Abstract
Body condition and reproductive maturation are parameters of reproductive success that are influenced by sexual hormones rising in the circulation during the time of puberty. Various endocrine systems can be programmed by conditions experienced during early life. Stress for instance is supposed to be capable of influencing fetal development, leading to adjustments of offspring’s later physiology. We examined whether prenatal stress (induced by exposure to strobe light) during early- to mid-gestation was capable of affecting later reproductive parameters in guinea pigs (Cavia aperea f. porcellus). Therefore, we measured the levels of testosterone and progesterone from the age of day 12–124 in prenatally stressed (PS, n = 20) and unaffected control animals (n = 24). Furthermore, we determined the timing of puberty and growth. Body weight development revealed significantly faster growth in PS females compared to control animals. The onset of first estrus was slightly earlier in PS females, however not significantly so. Cycle lengths and levels of progesterone differed between groups over the course of time with higher progesterone levels and more constant cycles among PS females compared to control females who displayed marked differences between first and subsequent cycles. Levels of testosterone did not differ between groups. We conclude that prenatal stress accelerates growth and maturity in females, but not in males.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Next to the genetic equipment of an individual, environmental factors play an important role in shaping different phenotypes and their reproductive capacity (Dufty et al. 2002). Nutrition, stress and bioactive substances can influence reproductive performance not only in adults, but also in developing organisms during pregnancy (reviewed in Rhind et al. 2001). This phenomenon is called “programming” and refers to the ability of an organism to alter its later phenotype according to the environmental condition experienced during early life. In many cases short-term adjustments may however increase the risk of disease as a long-term consequence of fetal programming (reviewed in Gluckman et al. 2008a). Programming effects could be demonstrated in nearly every organ system [e.g., kidney (Moritz et al. 2003), skeletal muscle (Du et al. 2010), brain (Harris and Seckl 2011), immune system (Palmer 2011)]. Also, several factors were found to act as cues of environmental conditions to the fetus—nutrition and stress being the ones best studied. Both cues may act via the same pathway, as glucocorticoids are believed to constitute an underlying mechanism (Seckl 2004). However, the literature is full of contradicting findings in this context. One reason for this is the vast amount of different experimental paradigms used in programming research; especially, sensitive time periods must be considered. The phenotype will be affected according to the organ system under development (Nathanielsz 2006). During early pregnancy, for instance, many cells still are omnipotent and the potential impact of environmental conditions may be even more detrimental.
Reproductive maturation commences with puberty, characterized by an increasing influence of gonadal hormones, which is reflected in body composition and reproductive cycles in females. Puberty varies in onset and duration and both of these variables depend on body condition. Consequently, this time period is of special interest in the study of programming.
The aim of our study was to examine the effects of prenatal stress during early- to mid-gestation on later development and reproductive performance of offspring. Because prenatal stress might act as a cue of harsh environmental conditions, we expected long-term effects of prenatal stress on both variables. If conditions are harsh and survival is at risk, it might be advantageous to invest in fast reproductive maturation even if at the cost of growth (see Gluckman and Hanson 2004). Therefore, we predicted reduced growth, increased sexual hormone levels and an earlier puberty in prenatally stressed as compared to control animals. This would enable prenatally stressed offspring to increase reproductive output during the first year of life. Glucocorticoids are believed to play a pivotal role in transferring maternal cues to the fetus. In mice, glucocorticoid transfer via the placenta is higher in female compared to male fetuses (Montano et al. 1993). If a similar sex difference exists in guinea pigs, we would predict more pronounced effects in females.
Materials and methods
The institutional ethics committee and the Austrian Federal Ministry of Science and Research approved and permitted all husbandry and experimental procedures performed for this work (GZ 68.205/0211-II/10b/2008).
Animals and housing conditions
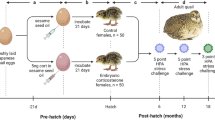
All animals could be identified by differences in natural coat pattern. A detailed description of animals, housing conditions and procedure of prenatal stress treatment can be found in Schöpper et al. (2011, 2012). Briefly, prenatally stressed and control guinea pigs (Cavia aperea f. porcellus, multicolored, bred in our facilities) of both sexes (prenatally stressed (PS), n = 20, ♀8 ♂12 and control, n = 24, ♀11 ♂13) were monitored for growth from birth to day 124, as well as sex hormone development from day 12 to 124. Mothers of PS animals were stressed once per week for 3 h (09:00–11:00 a.m. and 04:00–05:00 p.m.) with strobe light exposure in an unfamiliar dark room during early- to mid-pregnancy (from 1 week prior to conception until day 42 of gestation, total duration of gestation: ~68 days). They reacted with a short-term increase and a long-term downregulation of glucocorticoids and attenuated body weight during gestation, but no effect on reproductive effort (Schöpper et al. 2011). Mothers of control offspring were left undisturbed. After sexual maturation, females were allowed to mate with unrelated males to establish an F2 generation beginning with the sixth cycle of each individual female. Therefore, the receptive female was placed in the male’s cage for half an hour, if necessary repeatedly until copulation was observed or a copulatory plug was found. All animals were housed under standard conditions either in the same-sex groups or mothers with their litter from approximately 3 days prior to parturition (to avoid disturbance by partner females during course of birth) until weaning of the pups at an age of 21 days. As conception resulted from naturally occurring estrus of females, parturition was not synchronous in all litters. To minimize age differences in newly arranged groups of weaned offspring, animals were housed in the same-sex groups of two to four animals (ground area of enclosure: 100 cm × 50 cm, similar for both treatment groups). Feeding took place at 09:00 a.m. and comprised 15 g of standard chow (Altromin 3013, Altromin GmbH, Lage, Germany), 40 g of fresh fruit/vegetables and a handful of hay per animal as well as water ad libitum. As animals were housed in groups, we cannot exclude that individual feed intake varied between cage mates. Nevertheless, by only housing the animals from the same group together (either PS or control), the total amount per cage/group was always the same and we could exclude possible differences in individual feed intake to be reflected in our results analyzed by group. During the third trimester of gestation and during lactation, mothers received an additional 20 g of pellets, while pups were offered 10 g of standard chow and 10 g of fresh food/day.
