Abstract
Some mammals indigenous to desert environments, such as camels, cope with high heat load by tolerating an increase in body temperature (T b) during the hot day, and by dissipating excess heat during the cooler night hours, i.e., heterothermy. Because diurnal heat storage mechanisms should be favoured by large body size, we investigated whether this response also exists in Asian elephants when exposed to warm environmental conditions of their natural habitat. We compared daily cycles of intestinal T b of 11 adult Asian elephants living under natural ambient temperatures (T a) in Thailand (mean T a ~ 30°C) and in 6 Asian elephants exposed to cooler conditions (mean T a ~ 21°C) in Germany. Elephants in Thailand had mean daily ranges of T b oscillations (1.15°C) that were significantly larger than in animals kept in Germany (0.51°C). This was due to both increased maximum T b during the day and decreased minimum T b at late night. Elephant’s minimum T b lowered daily as T a increased and hence entered the day with a thermal reserve for additional heat storage, very similar to arid-zone ungulates. We conclude that these responses show all characteristics of heterothermy, and that this thermoregulatory strategy is not restricted to desert mammals, but is also employed by Asian elephants.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Elephants are the largest terrestrial animals with an extremely small surface-to-volume ratio, which, while it can reduce radiant heat load, also restricts heat loss. Still, both African and Asian elephants typically live in warm climates with average annual temperatures reaching up to 35°C (Oliver 2005). Under these conditions, small temperature gradients between the animal and its environment render heat loss via convection and radiation rather ineffective, at least during daytime. In elephants, heat dissipation is further restricted by their lack of sweat glands (Hiley 1975; Mariappa 1986; Wright and Luck 1984) and the absence of panting (Hiley 1975; Robertshaw 2006). While elephants have means of behavioural thermoregulation and passive diffusion of water through the skin that may slightly contribute to heat dissipation (Hiley 1975), they have no identified evaporative heat-loss mechanism, such as panting, which can be activated as a part of thermal homeostasis (Robertshaw 2006).
Given these physiological constraints it has been suggested that the principal thermoregulatory mechanism by which elephants cope with warm environments is simply the storage of heat during day and cooling during night, which would be highly efficient in an animal of this size (Elder and Rodgers 1975; Hiley 1975). Hence, Elder and Rodgers (1975) suggested that the elephant’s thermoregulation is like that of the camel, which probably is the best known case of increased daily fluctuations of body temperature (T b), i.e., heterothermy, as a strategy to conserve both energy and water under hot and dry conditions (Schmidt-Nielsen et al. 1956). Meanwhile, this physiological mechanism has been identified in number of mammals indigenous to desert environments (Langman and Maloiy 1989; Louw and Seely 1982; Ostrowski et al. 2003; Ostrowski and Williams 2006; Taylor 1969, 1970; Taylor and Lyman 1967; Willmer et al. 2005). However, the concept of heterothermy as an adaptive mechanism remained a matter of debate, because studies on captive animals, such as the study by Schmidt-Nielsen et al. (1956) on camels, may prevent opportunities for behavioural thermoregulation (e.g., Mitchell et al. 2002). Subsequently, this criticism was refuted by studies demonstrating the occurrence of heterothermy in free-living Arabian oryx (Oryx leucoryx) (Ostrowski et al. 2003; Hetem et al. 2010) and in small Arabian sand gazelles (Gazella subgutturosa marica) (Ostrowski and Williams 2006).
There are, however, several problems concerning the question whether or not the elephants indeed use diurnal heat storage and nocturnal heat dissipation to cope with increasing T a. First, while this type of thermoregulation has been reported for animals in hot desert environments, with high levels of solar radiation in which T a routinely exceeds T b, it may be questionable whether excessive heat storage is actually used by elephants under warm, but less severe climatic conditions. Second, the actual evidence for some degree of heterothermy in elephants is weak. While Elder and Rodgers (1975) reported a high variability of T b in African elephants, which was correlated with T a, these data were obtained by chasing and chemically immobilizing the animals. Hiley (1975) reported a strong increase of T b (by 2.1°C) during the day under conditions of high heat load in the African savannah, and evidence for facilitated cooling in the evening, but measurements were apparently restricted to a single animal. Also, none of these studies recorded continuous, 24 h data of T b. The only previous study that did employ continuous measurements of T b (Kinahan et al. 2007) failed to detect the evidence for a range of daily T b changes in African elephants (0.8°C) that would much exceed that expected for a mammal of this body size. However, this study was conducted in three individuals over a short period of similar and very moderate T a (mean ~ 21°C), and upto now, there was no direct, experimental comparison of thermoregulation in elephants exposed to different ranges of T a.
