determined.
To study the effect of ethanol as an oxidative stress inducer, 10% of ethanol in sucrose solution was employed based on mortality results. To study ethanol toxicity at different ages, flies of different age groups were transferred to the vials (ten flies per vial) containing Whatman filter paper discs soaked with 10% ethanol in 5% sucrose solution whereas the control group flies received only 5% sucrose solution. The number of dead flies was scored after 24 h of ethanol exposure. For each age group, six replicates of ten flies each per sex were tested and expressed as an average of six replicates.
For negative geotaxis assay and biochemical assays, an exposure time of 60 min for ethanol (10%) was used. The time of exposure was standardized based on preliminary assessment (data not shown); wherein 60 min exposure to 10% ethanol caused significant changes in the locomotor behavior and oxidative stress markers.
Locomotory behavioral assay
Flies were subjected to negative geotaxis assay wherein ability of flies to climb vertically was recorded. Briefly, ten flies were released at the bottom of a vertical glass column (standard length, 25 cm) and allowed to climb. The total number of flies that escaped beyond minimum distance of 12 cm in 20 s was counted. For each age group, six replicates of ten flies each per sex were tested and expressed as an average of six replicates (Feany and Bender 2000).
Biochemical assays
Flies were subjected to cold anesthesia for 10 min. 50 flies (ten flies per vial) were homogenized in 1 ml of respective assay buffers and centrifuged at 2500×g for 10 min at 4 °C. ROS and antioxidant enzymes (SOD, catalase) were assayed in the supernatant. Three replicates per group were tested with the total of 150 flies per group per assay.
Reactive oxygen species
ROS was assayed using 2′7′-Dichlorofluorescin diacetate (DCFH-DA) (Sigma Chemical, St. Louis, MO, USA) in which the reaction involves the conversion of non-fluorescent DCFH-DA into a highly fluorescent product, 2′7′-dichlorofluorescin (DCF), in the presence of ROS. The fluorescence was measured in a spectrofluorometer with the excitation wavelength of 488 nm and emission at 525 nm. ROS was quantified from a DCF standard curve and expressed as µmoles of DCF formed/min/mg protein (LeBel et al. 1992).
Antioxidant enzymes
SOD activity was measured using pyrogallol (2 mM) (Sigma Chemical, St. Louis, MO, USA) autoxidation in 0.1 M tris buffer (pH 8.2). Reaction was monitored for 3 min at 420 nm and expressed as units of enzyme required to inhibit 50% pyrogallol autooxidation (Marklund and Marklund 1974). Catalase activity was measured using 1% hydrogen peroxide (H2O2) (Sisco Research Laboratories, Mumbai, India) as a substrate in 0.05 M phosphate buffer (pH 7) by monitoring the change in absorbance at 240 nm for 3 min and expressed as mmoles of H2O2 decomposed/min/mg protein (Aebi 1983).
Protein estimation
Protein content in the homogenate was determined by Lowry’s method using BSA (Sigma Chemical, St. Louis, MO, USA) as the standard (Lowry et al. 1951).
Statistical analysis
Kaplan–Meier survival analysis was made to compare the survivability of male and female D. melanogaster flies. Factorial analysis of variance (ANOVA) was performed to test the interactions among sex, ethanol treatment and age using SPSS software (v.17.0 SPSS Inc., IBM, Armonk, NY, USA). Consequently, to facilitate subsequent interpretation, we also conducted ‘Tukey’ post-hoc test when a significant difference was found in the ANOVA test using SPSS software.
Results
Survival
The lifespan study as seen in the survivability curve reveals that females live longer than males (Fig. 1). The mean lifespan was 88.5 for females and 61.52 for males. Age-associated mortality was observed after 6 weeks of age wherein males exhibited higher mortality when compared to females (Fig. 2a).