Body weight and reproductive measurements
Body weight was determined accurately to 0.01 g from birth (maximum 2 h after birth) until weaning at day 21 and to the closest 0.5 g afterward. Measures were taken during the morning before feeding, starting with the day of birth followed by weekly intervals until weaning (day 0, 7, 14, 21) and again at weekly intervals from day 26 until day 124. We define puberty as the time period of increasing gonadal hormones for both sexes; in females external signs of estrus can be used to define onset of reproductive cycling. The reproductive stage of females was checked on a daily basis by visual inspection of the vaginal membrane, which is a suitable external marker of cycle stage in guinea pigs (Stockard and Papanicolaou 1917; Touma et al. 2001). The first day of a fully ruptured membrane was defined as estrus (day 0 of the cycle), which correlates well with physiological estrus (Young 1937). The duration of a cycle was determined by counting days between estrus (day 0) of one cycle and estrus (day 0) of the successive cycle. The day of conception (day 0 of gestation) was defined either as the day when mating was observed or when a vaginal plug was found. In three cases (one in the control and two in the PS group), when we could observe neither, the last day of fully ruptured vaginal membrane was defined as day 0 of gestation. The expected day of birth was gestational day 68. Parturition could be observed directly in nine control and six PS females. Out of these females, in three control and four PS females, total placentae could be collected and weighed within 5 min accurate to 0.01 g after cutting adjacent membranes. The gestational effort of females was calculated after Laurien-Kehnen and Trillmich (2004) as total litter weight (g)/maternal body weight at conception (g) × 100. Three newborn pups were found dead in the cage when birth had not been observed directly. All three pups originated from different litters (two control and one PS female). Those animals were included in measurements of reproductive performance to assess actual maternal effort during gestation.
Blood sampling and analysis of plasma progesterone and testosterone
To determine the concentrations of sexual hormones, blood samples were taken by puncturing marginal ear vessels and collecting the emerging blood in a heparinized capillary tube. This procedure is described by Sachser and Pröve (1984), takes less than 3 min per animal and can be performed without anesthesia. Reproductive maturation of females is characterized by the onset of the first estrus. In contrast, for males, adolescence is not a single event and is best described by increased plasma testosterone levels between days 40 and 80 (Rigaudière et al. 1976; Bauer et al. 2008a). Thus, it was necessary to use different sample regimes for males and females.
The following blood sampling regimes were performed for females: weekly intervals prior to the first estrus (d12, d19, d26, d33, d40) and every third day during the first, third and fifth reproductive cycle (Cy1d0, Cy1d3, …, Cy1d18; Cy3d0, …, Cy5d18). Progesterone was measured as a major female sex hormone fluctuating during the estrous cycle with peaks during mid-luteal phase. We abstained from measuring estrogens as large volumes of blood are needed for the analysis which could have affected the animal’s well-being (Bauer et al. 2008b). In addition, according to Graham and Clarke (1997), progesterone is probably the more important gonadal steroid for the regulation of reproductive cycles and female fertility. Supplementarily, we measured the levels of testosterone, as guinea pig females have been described to show increased levels of testosterone when being raised by mothers that experienced social instability during the time of gestation until the end of the lactational period (Kaiser et al. 2003). For analysis of testosterone in females, only samples of the days of estrus after puberty were used to avoid bias by, and influences of, the phase of reproductive cycle on hormone concentrations.
In males, testosterone is the primary sex hormone and was measured to monitor reproductive maturation. The weekly sampling regime was started the same day as in females and continued until the age of 4 months (d12, d19, d26, d33, …, d124). With this protocol, the same age range was covered in both males and females, while sex-specific characteristics were still addressed.
To minimize the influence of diurnal fluctuations of hormonal levels, all blood sampling took place at the same time of day (starting at 9:00 h). Samples (400 μl in females and 100 μl in males) were centrifuged immediately after collection and the plasma stored at −20 °C until further analysis. We extracted plasma (diluted in assay buffer to a total of 500 μl) with 5 ml of diethyl ether and measured progesterone and testosterone with an enzyme immunoassay (EIA). Details of the EIA, including cross-reactions of the antibody, are given by Schwarzenberger et al. (1996) for the measurement of progesterone concentration. The intra- and interassay coefficients of variation were 10.4 and 12.8 %, respectively. For the analysis of testosterone, we used an assay described by Palme and Möstl (1994). The intra- and interassay coefficients of variation were 7.9 and 12.9 %, respectively. All samples were run in duplicate.
Statistical analysis
Analysis of data was performed with R 2.9.1 (R Developmental Core Team 2007) with the additional packages nlme and multcomp (Pinheiro et al. 2007; Hothorn et al. 2008) and tested two-tailed.
For the analysis of body weight in offspring, we used a linear mixed effects (LME) model with time (used as a 2nd order polynomial as the quadratic term was significant), litter size (one to five pups per litter), sex (females and males) and group (control and PS) as fixed factors. Animal was entered as random factor to adjust for repeated measurements, and mother was entered as random factor to adjust for relatedness between litter mates. All interactions were included at first and the model then reduced stepwise by exclusion of non-significant interactions.