Finally, heterothermic desert mammals under heat load typically not only tolerate their T b to increase during the day (which, theoretically, could be an unavoidable response to heat stress), but allow T b to fall below normothermic levels at night, which enables the animals to start the next day with a thermal reserve (Louw and Seely 1982; Willmer et al. 2005). It is this characteristic of an increase of the daily T b rhythm in both directions which indicates its adaptive value. For elephants, however, there are no published data suggesting that increased heat load also affects the extent of nocturnal cooling.
Therefore, we continuously measured temperatures in the gastrointestinal tract of Asian Elephants (Elephas maximus, Linnaeus 1758), as a proxy for T b. Elephants were exposed to two different thermal conditions. One group was kept at moderate T a (mean ~ 21°C) in a Zoo with outdoor enclosure in Germany, the second group was living in Thailand under warm climatic conditions typical for their natural habitat (mean T a ~ 30°C). We predicted that, if elephants use daily heat storage and dissipation for thermoregulation, animals in the warm environment would show both higher maximum daily T b and lower minimum T b compared with elephants at cooler temperatures. We also predicted that, if heat storage was a regulated process, the range of daily rhythms in T b and thermal conductance during nocturnal cooling should be continuous functions of mean daily T a.
Materials and methods
Study site and elephants in Germany
Measurements under moderate climatic conditions were carried out in five female and one male Asian elephants (aged between 7 and 48 years) at the Munich Zoo Hellabrunn, Germany (48°1′N, 11°55′E) between April and August 2008. The animals were housed together day and night. During August the elephants had free access to both indoor and outdoor facilities, but chose to remain indoors during night. During the remaining observation period the elephants were allowed to stay outdoors during the daytime only (0800–1700 hours). The time spent outdoors averaged 8 h per day (i.e., ~90% of the time outdoor facilities were accessible). Their diet was a mixture of hay, tree branches, apples and carrots. Food was provided during the entire day but with a main feeding time at 1600 hours. The diet was complemented by pellets (1.5 kg/day/elephant) given to the animals at about 0600 hours in the morning. The animals were exposed to natural photoperiods via glass domes at the indoor facility. Body mass of the elephants ranged from 2,050 to 4,680 kg.
Study site and elephants in Thailand
Between February and March 2009 (i.e., at the onset of the hot season) we studied seven female and four male adult Asian elephants (aged between 14 and 60 years; body weight not known) at the Samphran Elephant Ground and Zoo in Thailand (13°43′N, 100°13′E). The animals were exposed to the warm climate of their natural habitat. Most parts of the elephant park were shaded by trees and awnings with animals exposed to direct sunlight for periods of maximally 15 min at a time, mostly between 1300 and 1600 hours. The park was on a flat terrain without any elevations. The elephants had a daily routine that included an elephant show and elephant riding (between 1000 and 1530 hours for a maximum of 15 min per ride). Not all of the experimental elephants participated in all shows. The daily time spent in shows ranged from 0 to 30 min (mean 19.3 SEM 0.06 min). The elephants were kept outdoors day and night. They were chained during the night (but still could move around, lie down or seek contact to each other) and kept under a roof on wooden beams, i.e., exposed to natural ambient temperature (from 1600 to 0900 hours). The main diet was hay complemented with pineapple leaves. Water was provided only during the daytime. As at Munich Zoo, food was provided during the entire day but with a main feeding time at 1600 hours local time. Body masses of Thai elephants could not be obtained, but there was no obvious difference in the average size of animals to the German group.
Measurements of T b and experimental procedure
T b was measured by feeding telemeters, a technique first used in elephants by Toscano et al. (2001). The telemetry system used was developed at the Research Institute of Wildlife Ecology. The system consisted of a temperature measurement unit, a non-volatile memory with a storage capacity of 32,000 temperature readings, a VHF radio transmitter, and an integrated microcontroller. The components were embedded in epoxy resin in a cylindrical stainless steel capsule (length 30 mm, diameter 22 mm, mass 30 g) to protect the electronic parts from shock and moisture. All capsules were calibrated against a certified precision thermometer (Testo 950, Testo AG, Lenzkirch, Germany) in a temperature-controlled water bath between 31 and 41°C at 2°C intervals. A third-order polynomial function was fitted to these data for obtaining a calibration function. The resolution of temperature measurements with the capsules was 0.01°C, with an accuracy of 0.1°C.