Sex difference in the survivability of D. melanogaster as determined by the Kaplan–Meier survival analysis. The two curves represent the survivorship of male and female flies housed separately in vials (n = 100; 5 vials with 20 flies each per sex). The log-rank test revealed a statistically significant difference in the survivorship between males and females (Chi square value = 21.227 and P < 0.001)
Sex differences in ethanol-induced mortality and climbing activity among different age groups of D. melanogaster. a Age-assicated mortality in males and females. Values represent mean number of dead flies (±SEM; n = 6, 10 flies per replicate) at different ages. b Ethanol-induced mortality in males and females. Values represent average number of dead flies (±SEM; n = 6, 10 flies per replicate) after 24 h of ethanol exposure. c The climbing ability of flies at different ages as determined by the negative geotaxis assay. d Ethanol-induced changes in the climbing activity in the sexes at different ages. Values represent average number of flies (±SEM; n = 6, 10 flies per replicate) escaped beyond the minimum distance in a given time. Groups of 0–9 week old flies were exposed to the stressor (10% ethanol). Sex differences in mortality and climbing activity was evaluated by ANOVA with ‘Tukey’ post hoc test and bars denoted by different alphabets differ significantly (P < 0.05)
Ethanol tolerance
Mortality of young flies (1 week old) exposed to different concentrations of ethanol for 24 h was concentration-dependent (Table 1). The LC50 for ethanol was found to be 9.7 and 10.9% for males and females, respectively. To study the effect of sex and age on mortality, behavior and antioxidant status, a mean value of 10% ethanol was employed.
Ethanol-induced mortality was sex-dependent as indicated by the significant sex × treatment interaction in Table 2 (***P < 0.001). Results showed significant differences in mortality wherein longer-lived females exhibited higher resistance to ethanol toxicity than males, at all ages (Fig. 2b). Overall, there was a significant effect of sex and age on ethanol-induced mortality as indicated by the sex × treatment × age interaction in Table 3 (*P < 0.05).
Locomotor activity
Both males and females exhibited decreased locomotor activity as they aged. Effect of age on locomotor ability was sex-dependent as indicated by the significant sex × age interaction in Table 2 (***P < 0.001). While both sexes exhibit robust negative geotaxis at young age (0 week), males become worse at this task when compared to females from third week onwards (Fig. 2c).
Both males and females showed locomotory impairment after ethanol treatment as evident from the decreased climbing activity (Fig. 2d). Effect of ethanol treatment on locomotor activity was significantly varied with sex and age as indicated by sex × treatment × age interactions in Table 3 (*** P < 0.001). However, there was no overall significant sex × treatment interaction on locomotor behavior (Table 3). Consequently, we conducted sex × treatment interaction at different age points separately. Results showed that ethanol-induced locomotor behavior was significantly affected by sex at 0, 3 and 9 weeks but the interaction was not statistically significant at sixth week (Online Resource 1). Interestingly, females showed higher resistance against ethanol-induced locomotory impairment relative to the males at all ages.
ROS
Endogenous ROS levels show gradual increase during aging (0–9 weeks) in both sexes. A significant sex × age interaction (**P < 0.01) indicated that the effect of age on ROS production was sex-dependent (Table 2). Females showed lower ROS with age compared to males (Fig. 3a). A significant increase in the ROS production was seen in flies upon exposure to ethanol. Overall interaction between sex, ethanol treatment and age (sex × treatment × age interactions) was not significant (Table 3). However, ethanol-induced ROS levels varied with sex as indicated by the significant sex × treatment interaction (*P < 0.05) in Table 3. Interestingly, males showed higher level of oxidative stress than females at all ages, shown by marked induction of ROS (Fig. 3b).
Sex difference in ROS levels among different age groups of D. melanogaster. a Endogenous ROS levels in the sexes at different ages. b Ethanol-induced ROS levels in males and females. Groups of 0–9 week old flies were exposed to the stressor (10% ethanol). The difference in ROS production was evaluated by ANOVA with ‘Tukey’ post hoc test. Values are mean ± SEM. and bars denoted by different alphabets differ significantly (P < 0.05). Correlation between mortality and ROS as a function of age in males (c) and in females (d). The relationship between ROS and mortality was analyzed using linear regression. Endogenous ROS show a positive correlation with mortality in both sexes
There was a positive correlation between age-associated mortality and the endogenous ROS levels in both sexes (Fig. 3c, d). Endogenous ROS tends to be higher in aged males and females corresponding with low survival.