For the analysis of reproductive parameters in females, the onset of first estrus and body weight at this point of time (one female had to be excluded from analysis due to unclear onset of reproductive cycles) as well as reproductive performance were analyzed using LME models with group (control and PS) as fixed factor and mother as random factor. For the analysis of cycle length, additionally the number of the cycle was included as fixed factor and animal as random factor. Post hoc comparison of groups at the different cycles was performed to unravel significant interactions. Sex ratio of F2 offspring was analyzed using Fisher’s exact test.
Differences in concentrations of plasma progesterone in females were tested using an LME model with day, group and—only in cycling females—the number of the cycle as fixed factors, and animal and mother as random factors. Post hoc comparisons were performed to analyze significant group:cycle interactions.
Analysis of plasma testosterone concentrations both in F1 males and F1 females was carried out using an LME model with day and group as fixed factors and animal as random factor. As inclusion of mother as the second random factor increased model complexity but did not decrease residual deviance, we used the model without this random factor. For data collected during reproductive maturity of females, the LME model for testosterone included the number of the cycle and group as fixed factors and both mother and animal as random factors.
All response variables were Box–Cox transformed for the analysis if necessary for normal distributions of residuals. Achievement of this was checked via visual inspection of histograms, qq plots and random distribution of fitted values. Results are presented as F values with degrees of freedom and corresponding p value. If both the interaction and the main effect were significant, only interactions were the subject of interpretation. Note that the numerator degrees of freedom of 2 for the time effect indicate the use of a quadratic polynomial. The observed differences were considered significant at p values <0.05.
Results
Body weight
Body weight increase from birth to the age of 124 days was significantly influenced by litter size, with animals from large litters growing more slowly than those from small ones. Additionally, body weight differed significantly between groups over the course of time, with sex as an influencing factor (LME: time:sex:group: F 2/1615 = 22.482, p < 0.001). In males, body weight development was similar in PS and control animals (Fig. 1a). Among females, however, despite similar birth weight, PS animals put on significantly more weight over the course of time than control females (Fig. 1b).
Reproductive parameters
In females, onset of the first estrus occurred somewhat earlier in PS than control animals, at a mean age of 34 days in PS-compared with 41 days in control animals. However, the difference between 1(LME: group: F 1/9 = 1.447, p = 0.260).
Body weight was not significantly different between groups at this point of time (mean ± SEM: control 344.8 ± 7.7, PS 347.9 ± 19.1, LME: group: F 1/9 = 0.444, p = 0.522).
Duration of the first five cycles was significantly different between groups depending on the cycle number. The length of cycles was relatively constant in PS females, while in control animals, the first cycle was longer and the following cycles were slightly shorter than in the PS animals (Fig. 2, LME: number of cycle:group: F 4/67 = 4.087, p < 0.005). Post hoc test between groups over the cycles revealed a significant difference for the second cycle, with control females showing shorter cycle length than PS females (post hoc: Cy1C–Cy1PS: p = 0.229, Cy2C–Cy2PS: p = 0.003, Cy3C–Cy3PS: p = 0.574, Cy4C–Cy4PS: p = 0.1, Cy5C–Cy5PS: p = 0.512).
All mated females conceived and maintained pregnancy until natural parturition. None of the reproductive parameters measured was significantly different between PS and control group (LME’s: p > 0.2).
Hormonal data
Concentration of progesterone and testosterone in females
Prior to the first estrus, levels of progesterone rose with time and were significantly lower in PS compared to control females (Fig. 3a, LME: day: F 3/56 = 5.234, p = 0.003, group: F 1/7 = 6.397, p = 0.039). During the first, third and fifth cycle, progesterone concentrations differed significantly between days of cycle and between groups, both depending on the number of the cycle (Fig. 3b–d, LME: cycle:day: F 12/349 = 2.701, p = 0.002, cycle:group: F 2/349 = 4.923, p = 0.008).
Plasma progesterone concentrations (mean ± SEM) of F1 females separated by group (control, open circles; PS, closed circles) a during the prepubertal phase (n (control) = 11/11/11/9 + 6, n (PS) = 8/8/7/5 + 3) and b–d over the course of the first, third and fifth cycle (n (control) = 11 (except Cy3d6, Cy5d0/d18 n = 10 and Cy3d18 n = 8), n (PS) = 8 (except Cy1d0/d6 n = 7)
Generally, levels of progesterone varied over the course of the cycle, starting with levels <1 ng/ml plasma at estrus and rising thereafter to more than threefold measures at day 9 and declined again to baseline levels for the next estrus. This time course of progesterone levels was less pronounced during the first cycle than the following ones. Especially in control females, progesterone levels did not increase as much as in PS females, during the first cycle, who showed already almost similar peak progesterone values compared to the following cycles. However, post hoc comparison of mean progesterone levels per cycle between groups revealed no significant differences between groups during each cycle (post hoc: Cy1C–Cy1PS: p = 0.120; Cy3C–Cy3PS: p = 0.855, Cy5C–Cy5PS: p = 0.991).
Concentrations of testosterone in females during the first 40 days of life did not differ significantly between groups, but varied over time with mean levels of testosterone ranging between 25 and 85 pg/ml plasma (Fig. 4, LME: day: F 1/57 = 9.991, p = 0.003, group: F 1/17 = 0.424, p = 0.523). Over the course of the first six cycles, concentrations of testosterone increased significantly, with PS females showing consistently higher levels than control females. However, this difference did not reach significance (LME: cycle: F 5/89 = 4.090, p = 0.002; group: F 1/10 = 2.298, p = 0.161).