Altogether, capsules were fed 69 times to 17 elephants. The capsules transmitted a temperature measurement every 60 s and stored one every 600 s or 300 s at the German and Thai site, respectively. Capsules were recovered from dung with the help of a portable receiver and a hand-held H-bar antenna. After downloading of data, capsules were re-used in further experiments. We obtained 27,326 recordings of T b during intestinal passage of the devices. Following Kinahan et al. (2007) we removed all records from the first (on average ~4) hours after feeding of the capsules when it was located in the stomach, as indicated by large temperature variations following ingestion of food and drinking of water. The passage time of sensors through the gut was extremely variable, ranging from 20 h to 10 days. Presumably, during passages lasting several days the capsule may have remained in the caecum for prolonged times, but we were unable to actually determine the sensor’s position in the digestive tract once it entered the gut. We obtained 98 complete 24 h records from the 17 elephants (range 1–10, median 6 per individual). Apart from the initial (stomach) phase, there was no detectable relation between mean daily T b and the duration since the capsule entered the gut.
In addition to the onboard storage, we measured T b at the Munich Zoo telemetrically. We used omnidirectional antennae mounted permanently in both the outdoor and indoor enclosure connected to a telemetry receiver to pick up the signals from the capsules in the animals’ gut. Data were stored in an onsite PC, which also automatically adjusted the gain and fine tuning of the telemetry receiver using self-developed software. In cases of failing to recover the capsules we reverted to T b data recorded telemetrically (about 25% of T b data). However, there were no statistically significant differences between the T b recorded by telemetric methods and the T b logged onboard (P = 0.5). For a full description of the design, the functionality of the temperature devices and further information about the experimental procedure see Weissenböck et al. (2010a, 2010b).
T a and relative air humidity (RH) were measured throughout the study in close proximity to the elephants (<100 m) with temperature/humidity data loggers (Gemini Data Loggers Ltd, Chichester, West Sussex, UK). All data loggers, protected by a perforated plastic housing, were placed away from direct sources of heat, sunlight and water, and mounted at a height of approximately 2 m from the ground in all enclosures. Mean T a ± SD in Thailand throughout the study period was 30.3 ± 2.3°C and mean RH ± SD was 70.5 ± 13.9%. Mean daily T a for the Munich site was calculated from measurements in the indoor facility at night (1700–0800 hours) and from the outdoor enclosure during the day (0800–1700 hours) where animals spent ~ 90% of the daytime. This computation of T a thus represents a close proxy to the environmental air temperature actually experienced by the animals. Notably, however, none of our statistical analysis was principally affected by using either indoor T a or outdoor T a measured throughout the day (mean T a ± SD in the outdoor enclosure was 18.0 ± 7.5°C and in the indoor enclosure 20.9 ± 1.5°C). Also, it should be noted that even the mean indoor T a was substantially lower than night time T a at the Thai study site (Fig. 2). Mean RH ± SD in Munich was 64.7 ± 16.9% (calculated as for T a; mean RH ± SD in the outdoor enclosure was 63.8 ± 18.4% and in the indoor enclosure 74.4 ± 11.1%).
Statistical analysis
Statistical tests were performed using the statistical package R (R Development Core Team 2011). First, we computed daily mean profiles for T b, T a, and RH during the measurement period of each animal from complete 24 h records. These profiles were then used to calculate daily means, maxima, minima, and ranges (mean maximum–mean minimum) of variables. Where we report standard errors of means for the groups at different sites, they reflect between elephant SEM. We used multiple regression models to test for the effects of study site, or after removing the factor “site”, for T a, daily T a range, RH, age, sex and “daily time spent in a show” on T b parameters. Starting with the full model, we removed nonsignificant terms in a stepwise procedure. We verified normality and homoscedasticity of model residuals with Shapiro–Wilk and Levene’s tests, respectively. To further assess the relative importance of climatic and other variables on mean daily T b ranges, we also used a multi-model inference approach (Burnham and Anderson 2002) implemented by the R package “MuMIn” (Barton 2010). This procedure allows estimating the relative explanatory strength of each parameter while simultaneously accounting for uncertainty in model selection. Models were ranked by minimizing the value of Akaike’s Information Criterion corrected for small samples size (i.e. AICc). We calculated model-averaged standardized coefficients for all parameters by averaging the estimates, weighted by multiplication with the model’s Akaike weight (i.e. the relative likelihood of each model), over the set of well-supported models (ΔAICc < 10) including each parameter (Burnham and Anderson 2002). We also derived the unconditional standard error and 95% CI associated with each coefficient (i.e. not dependent on a single model). The relative importance of all parameters was calculated by summing the Akaike weights of each model containing that parameter.