The activity of antioxidant enzymes
Activity of the antioxidant enzymes was significantly decreased after 3 weeks of age in both sexes. As indicated by a significant sex × age interaction (**P < 0.01), age-associated changes in the activity of antioxidant enzymes were sex-dependent (Table 2). Females exhibited higher activity of the SOD and catalase when compared to males at all ages (Fig. 4a, c).
Sex difference in the activity of antioxidant enzymes at different ages in D. melanogaster. a Endogenous SOD activity in the sexes at different ages. b Ethanol-induced changes in the activity of SOD at different ages. c Endogenous catalase activity in the sexes at different ages. d Ethanol-induced changes in the acivity of catalase at different ages. Groups of 0–9 week old flies were exposed to the stressor (10% ethanol). The difference in the activity of antioxidant enzymes were evaluated by ANOVA with ‘Tukey’ post hoc test. Values are mean ± SEM. and bars denoted by different alphabets differ significantly (P < 0.05)
In flies exposed to ethanol, the antioxidant enzymes were markedly higher in both sexes. Increased SOD and catalase activity in response to ethanol treatment were significantly influenced by sex and age as indicated by the sex × treatment × age interactions (Table 3; ***P < 0.001). Analysis of the sex × treatment interaction at different age points revealed that the induction of SOD in response to ethanol treatment was significantly affected by sex at 0, 3 and 6 weeks (Online Resource 1). Males exhibited higher SOD activity at zero (emergence) and third week of age whereas females showed increased activity up to sixth week (Fig. 4b). Likewise, catalase activity in response to ethanol exposure was sex-dependent as indicated by the significant sex × treatment interaction in Table 3 (***P < 0.001). Males exhibited significant induction of catalase up to sixth week of age, whereas, in females, activity of catalase was induced after middle age and significant induction was observed up to 9 weeks of age (Fig. 4d).
Discussion
Differences in lifespan between males and females are commonly observed in laboratory species such as C. elegans, D. melanogaster and Mus musculus (Tower and Arbeitman 2009; Austad and Fischer 2016). D. melanogaster (Oregon K) used in our study, is a good model to study the gender difference in aging since females live longer than males as evident from the survival curves. Our results on survivability of Drosophila show that the mortality rates exhibited gender differences with age which could be attributed to differential aging in the sexes. Age-related mortality was positively correlated with endogenous ROS which was consistent with earlier studies showing inverse relationship between oxidative stress and lifespan (Ku et al. 1993; Sohal et al. 1995). Recent studies by Archer et al. (2013) in the cricket, Gryllodes sigillatus, have presented evidence for sex differences in oxidative stress in relation to lifespan. Our study shows age-dependent decline in total SOD and catalase activities in both sexes. However, earlier studies showed no consistent relationship of endogenous antioxidant defenses with aging (Le Bourg 2001; Speakman and Selman 2011). It was suggested that normal enzyme activity is sufficient to counter free radicals and the antioxidant enzymes could be considered as stress enzymes (Le Bourg 2001). We found negative correlations between the two major antioxidant enzymes and aging. We also observed a significant sex differences in the activity of antioxidant enzymes with age wherein long-lived females exhibited higher activity of endogenous SOD and catalase enzymes than males. Previous studies have reported that long-lived strains of Drosophila are associated with lower ROS levels, but it may not always reflect the higher antioxidant defense mechanisms in those strains (Sanz et al. 2010). In contrast, our study shows that the levels of two major antioxidant enzymes correlated negatively with ROS levels and positively with lifespan. A previous study in humans have shown that men exhibited higher ROS levels with slightly lower SOD and catalase activity compared to women (Ide et al. 2002). Another study in SAMP8 mice revealed sex differences in the activity of antioxidant enzymes with aging wherein males exhibited lower levels of SOD and catalase activities compared to the females (Tomás-Zapico et al. 2006). Furthermore, gender differences in oxidative stress in relation to longevity was reported in human subjects wherein long-lived females exhibited lower levels of mitochondrial H2O2, higher levels of Mn-SOD and GPx activities compared to males (Viña et al. 2005). Our study in D. melanogaster is consistent with the observations in human subjects supporting the role of oxidative stress in gender-specific aging.