Plasma testosterone concentrations (mean ± SEM) of F1 females separated by group (control, open circles; PS, closed circles) over the course of prepubertal phase and over the days of estrus of the first six cycles (n (control) = 11/11/11/9/5//11 except Cy5 n = 10, n (PS) = 8/8/7/5/3//8 except Cy1 n = 7)
Concentration of testosterone in males
In males, the levels of testosterone revealed no significant difference between control and PS animals between day 12 and 124. In both groups, overall levels of testosterone increased with a peak at day 75 and subsequently decreased to moderate levels again (Fig. 5, LME: day: F 16/357 = 11.441, p < 0.001; group: F 1/23 = 1.161, p = 0.292).
Plasma testosterone concentrations (mean ± SEM) over the course of the first 4 months (124 days) of F1 males separated by group (control, open circles, n = 13 except d12, 33, 110 n = 12 and d40, 47, 68, 89, 96 n = 11; PS, closed circles, n = 12, except d33, 54, 96, 110 n = 11, d26, 40 n = 10 and d89, 124 n = 9)
Discussion
Our prenatal stress treatment (i.e., exposure of the pregnant mothers to strobe light during the first two-thirds of gestation once a week in an unfamiliar dark room) showed comprehensive effects in female, but negligible effects in male guinea pigs. In females, body weight and reproductive parameters were altered, indicating advanced maturity in prenatally stressed animals. In contrast, among males no significant effects of prenatal stress on body weight or hormonal measures were found.
The stress treatment of the pregnant mothers resulted in a short-term increase in mothers’ cortisol levels, but in a long-term downregulation of mothers’ hypothalamic–pituitary–adrenal (HPA) axis (Schöpper et al. 2011). Along with these hormonal changes, maternal weight gain was reduced during gestation in stressed compared to control dams. Maternal behavior and feeding offspring were also slightly altered as stressed mothers showed slightly higher levels of aggression and milk yield was reduced even though offspring suckled for a longer duration (for details see Klaus 2010; Schöpper et al. 2011). We therefore assume that the effects seen in the F1 generation are not only related to the original strobe light exposure of the dams, but rather to the stress treatment with all its effects on the physiology and behavior of the mother. It is clear that chronic moderate stress does not affect a single parameter and all remaining functions remain unaffected, rather glucocorticoids interfere with almost any physiological function and therefore the whole stress–effect complex is addressed here. This is an approach taking the biological unit—the whole animal—into account, instead of trying to split mechanistic pathways that actually interact. The stress treatment of pregnant mothers was capable of having long-term effects on the F1 generation, changing characteristics of growth and reproduction.
Body weight
Animals deriving from large litters grew slower compared to those from small litters. This inverse relationship between litter size and body weight has been shown for guinea pigs before (Fey and Trillmich 2008). Prenatal crowding conditions in the womb are believed to be the cause for these differences in early body weight. Furthermore, this difference depending on litter size could be shown to even increase over time in rats probably due to competition for milk as an energy resource (Chahoud and Paumgartten 2009). Independent of litter size, we found a treatment effect on offspring body weight development as a function of sex. For males, body weight did not differ significantly between prenatally stressed and control animals. This is in accordance with the findings of Kemme et al. (2007, 2008) and Banjanin et al. (2004) who likewise did not find any differences in growth of male guinea pig offspring after prenatal doses of synthetic glucocorticoids or social instability. However, if stress was induced during late gestation, prenatally stressed male guinea pigs were lighter than their control counterparts (Kapoor and Matthews 2005; Emack et al. 2008). The time and origin of stress imposition has already been described as crucial for certain effects (Kapoor and Matthews 2005) and thus are believed to be the cause of the different findings.
In females, however, we found significant differences in body weight development depending on the group. However, the differences were in the opposite direction than we had predicted. Prenatally stressed females put on more and not less weight over time compared to control females. This highly significant result is noteworthy as it shows a distinct sex-specific effect and has not been described for female guinea pigs before. There is some literature showing reduced body weight after prenatal disturbances, especially right after birth, but also findings of increased weight gain are described [lower: e.g., Ikegami et al. 1997 (lambs); Schneider et al. 1999 (primates); McCabe et al. 2001 (guinea pigs); higher: Götz et al. 2008 (rats)]. Götz et al. (2008) for example investigated prenatal stress treatment in rats and found increased body weights in stressed animals comparable to our results, however, in both sexes. Sex-specific effects have been described for female rat offspring with prenatally stressed females putting on more weight over time compared to the control females (Mueller and Bale 2006). One of many possible ideas to explain sex differences and the assumed increased vulnerability of females to prenatal stress is given by Montano et al. (1993). The authors described higher transfer of maternal glucocorticoids via the placenta to female fetus compared to male offspring. Due to our study design, it was not possible to cross-foster the pups, so that mother–pup interaction might also influence offspring’s physiology. Milk intake could be argued as a critical factor influencing growth curves in juveniles (Götz et al. 2008). Yet, Klaus (2010) found no differences in the overall milk yield between groups, but found a slightly lower milk yield on lactational day 12 in PS mothers. As both sexes as well as both groups were fed the same solid diet and we still see a highly significant effect on PS females, we doubt the influence of single day differences in milk intake to be decisive.