Second, we computed daily mean, minimum, and maximum T b, as well as daily T b ranges and mean daily T a for all complete measurement days per individual. We analysed these repeated measurements per individual using linear mixed effect models (lme; library nlme, Pinheiro and Bates 2000, Pinheiro et al. 2009). In lme models, individual was entered as a random effect to avoid pseudoreplication. We used these models to test for associations between T b variables and mean daily T a. As these regression models represent simple hypothesis tests, we used no multimodel inference approach in these cases (Burnham and Anderson 2002). For all statistical models, we provide F and P values from type III sum of squares ANOVA tables.
Ethical approval
This study was approved by the Ethics Committee of the University of Veterinary Medicine Vienna, Austria and the FVS-MU Committee on Ethics of Laboratory Animals Use of the Mahidol University, Thailand.
Results
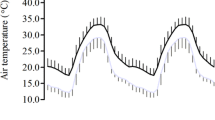
The overall mean T b in Thai elephants was 36.17°C (SEM 0.04) and mean T b in the German group was 36.34°C (SEM 0.10) (F 1,15 = 2.80, P = 0.11). There was no statistically significant effect of T a (F 1,15 = 1.54, P = 0.23), RH (F 1,15 = 3.21, P = 0.09), sex (F 1,15 = 1.69, P = 0.21), age (F 1,15 = 3.62, P = 0.08) and “daily time spent in a show” (F 1,15 = 2.83, P = 0.12) on mean T b. A distinct daily rhythm of T b was evident in both elephant groups, whereby in the Thai elephants the time course of T b ran fairly parallel to the daily course of T a (Figs. 1, 2). T b in Thai elephants was lowest at 0623 hours (SEM 0.12 h), and T b peaked at 1839 hours (SEM 0.08 h). Among elephants in Germany the daily minimum T b occurred at 0928 hours (SEM 1.35), and maximum T b was reached at 1730 hours (SEM 1.09 h) (Fig. 2).
Representative pattern of body temperature and ambient temperature. Daily fluctuations of core body temperature (white circles) in an adult female over 7 days and corresponding ambient temperature (line) measured under natural ambient temperatures in Thailand. The white and black bars at the top indicate the daily light–dark cycle
Body and ambient temperatures in Thailand and Germany. To ease visualization of daily rhythms, all data are double-plotted. a Mean daily body temperature profiles in the Thai (black circles) and the German group (white circles). Means ± SEM from individual animal 24 h patterns. Horizontal bars above the time axis indicate mean hours of darkness during the study periods in Thailand (lower bars) and Germany (upper bars). b Mean daily profiles of ambient temperatures at the study sites. For Germany data are shown for both the indoor facility in which the elephants spent all nights (1700–0800 hours) and an outdoor enclosure in which the animals spent ~90% of the daytime (0800–1700 hours)
Figure 3 illustrates the relation between T a and T b using means over 6 min intervals of both variables for the example of the Thai group, in which daily excursions were much more pronounced. This plot indicates that, at T a > ~30°C, there was a considerable hysteresis with changes in T b lagging behind changes in T a during both heating and cooling phase.