Locomotor performance has been positively correlated with lifespan in six species of snakes wherein long-lived species exhibited better performance than short-lived species (Robert et al. 2007). It is also positively correlated with survival in lizards (Warner and Andrews 2002; Miles 2004) and turtles (Janzen 1995) suggesting that locomotor performance could be used as a measure of fitness and survivability (Robert et al. 2007). Negative geotaxis has been used as a locomotory behavioral measure of aging in Drosophila. Consistent with previous reports (Gargano et al. 2005; Jones and Grotewiel 2011), flies exhibited age-dependent decline in the locomotory activity. We also investigated the effect of age on the locomotory ability in relation to the sex differences in longevity. Age-related decline in the locomotory activity was more pronounced in males when compared to females suggesting the better fitness of long-lived sex. In addition, older flies overexpressing catalase and Cu/Zn-SOD fared better than controls (Orr and Sohal 1994) indicating that greater antioxidant defenses also reflect the fitness and survivability of an organism. Our study provides correlative evidence that long-lived sex exhibits greater locomotor performance as well as antioxidant defense mechanisms and thus greater fitness and survivorship compared to the short-lived sex.
Oxidative stress resistance to a stressor such as paraquat is implicated in affecting lifespan in Drosophila (Vermeulen et al. 2005). We have used sensitivity to ethanol as a measure of resistance to oxidative stress in Drosophila. Similar to paraquat, ethanol exposure gives rise to the generation of excessive amount of free radicals as evident from the induction of ROS after ethanol exposure in both sexes. Our results are in agreement with similar data reported in different laboratory models of ethanol exposure leading to oxidative stress mediated damages (Das and Vasudevan 2007; Kasdallah-Grissa et al. 2007; Jahromi et al. 2015). Interestingly, we found sex differences in ethanol-induced oxidative stress wherein females exhibited higher resistance to oxidative stress as evident from mortality and negative geotaxis assay. A similar sex differences in oxidative stress-mediated mortality was also reported in Drosophila treated with paraquat (Chaudhuri et al. 2007). Also, it has been reported that males are more resistant to ethanol-induced sedation but show higher susceptibility to mortality compared to females (Devineni and Heberlein 2012). Despite a number of studies examining sex differences in oxidative stress-mediated mortality and ethanol responses in Drosophila, relationship between oxidative stress and antioxidant defense system in relation to sex differences in longevity has not been studied. In our study, we found sex differences in oxidative stress resistance that showed a correlation with ethanol-induced mortality, ROS production and antioxidant protection. To see how these effects extend across age groups, we also considered young and older flies in our study. Longer-lived females showed higher activity of SOD which remained so after ethanol exposure while induction of catalase activity was significantly higher in older females than in males of the same age. In contrast, the shorter lived males had elevated ROS level and also higher mortality after ethanol exposure than the females, thus providing evidence that the management of oxidative stress may play a role in the sex-specific aging. It is well possible that, responses to applied stress are specific to organism under study; however, it supports the idea that the differential protective mechanisms against environmental stresses such as oxidative stress are attributed to differences in lifespan in many organisms. Furthermore, other molecular mechanisms may also be involved in ethanol resistance, such as the rate of alcohol metabolism in the sexes, due to the activity of the enzyme, alcohol dehydrogenase (ADH). Previous studies have reported the positive association between ethanol tolerance and higher ADH activity in mice and Drosophila (Eriksson and Pikkarainen 1968; McDonald et al. 1977), although others have not reported this correlation (Gibson et al. 1979; Barbancho et al. 1987). Overall, our study presents evidence that the oxidative stress and antioxidant defenses are important factors influencing sex-specific aging and longevity in D. melanogaster. Despite divergent views on the role of free radicals in aging, our results favor the recent hypothesis (Kirkwood and Kowald 2012) which suggests that oxidative stress could be one of the mechanisms that may synergistically influence the aging process.