We rather suppose the offspring to differ in energy metabolism. Glucocorticoids play a modulating role in weight gain, because neural pathways of stress response and energy homeostasis are tightly linked (Phillips et al. 1998; Nyrienda and Seckl 1998; Nieuwenhuizen and Rutters 2008). There are studies analyzing the effects of prenatal stress on later metabolism [Lesage et al. 2004 (no effect on later body weight, but insulin resistance in rats), D’mello and Liu 2006 (no effect), Mueller and Bale, 2006 (effects seen after stress during mid to late pregnancy in males only and also leptin and glucose measurements were affected)]. We think it possible that the early prenatal stress in our study may have been able to alter metabolic pathways that lead to better energy utilization, especially in females. From studies of undernourishment in humans and animals—for which also glucocorticoids are believed to transfer nutritional cues to the developing brain—it is known that especially early gestation is a crucial time window for programming later body weight of offspring and risk of obesity (Ravelli et al. 1976; Symonds et al. 2007).
According to the mismatch hypothesis of Gluckman and Hanson (2004), if postnatal conditions do not match what was predicted prenatally, effects can become accelerated and even be detrimental at long term. Concerning growth, prenatal deprivation will favor an offspring phenotype that is adapted to poor conditions and is able to cope with low amounts of food. Therefore, metabolic rate will decrease and energy storage will be improved. This phenotype is well suited for harsh conditions—but what if the predicted future is better than expected? The mismatch of predicted and actual condition can lead to a phenotype that is at higher risk to develop obesity, diabetes and cardiovascular disease—taken together as metabolic disease (Gluckman et al. 2008b). Postnatal conditions in our study met all physiological needs of a young guinea pig and therewith were presumably better than the ones predicted by the PS animals. Without intention we might have created a mismatch that led to the PS females putting on significantly more weight than the control animals. We could speculate that the sex difference in body weight of PS animals is due to higher fat mass in females. Stored fat can be directly converted to milk during reproductive activity (Valencak et al. 2009) and might therefore be advantageous especially for females. More mechanistic studies are needed to evaluate this assumption and possible translatability for human medical conditions like obesity and metabolic diseases.
Reproductive parameters
While we found that females of both groups were similar in body weight at first estrus, PS females were younger compared with their control counterparts at this point of time. The reasons why the results did not reach significance were speculated to follow from the high inter-individual variability (range: control 28–55, PS 23–42). According to Trillmich et al. (2006), there is no critical body mass to be obtained for first estrus in guinea pigs. However, the steeper the growth curve until weaning, the earlier onset of estrus occurred in their study (Trillmich et al. 2006). The same might be true in our study, with faster growing PS females being slightly younger at first estrus than control females.
Female guinea pigs are theoretically able to reproduce from the very first cycle onward (Rood and Weir 1970; Trillmich et al. 2006), but successful mating is dependent on normal cycle length (15–17 days) which the majority of females do not reach until the second or third cycle (Rood and Weir 1970). In our study, cycle length was influenced by group in interaction with the number of cycle. In PS females, cycle length was almost constant for all six measured cycles, lasting around 17 days. Control females however had a longer first cycle of a mean over 18 days and slightly shorter subsequent cycles compared to PS females. As proper functioning of the hypothalamic–pituitary–gonadal axis is crucial for regular cycling, we assume hormonal cues to be more mature in PS females during the first cycle (see below).
Concerning reproductive performance of females, there was no indication for differences between groups. None of the parameters taken was significantly different in PS versus control females, which is in accordance with the findings in rats (Götz et al. 2008). There are reports of reduced reproductive success in prenatally stressed female mink and mice (e.g., Politch and Herrenkohl 1984; Jeppesen and Heller 1986). In guinea pigs, however—to the best of our knowledge—no comparable studies are available. Species differences in vulnerability as well as timing, intensity and type of stressor are crucial factors that may influence the variability of the results described.
Hormonal data
Concentration of progesterone and testosterone in females
In our study, concentrations of progesterone were very low before onset of first estrus. Progesterone generally starts to rise during reproductive maturation. It reflects the growth of corpora lutea of the ovaries, showing the highest levels during the mid-luteal phase around days 8 and 9 (Feder et al. 1968; Challis et al. 1971; Blatchley et al. 1976). During the first cycle, the levels of progesterone do not yet approximate the levels of subsequent mature cycles and are normally at medium levels (Westfahl and Vekasy 1988). This was also the case in the control females of our study, whereas PS females showed higher levels of progesterone both prior to first estrus and during the first cycle. Bauer et al. (2009) found a similar situation in guinea pig offspring whose mothers were fed a low quality diet during gestation. Both low protein diet as well as stress exposure of the mothers should act as cues of harsh environments, which might adapt their offspring to the conditions foreseen and therefore accelerate maturation. For both, nutritional interventions and stressful events, a common mechanism in transfer of programming effects is suspected to involve glucocorticoids (Seckl 2004). As cycle length of guinea pigs can be shortened by administration of progesterone during the first 6 days of the cycle (Woody et al. 1967), the elevated levels of progesterone levels found in our PS females may be the reason for the shorter first cycle.
We further tested for testosterone levels in females, as an increase in the levels of this hormone is associated with the disposition to masculinization that has been described in prenatally stressed female guinea pigs (Sachser and Kaiser 1996; Kaiser et al. 2003). Testosterone was also increased in prenatally stressed girls when mothers were pregnant at the time of the Chernobyl disaster (Huizink et al. 2008). However, no such differences could be seen in our females.