Body temperature as a function of ambient temperature during daily fluctuations of both variables in the Thai elephant group. Means ± SEM (grey areas) from 11 individual 24 h patterns determined at 6 min intervals. Triangles pointing up show data for the daily heating phase, i.e. between the mean daily minimum and maximum T b; triangles pointing down show the daily cooling phase
The mean range of daily T b fluctuations in elephants exposed to higher T a in Thailand was 1.15°C (SEM 0.07), which was more than twice as large as in the German group (0.51°C [SEM 0.03]; F 1,15 = 41.5, P < 0.0001; Fig. 2a). This increased range among Thai elephants was due to both significantly higher daily peak T b (Thailand 36.88°C (SEM 0.057); Germany 36.58°C (SEM 0.096; F 1,15 = 7.57, P = 0.014) and lower minimum T b (Thailand 35.73°C (SEM 0.06); Germany 36.08°C (SEM 0.11; F 1,15 = 8.69, P = 0.01). Differences in the range of T b rhythms were almost entirely due to the effects of mean T a during the measurement period (F 1,15 = 35.2, P < 0.0001), while the range of daily T a changes had no effect on the range of T b rhythms (F 1,15 = 0.34, P = 0.57). Daily T b ranges tend to be slightly higher among males (F1,14 = 4.73, P = 0.047). Age (F 1,14 = 0.39, P = 0.53), RH (F 1,14 = 1.76, P = 0.20), and “daily time spent in a show” (F 1,14 = 0.74, P = 0.40) had no significant effects on the range of T b rhythms. These results were confirmed by a multimodel inference approach indicating that mean T a had by far the highest relative importance (0.98) and was the only variable for which the 95% confidence interval of the parameter estimate did not overlap 0 (Table 1).
Regression models of daily means (of mean, minimum, and maximum T b, as well as daily T b ranges) as a function of mean daily T a gave no evidence for any significant difference in slopes between the data from the German and the Thai site in all cases (Table 2). Therefore, we computed linear mixed models without interactions across all data from both elephant groups. These models indicated that the daily range of T b significantly increased with rising mean daily T a (Fig. 4; Table 2). Further, maximum daily T b increased and minimum daily T b decreased with increasing daily mean T a, but mean daily T b was unaffected by T a (Fig. 4, for parameter estimates and statistics see Table 2).
Relation between the mean daily body temperature and ambient temperature. White circles and dotted line: daily maximum of core body temperature and regression line. Black circles and dashed line: daily mean of core body temperature and regression line. White triangles and solid line: daily minimum of core body temperature and regression line. Data points include both elephant observation groups (Germany and Thailand). Note that, while this figure shows all daily means, statistical analyses were carried out using linear mixed effects models, which adjust for repeated measurements in the same individuals. For parameter estimates and statistics see Table 2
Discussion
Our study demonstrates that Asian elephants respond to exposure to elevated T a by increasing heat storage during the day and by decreasing T b during the night, with T a leading T b due to body inertia (Fig. 3). At high T a, nocturnal decreases of T b reached levels below those maintained at cooler T a, and enabled elephants to encounter subsequent days with a large thermal reserve. Therefore, we would consider this type of thermoregulation to represent heterothermy, very similar to the mechanisms used by desert mammals, such as camels and antelopes to cope with high environmental temperatures (Langman and Maloiy 1989; Louw and Seely 1982; Ostrowski et al. 2003; Ostrowski and Williams 2006; Taylor 1969, 1970; Taylor and Lyman 1967; Willmer et al. 2005). The IUPS Thermal Commission (1987) defined heterothermy as variation in T b of more than 2°C, but in a later definition the use of arbitrary absolute ranges of T b was avoided (IUPS Thermal Commission 2003). We concur with the latter approach, particularly because ranges of daily rhythms in T b (in the absence of high heat load) differ between mammalian species, and are known to significantly decrease with body size (Aschoff 1982; Mortola and Lanthier 2004; Refinetti 2010). We also concur with the current definition of heterothermy (IUPS Thermal Commission 2003) in that it does not specify whether increased variation in T b is caused by external heat load or by endogenous heat production. In our view, this broad definition of heterothermy should be augmented, however, by the notion that heterothermy as an adaptive thermoregulatory response (rather than an inevitable consequence of heat stress) can be identified by a significant decrease of the set-point of T b during the trough of the cycle.
While the 0.51°C range of T b rhythms in our elephants in Germany is within the range expected for an animal of this size, the 1.15°C range observed in our Thai animals at high T a was 2.5 times larger than that predicted from (extrapolated) allometric relations (Mortola and Lanthier 2004). However, even disregarding any allometric predictions, the finding that our Thai elephants exposed to a higher T a showed more than two-fold increase in T b ranges compared with the German group, by itself seems to justify the classification of thermoregulatory responses to heat load in Asian elephants as heterothermy. Although heterothermy includes all deviations of the set-point irrespective of the causative factor, we can exclude heat increment of feeding and activity as the proximate cause of increased daily T b ranges in the Thai elephants. By supplementation of high caloric food pellets to the elephant’s diet, as was the case for the elephants in Munich, one could expect a larger increase of T b during daytime due to a larger heat increment of feeding. This was, however, not the case. Also, while there are reports of food quality on overall levels of T b even under ad libitum feeding conditions (e.g. Ahmed and El Kheir 2004), mean T b did not differ between the elephant groups in our study, and we are not aware of experimental evidence for food quality differences alone causing the magnitude of difference in daily T b profiles observed here. Similarly, we can rule out an important role of locomotor activity in generating larger T b fluctuations, as the daily time spent in a show had no detectable effect on the degree of daily T b ranges in our Thai elephants. As indicated by Fig. 3, the daily T b range seemed to be primarily driven by T a, although diurnal cycles of T b did not simply follow the daily mean or amplitude of T a (cf., Fig. 1). At least part of these discrepancies may be due to the fact that individuals moving on the sites experienced environmental temperatures that differed from the T a measured at a single point at each site.