References
Aebi H (1983) Catalase. In: Bergmeyer HU (ed) Methods of enzymatic analysis, 3rd edn. Verlag, Chemie, Weinheim, pp. 273–286
Andziak B, O’Connor TP, Qi W et al (2006) High oxidative damage levels in the longest-living rodent, the naked mole-rat. Aging Cell 5:463–471
Archer CR, Sakaluk SK, Selman C et al (2013) Oxidative stress and the evolution of sex differences in life span and ageing in the decorated cricket, Gryllodes sigillatus. Evol Int J org Evol 67:620–634
Austad SN, Fischer KE (2016) Sex differences in lifespan. Cell Metab 23:1022–1033
Bainton RJ, Tsai LT, Singh CM et al (2000) Dopamine modulates acute responses to cocaine, nicotine and ethanol in Drosophila. Curr Biol 10:187–194
Ballard JWO, Melvin RG, Miller JT, Katewa SD (2007) Sex differences in survival and mitochondrial bioenergetics during aging in Drosophila. Aging Cell 6:699–708
Barbancho M, Sánchez-Cañete FJ, Dorado G, Pineda M (1987) Relation between tolerance to ethanol and alcohol dehydrogenase (ADH) activity in Drosophila melanogaster: selection, genotype and sex effects. Heredity (Edinb) 58(Pt 3):443–450
Barja G (2013) Updating the mitochondrial free radical theory of aging: an integrated view, key aspects, and confounding concepts. Antioxid Redox Signal 19:1420–1445
Barja G, Herrero A (2000) Oxidative damage to mitochondrial DNA is inversely related to maximum life span in the heart and brain of mammals. FASEB J 14:312–318
Beckman KB, Ames BN (1998) The free radical theory of aging matures. Physiol Rev 78:547–581
Borrás C, Sastre J, García-Sala D et al (2003) Mitochondria from females exhibit higher antioxidant gene expression and lower oxidative damage than males. Free Radic Biol Med 34:546–552
Charlesworth B (1994) Evolution in age-structured populations, 2nd edn. Cambridge University Press, UK
Chaudhuri A, Bowling K, Funderburk C et al (2007) Interaction of genetic and environmental factors in a Drosophila parkinsonism model. J Neurosci 27:2457–2467
Chauhan V, Chauhan A (2016) Effects of methylmercury and alcohol exposure in Drosophila melanogaster: Potential risks in neurodevelopmental disorders. Int J Dev Neurosci 51:36–41
Das SK, Vasudevan DM (2007) Alcohol-induced oxidative stress. Life Sci 81:177–187
Devineni AV, Heberlein U (2012) Acute ethanol responses in Drosophila are sexually dimorphic. Proc Natl Acad Sci USA 109:21087–21092
Eriksson K, Pikkarainen PH (1968) Differences between the sexes in voluntary alcohol consumption and liver ADH-activity in inbred strains of mice. Metabolism 17:1037–1042
Ernsting G, Isaaks JA (1991) Accelerated ageing: a cost of reproduction in the carabid beetle Notiophilus biguttatus F. Funct Ecol 5:299
Feany MB, Bender WW (2000) A Drosophila model of Parkinson’s disease. Nature 404:394–398
Finch CE (1990) Longevity, senescence and the genome. University of Chicago Press, Chicago
Finkel T, Holbrook NJ (2000) Oxidants, oxidative stress and the biology of ageing. Nature 408:239–247
Gargano JW, Martin I, Bhandari P, Grotewiel MS (2005) Rapid iterative negative geotaxis (RING): a new method for assessing age-related locomotor decline in Drosophila. Exp Gerontol 40:386–395
Gibson JB, Lewis N, Adena MA, Wilson SR (1979) Selection for ethanol tolerance in two populations of Drosophila melanogaster segregating alcohol dehydrogenase allozymes. Aust J Biol Sci 32:387–398
Harman D (1956) Aging: a theory based on free radical and radiation chemistry. J Gerontol 11:298–300
Hughes KA, Reynolds RM (2005) Evolutionary and mechanistic theories of aging. Annu Rev Entomol 50:421–445
Ide T, Tsutsui H, Ohashi N et al (2002) Greater oxidative stress in healthy young men compared with premenopausal women. Arterioscler Thromb Vasc Biol 22:438–442
Isaksson C, Sheldon BC, Uller T (2011) The challenges of integrating oxidative stress into life-history biology. Bioscience 61:194–202