Concentration of testosterone in males
In contrast to the distinctive differences in hormonal levels between prenatally stressed and control animals found in females, no such effects could be seen in testosterone levels of males. These results are in accordance with earlier programming studies in guinea pigs, using prenatal stress treatment as well as nutritional challenge (Banjanin et al. 2004; Kemme et al. 2008; Bauer et al. 2009). However, also contradictory findings are available for this species. While increased testosterone levels were apparent in male offspring of mothers that experienced social instability during whole gestation or until weaning (Kaiser et al. 2003; Kemme et al. 2007), Kapoor and Matthews (2005) described decreased levels after strobe light treatment during late gestation. The inconsistency of the results may be due to different stressors used and the time windows during which stress was applied. Furthermore, it might point to more complex interrelations than we are currently able to address and further investigations are needed, especially in the mechanistic approach to evaluate the way of programming testosterone levels in males.
Conclusions
In summary, we could show in this study that prenatal stress during early- to mid-gestation has distinctive effects on hormonal traits of reproductive function in guinea pig offspring. The effects seen proved to be highly sex specific and point to a different biological relevance for males and females.
For males, prenatal stress did not affect growth or levels of gonadal steroids. In females, on the other hand, prenatally stressed individuals showed significantly advanced growth, a slightly earlier onset of estrus and more mature first estrus cycle, probably indicating advanced competence to reproduce. If a female in good condition is additionally early in estrus and the first cycle is more mature compared to the control animals, the potential to successfully reproduce is multiplied. It would be of great interest to compare the actual reproductive output at such an early stage of maturation to assess the biological relevance of such differences between groups. Our animals, however, were not mated before the sixth cycle, when both groups had normal cycle lengths and comparable levels of progesterone. This may explain why reproductive performance per se was not different between groups.
Taken together, our results suggest that prenatal experience of harsh environmental conditions via increased maternal stress might predict a postnatal continuation of such a situation. In line with our findings in females, evidence from animal and human studies indicates that poor conditions during fetal development can lead to premature offspring (Cooper et al. 1996; Gluckman and Hanson 2006; Sloboda et al. 2007). The biological relevance of an early onset of reproduction is evident, especially if the overall expectation for lifespan is expected to be poor, as lifetime reproductive success might be improved if females start reproducing early.
However, when looking at long-term consequences of prenatal disturbances, especially beyond the age of reproductive activity as is the case in humans, negative effects of hormonal alterations may be anticipated. Epidemiological as well as experimental studies indicate that diseases like diabetes, obesity, coronary heart disease and stress reactivity are possibly triggered by prenatal programming (Entringer et al. 2008; Barker 2002; Lesage et al. 2004; Darnaudéry and Maccari 2008). In the majority of cases endocrine function, especially HPA function, is affected, thereby altering physiology and probably accounting for later diseases (review: Bertram and Hanson 2002). Yet, apart from such pathological long-term consequences of poor prenatal condition as seen in our study, shorter-term effects that are rather adaptive for the predicted conditions may well be advantageous.
Abbreviations
- HPA axis:
-
Hypothalamic–pituitary–adrenal axis
- LME model:
-
Linear mixed effects model
- PS:
-
Prenatally stressed
References
Banjanin S, Kapoor A, Matthews SG (2004) Prenatal glucocorticoid exposure alters hypothalamic–pituitary–adrenal function and blood pressure in mature male guinea pigs. J Physiol 558:305–318
Barker DJP (2002) Fetal programming of coronary heart disease. Trends Endocrinol Metab 13:364–368
Bauer B, Womastek I, Dittami J, Huber S (2008a) The effects of early environmental conditions on reproductive and somatic development of juvenile guinea pigs (Cavia aperea f. porcellus). Gen Comp Endocrinol 155:680–685
Bauer B, Palme R, Machatschke IH, Dittami J, Huber S (2008b) Non-invasive measurement of adrenocortical and gonadal activity in male and female guinea pigs (Cavia aperea f. porcellus). Gen Comp Endocrinol 156:482–489
Bauer B, Dittami J, Huber S (2009) Effects of nutritional quality during early development on body weight and reproductive maturation in guinea pigs (Cavia aperea f. porcellus). Gen Comp Endocrinol 161:384–389
Bertram CE, Hanson MA (2002) Prenatal programming of postnatal endocrine responses by glucocorticoids. Reproduction 124:459–467
Blatchley FR, Donovan BT, Ter Haar MB (1976) Plasma progesterone and gonadotrophin levels during the estrous cycle of the guinea pig. Biol Reprod 15:29–38
Chahoud I, Paumgartten FJR (2009) Influence of litter size on postnatal growth of rat pups: is there a rationale for litter-size standardization in toxicity studies? Environ Res 109:1021–1027