Jahromi SR, Haddadi M, Shivanandappa T, Ramesh SR (2015) Modulatory effect of Decalepis hamiltonii on ethanol-induced toxicity in transgenic Drosophila model of Parkinson’s disease. Neurochem Int 80:1–6
Janzen FJ (1995) Experimental evidence for the evolutionary significance of temperature dependent sex determination. Evolution (N Y) 49:864
Johnson TE, de Castro E, Hegi de Castro S et al (2001) Relationship between increased longevity and stress resistance as assessed through gerontogene mutations in Caenorhabditis elegans. Exp Gerontol 36:1609–1617
Jones MA, Grotewiel M (2011) Drosophila as a model for age-related impairment in locomotor and other behaviors. Exp Gerontol 46:320–325
Kasdallah-Grissa A, Mornagui B, Aouani E et al (2007) Resveratrol, a red wine polyphenol, attenuates ethanol-induced oxidative stress in rat liver. Life Sci 80:1033–1039
Kavanagh MW (1987) The efficiency of sound production in two cricket species, Gryllotalpa australis and Teleogryllus commodus (Orthoptera: Grylloidea). J Exp Biol 130:107–119
Kirkwood TBL, Kowald A (2012) The free-radical theory of ageing–older, wiser and still alive: modelling positional effects of the primary targets of ROS reveals new support. Bioessays 34:692–700
Ku H-H, Brunk UT, Sohal RS (1993) Relationship between mitochondrial superoxide and hydrogen peroxide production and longevity of mammalian species. Free Radic Biol Med 15:621–627
Kurepa J, Smalle J, Van Montagu M, Inzé D (1998) Oxidative stress tolerance and longevity in Arabidopsis: the late-flowering mutant gigantea is tolerant to paraquat. Plant J 14:759–764
Le Bourg E (2001) Oxidative stress, aging and longevity in Drosophila melanogaster. FEBS Lett 498:183–186
LeBel CP, Ischiropoulos H, Bondy SC (1992) Evaluation of the probe 2′,7′-dichlorofluorescin as an indicator of reactive oxygen species formation and oxidative stress. Chem Res Toxicol 5:227–231
Lewis KN, Andziak B, Yang T, Buffenstein R (2013) The naked mole-rat response to oxidative stress: just deal with it. Antioxid Redox Signal 19:1388–1399
Lints FA, Bourgois M, Delalieux A et al (1983) Does the female life span exceed that of the male? A study in Drosophila melanogaster. Gerontology 29:336–352
Liochev SI (2015) Reflections on the theories of aging, of oxidative stress, and of science in general. Is it time to abandon the free radical (oxidative stress) theory of aging? Antioxid Redox Signal 23:187–207
Logan-Garbisch T, Bortolazzo A, Luu P et al (2014) Developmental ethanol exposure leads to dysregulation of lipid metabolism and oxidative stress in Drosophila. G3 (Bethesda) 5:49–59. doi:10.1534/g3.114.015040
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Malherbe Y, Kamping A, van Delden W, van de Zande L (2005) ADH enzyme activity and Adh gene expression in Drosophila melanogaster lines differentially selected for increased alcohol tolerance. J Evol Biol 18:811–819
Marklund S, Marklund G (1974) Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur J Biochem 47:469–474
McDonald JF, Chambers GK, David J, Ayala FJ (1977) Adaptive response due to changes in gene regulation: a study with Drosophila. Proc Natl Acad Sci USA 74:4562–4566
Migliaccio E, Giorgio M, Mele S et al (1999) The p66shc adaptor protein controls oxidative stress response and life span in mammals. Nature 402:309–313
Miles DB (2004) The race goes to the swift: fitness consequences of variation in sprint performance in juvenile lizards. Evol Ecol Res 6:63–75
Monaghan P, Metcalfe NB, Torres R (2009) Oxidative stress as a mediator of life history trade-offs: mechanisms, measurements and interpretation. Ecol Lett 12:75–92
Montooth KL, Siebenthall KT, Clark AG (2006) Membrane lipid physiology and toxin catabolism underlie ethanol and acetic acid tolerance in Drosophila melanogaster. J Exp Biol 209:3837–3850
Moore MS, DeZazzo J, Luk AY et al (1998) Ethanol intoxication in Drosophila: Genetic and pharmacological evidence for regulation by the cAMP signaling pathway. Cell 93:997–1007
Murakami S, Salmon A, Miller RA (2003) Multiplex stress resistance in cells from long-lived dwarf mice. FASEB J 17:1565–1566
Oka S, Hirai J, Yasukawa T et al (2015) A correlation of reactive oxygen species accumulation by depletion of superoxide dismutases with age-dependent impairment in the nervous system and muscles of Drosophila adults. Biogerontology 16:485–501
Okada K, Pitchers WR, Sharma MD et al (2011) Longevity, calling effort, and metabolic rate in two populations of cricket. Behav Ecol Sociobiol 65:1773–1778
Orr WC, Sohal RS (1994) Extension of life-span by overexpression of superoxide dismutase and catalase in Drosophila melanogaster. Science 263:1128–1130
Pérez VI, Bokov A, Van Remmen H et al (2009) Is the oxidative stress theory of aging dead? Biochim Biophys Acta Gen Subj 1790:1005–1014
Robert KA, Brunet-Rossinni A, Bronikowski AM (2007) Testing the “free radical theory of aging” hypothesis: physiological differences in long-lived and short-lived colubrid snakes. Aging Cell 6:395–404
Rose MR, Vu LN, Park SU, Graves JL (1992) Selection on stress resistance increases longevity in Drosophila melanogaster. Exp Gerontol 27:241–250
Sanz A, Fernández-Ayala DJM, Stefanatos RK, Jacobs HT (2010) Mitochondrial ROS production correlates with, but does not directly regulate lifespan in Drosophila. Aging 2:200–223
Service PM, Hutchinson EW, Mackinley MD, Rose MR (1985) Resistance to environmental stress in Drosophila melanogaster selected for postponed senescence. Physiol Zool 58:380–389
Sohal RS (2002) Role of oxidative stress and protein oxidation in the aging process. Free Radic Biol Med 33:37–44
Sohal RS, Sohal BH, Orr WC (1995) Mitochondrial superoxide and hydrogen peroxide generation, protein oxidative damage, and longevity in different species of flies. Free Radic Biol Med 19:499–504
Speakman JR, Selman C (2011) The free-radical damage theory: accumulating evidence against a simple link of oxidative stress to ageing and lifespan. Bioessays 33:255–259
Tomás-Zapico C, Alvarez-García O, Sierra V et al (2006) Oxidative damage in the livers of senescence-accelerated mice: a gender-related response. Can J Physiol Pharmacol 84:213–220
Tower J, Arbeitman M (2009) The genetics of gender and life span. J Biol 8:38
Trivers R (1972) Parental investment and sexual selection. In: Campbell B (ed) Sexual selection and the descent of man 1871–1971. Aldine, Chicago, pp 136–179
Vermeulen CJ, Van De Zande L, Bijlsma R (2005) Resistance to oxidative stress induced by paraquat correlates well with both decreased and increased lifespan in Drosophila melanogaster. Biogerontology 6:387–395
Viña J, Borrás C, Gambini J et al (2005) Why females live longer than males? Importance of the upregulation of longevity-associated genes by oestrogenic compounds. FEBS Lett 579:2541–2545
Warner DA, Andrews RM (2002) Laboratory and field experiments identify sources of variation in phenotypes and survival of hatchling lizards. Biol J Linn Soc 76:105–124
Wolf FW, Rodan AR, Tsai LT-Y, Heberlein U (2002) High-resolution analysis of ethanol-induced locomotor stimulation in Drosophila. J Neurosci 22:11035–11044
Acknowledgements
The first and second authors thank Department of Science and Technology, Government of India, for the financial support under INSPIRE fellowship program. Thanks are also due to The Chairperson, Department of Zoology, University of Mysore, Mysuru, for the facilities.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Communicated by H. V. Carey.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Niveditha, S., Deepashree, S., Ramesh, S.R. et al. Sex differences in oxidative stress resistance in relation to longevity in Drosophila melanogaster . J Comp Physiol B 187, 899–909 (2017). https://doi.org/10.1007/s00360-017-1061-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00360-017-1061-1