Challis JRG, Heap RB, Illingworth DV (1971) Concentrations of oestrogen and progesterone in the plasma of non-pregnant, pregnant and lactating guinea pigs. J Endocrinol 51:333–348
Cooper C, Kuh D, Egger P, Wadsworth M, Barker D (1996) Childhood growth and age at menarche. Br J Obstet Gynaecol 103:814–817
D’mello AP, Liu Y (2006) Effects of maternal immobilization stress on birth weight and glucose homeostasis in the offspring. Psychoneuroendocrinology 31:395–406
Darnaudéry M, Maccari S (2008) Epigenetic programming of the stress response in male and female rats by prenatal restraint stress. Brain Res Rev 57:571–585
Du M, Tong J, Zha J, Underwood KR, Zhu SP, Ford SP, Nathanielsz PW (2010) Fetal programming of skeletal muscle development in ruminant animals. J Anim Sci 88:E51–E60
Dufty AMJr, Clobert J, Møller AP (2002) Hormones, developmental plasticity and adaptation. Trends Ecol Evol 17:190–196
Emack J, Kostaki A, Walker CD, Matthews SG (2008) Chronic maternal stress affects growth, behaviour and hypothalamo–pituitary–adrenal function in juvenile offspring. Horm Behav 54:514–520
Entringer S, Wüst S, Kumsta R, Layes IM, Nelson EL, Hellhammer DH, Wadhwa PD (2008) Prenatal psychological stress exposure is associated with insulin resistance in young adults. Am J Obstet Gynecol 199:498.e1–498.e7
Feder HH, Resko JA, Goy W (1968) Progesterone concentrations in the arterial plasma of guinea-pigs during the oestrous cycle. J Endocrinol 40:505–513
Fey K, Trillmich F (2008) Sibling competition in guinea pigs (Cavia aperea f. porcellus): scrambling for mother’s teats is stressful. Behav Ecol Sociobiol 62:321–329
Gluckman PD, Hanson MA (2004) Living with the past; evolution, development, and patterns of disease. Science 305:1733–1736
Gluckman PD, Hanson MA (2006) Evolution, development and timing of puberty. Trends Endocrinol Metababol 17:7–12
Gluckman PD, Hanson MA, Cooper C, Thornburg KL (2008a) Effect of in utero and early-life conditions on adult health and disease. N Engl J Med 359:61–73
Gluckman P, Hanson M, Beedle A, Raubenheimer D (2008b) Fetal and neonatal pathways to obesity. In: Korbonits M (ed) Obesity and metabolism. Front Horm Res, vol 36. Karger, Basel, pp 61–72
Götz AA, Wolf M, Stefanski V (2008) Psychosocial maternal stress during pregnancy: effects on reproduction for F0 and F1 generation laboratory rats. Physiol Behav 93:1055–1060
Graham JD, Clarke CL (1997) Physiological action of progesterone in target tissues. Endocr Rev 18:502–519
Harris A, Seckl J (2011) Glucocorticoids, prenatal stress and the programming of disease. Horm Behav 59:279–289
Hothorn T, Bretz F, Westfall P, Heiberger RM (2008) Multcomp: simultaneous inference in general parametric models. R package version 1.0-0. http://cran.rproject.org/web/packages/multcomp/vignettes/generalsiminf.pdf
Huizink AC, Bartels M, Rose RJ, Pulkkinen L, Eriksson CJP, Kaprio J (2008) Chernobyl exposure as stressor during pregnancy and hormone levels in adolescent offspring. J Epidemiol Community Health 62:e5
Ikegami M, Jobe AH, Newnham J, Polik DH, Willet KE, Sly P (1997) Repetitive prenatal glucocorticoids improve lung function and decrease growth in preterm lambs. Am J Respir Crit Care Med 156:178–184
Jeppesen LL, Heller KE (1986) Stress effects on circulating eosinophil leukocytes, breeding performance, and reproductive success of ranch mink. Scientifur 10:15–18
Kaiser S, Kruijver FPM, Swaab DF, Sachser N (2003) Early social stress in female guinea pigs induces a masculinization of adult behavior and corresponding changes in brain and neuroendocrine function. Behav Brain Res 144:199–210
Kapoor A, Matthews SG (2005) Short periods of prenatal stress affect growth, behaviour and hypothalamo–pituitary–adrenal axis activity in male guinea pig offspring. JPhysiol 566:967–977
Kemme K, Kaiser S, Sachser N (2007) Prenatal maternal programming determines testosterone response during social challenge. Horm Behav 51:387–394
Kemme K, Kaiser S, Sachser N (2008) Prenatal stress does not impair coping with challenge in later life. Physiol Behav 93:68–75
Klaus T (2010) Effects of gestational stress on maternal performance and offspring growth and behaviour in the guinea pig (Cavia aperea f. porcellus) Diploma thesis, Vienna University
Laurien-Kehnen C, Trillmich F (2004) Maternal food restriction delays weaning in the guinea pig, Cavia porcellus. Anim Behav 68:303–312
Lesage J, Del-Favero F, Leonhardt M, Louvart H, Maccari S, Vieau S, Darnaudery M (2004) Prenatal stress induces intrauterine growth restriction and programmes glucose intolerance and feeding behaviour disturbances in the aged rat. J Endocrinol 181:291–296
McCabe L, Marash D, Li A, Matthews SG (2001) Repeated antenatal glucocorticoid treatment decreases hypothalamic corticotrophin releasing hormone mRNA but not corticosteroid receptor mRNA expression in the fetal guinea-pig brain. J Neuroendocrinol 13:425–431
Montano MM, Wang MH, vom Saal FS (1993) Sex differences in plasmacorticosterone in mouse fetuses are mediated by differential placental transport from the mother and eliminated by maternal adrenalectomy or stress. J Reprod Fertil 99:283–290
Moritz KM, Dodic M, Wintour EM (2003) Kidney development and the fetal programming of adult disease. BioEssays 25:212–220
Mueller BR, Bale TL (2006) Impact of prenatal stress on long term body weight is dependent on timing and maternal sensitivity. Physiol Behav 88:605–614
Nathanielsz PW (2006) Animal models that elucidate basic principles of the developmental origins of adult disease. ILAR J 47:73–82
Nieuwenhuizen AG, Rutters F (2008) The hypothalamic–pituitary–adrenal-axis in the regulation of energy balance. Physiol Behav 94:169–177
Nyrienda MJ, Seckl JR (1998) Intrauterine events and the programming of adulthood disease: the role of fetal glucocorticoid exposure (review). Int J Mol Med 2:607–614
Palme R, Möstl E (1994) Biotin–streptavidin enzyme immunoassay for the determination of oestrogens and androgens in boar faeces. In: Görög S (ed) Advances of steroid analysis ‘93. Akadémiai Kiadó, Budapest, pp 111–117
Palmer AC (2011) Nutritional mediated programming of the developing immune system. Adv Nut 2:377–395
Phillips DIW, Barker DJP, Fall CHD, Seckl JR, Whorwood CB, Wood PJ, Walker BR (1998) Elevated plasma cortisol concentrations: a link between low birth weight and the insulin resistance syndrome. J Clin Endocinol Metab 83:757–760
Pinheiro J, Bates D, DebRoy S, Sarkar D, the R core team (2007) Nlme: linear and nonlinear mixed effects models. R package version 3.1-89. http://CRAN.R-project.org/package=nlme
Politch JA, Herrenkohl LR (1984) Effects of prenatal stress on reproduction in male and female mice. Physiol Behav 32:95–99
R Development Core Team (2007) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. ISBN 3-900051-07-0 http://www.R-project.org
Ravelli GP, Stein ZS, Susser MW (1976) Obesity in young men after famine exposure in utero and early infancy. N Engl J Med 295:349–353
Rhind SM, Rae MT, Brooks AN (2001) Effects of nutrition and environmental factors on the fetal programming of the reproductive axis. Reproduction 122:205–214
Rigaudière N, Pelardy G, Robert A, Delost P (1976) Changes in the concentrations of testosterone and androstenedione in the plasma and testis of the guinea-pig from birth to death. J Reprod Fertil 48:291–300
Rood JP, Weir B (1970) Reproduction in female wild guinea-pigs. Reproduction 23:393–409
Sachser N, Kaiser S (1996) Prenatal social stress masculinizes the females’ behaviour in guinea pigs. Physiol Behav 60:589–594
Sachser N, Pröve E (1984) Short-term effects of residence on the testosterone responses to fighting in alpha male guinea pigs. Aggr Behav 10:285–292
Schneider ML, Roughton EC, Koehler A, Lubach GR (1999) Growth and development following prenatal stress in primates: an examination of ontogenetic vulnerability. Child Dev 70:263–274
Schöpper H, Palme R, Ruf T, Huber S (2011) Chronic stress in pregnant guinea pigs (Cavia aperea f. porcellus) attenuates long-term stress hormone levels and body weight gain, but not reproductive output. J Comp Physiol B 181:1089–1100
Schöpper H, Palme R, Ruf T, Huber S (2012) Effects of prenatal stress on hypothalamic–pituitary–adrenal (HPA) axis function over two generations of guinea pigs (Cavia aperea f. porcellus) Gen Comp Endocrinol 176:18–27
Schwarzenberger F, Tomasova K, Holeckova D, Matern B, Möstl E (1996) Measurement of fecal steroids in the black rhinoceros (Diceros bicornis) using group-specific enzyme immunoassay for 20-oxo-pregnanes. Zoo Biol 15:159–171
Seckl JR (2004) Prenatal glucocorticoids and long-term programming. Eur J Endocrinol 151:U49–U62
Sloboda DM, Hart R, Doherty DA, Pennell CE, Hickey M (2007) Age at menarche: influences of prenatal and postnatal growth. J Clin Endocrinol Metab 92:46–50
Stockard CR, Papanicolaou GN (1917) The existing of a typical oestrus cycle in the guinea-pig—with a study of histological and physiological changes. Am J Anat 22:225–283
Symonds ME, Stephenson T, Gardner DS, Budge H (2007) Long-term effects of nutritional programming of the embryo and fetus: mechanisms and critical windows. Reprod Fertil Dev 19:53–63
Touma C, Palme R, Sachser N (2001) Different types of oestrus cycle in two closely related South American rodents (Cavia aperea and Galea musteloides) with different social and mating systems. Reproduction 121:791–801
Trillmich F, Laurien-Kehnen C, Adrian A, Linke S (2006) Age at maturity in cavies and guinea-pigs (Cavia aperea and Cavia aperea f. porcellus): influence of social factors. J Zool 268:285–294
Valencak TG, Tataruch F, Ruf T (2009) Peak energy turnover in lactating European hares: the role of fat reserves. J Exp Biol 212:231–237
Westfahl PK, Vekasy MS (1988) Changes in serum and ovarian steroids during reproductive development in the female guinea pig. Biol Reprod 39:1086–1092
Woody CO, First NL, Pope AL (1967) Effect of exogenous progesterone on estrous cycle length. J Anim Sci 26:139–141
Young WC (1937) The vaginal smear picture, sexual receptivity and the time of ovulation in the guinea pig. Anat Rec 67:305–325
Acknowledgments
We are grateful to the anonymous reviewers for their comments that helped to improve this manuscript. The authors thank Edith Klobetz-Rassam for the great support with biochemical analyses. We appreciate the help of Jasmin Höfler in animal care taking. Facilities for animal keeping were kindly provided by the Institute of Virology. This study was financially supported by the Ph.D. Initiative Program BIOREC of the University of Veterinary Medicine, Vienna.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by G. Heldmaier.
Rights and permissions
About this article
Cite this article
Schöpper, H., Klaus, T., Palme, R. et al. Sex-specific impact of prenatal stress on growth and reproductive parameters of guinea pigs. J Comp Physiol B 182, 1117–1127 (2012). https://doi.org/10.1007/s00360-012-0680-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00360-012-0680-9