One notable aspect of heterothermic responses observed in our Asian elephants is that they occurred at comparatively moderate temperatures, when T a was still below T b. This is in contrast to free-ranging arid-zone antelopes that used heterothermy only when exposed to T a > 40°C (Ostrowski et al. 2003; Ostrowski and Williams 2006), but not when climate conditions were moderate (T a < 35°C) (see also Fuller et al. 1999, 2005; Mitchell et al. 1997). We suggest that this difference merely reflects the effects of extremely large body size in elephants, which inevitably constrains the dissipation of heat resulting from endogenous heat production when T b–T a gradients are small. Still, these results suggest that heterothermy is a strategy not necessarily restricted to desert mammals, but also employed by Asian elephants when T a remains below T b. Since large parts of their distribution range are characterized by mean T as that can well exceed the T as at our Thai study site it seems likely that Asian elephants under severe heat load may undergo daily cycles of T b that are significantly larger than observed here.
In African savannah elephants, which often inhabit areas characterized by high solar radiation (with effective T a > T b), both the benefits of heterothermy and its extent may be even larger. In African elephants under relatively cool mean T a ( ~21°C) there was no clear evidence for heterothermy (Kinahan et al. 2007). The mean daily T b range of animals in that study (Kinahan et al. 2007) was 0.8°C, which is intermediate between the T b range observed in our elephants form the Munich study site (range 0.51°C at T a ~ 21°C), and the Thai elephants (range 1.15°C at T a ~ 30°C). The somewhat higher range of 0.8°C in African elephants compared with our elephants at the Munich site may have been due to the comparison of different species, or simply coincidental, as data in the study by Kinahan et al. (2007) were obtained from 3 individuals only. Under conditions of high T a and intense solar radiation (ground temperature ~43°C) in the African savanna, Hiley (1975) reported, however, an increase of T b during the day from 36.1 to 38.2°C (i.e., a range of 2.1°C), which clearly exceeds the variation in T b of our Thai Asian elephants. As mentioned before, Hiley’s (1975) data were, however, apparently obtained from a single animal only, which underlines the need for further studies on free-living elephants under conditions of high environmental temperatures.
The nocturnal decline of T b in our warm-exposed Thai elephants was more rapid and reached lower levels than in the German group (Fig. 2). Theoretically, this facilitated the decrease of T b which could be due to both a decrease in metabolic rate and hence endogenous heat production, and by increased thermal conductance. While it is likely that elephants, like most mammals (Mortola and Lanthier 2004), have a daily cycle of metabolic rate, it is unclear to which degree such a cycle may have contributed to daily T b rhythms, or if it was affected by differences in T a. As far as rates of heat loss are concerned, our present data suggest that Asian elephants may undergo profound changes of thermal conductance. During night, and perhaps even at mean T a at both sites, both groups probably were at thermoneutral temperatures. As elephants show signs of complete vasoconstriction (determined by infrared imaging) only at T a < ~10°C (Weissenböck et al. 2010a, 2010b), even the Munich group was probably never cold-exposed. If mean T a correspond to thermoneutrality at both sites, mean thermal conductance at those environmental temperatures would have been ~2.5 times higher in the Thai group, corresponding to the smaller T b–T a gradient (at mean T b). During times of rapid nocturnal cooling in the Thai group, changes in thermal conductance temporarily may well have been larger than that, since elephants have a greater capability to control the temperature of their surface than all other terrestrial mammals (Phillips and Heath 1995). Active elevations of thermal conductance in elephants typically not only involve increased vasodilatation and blood flow in ear vessels, but also involve thermal windows on other parts of the body surface, which seems to be the primary mechanism by which at least African elephants dissipate excess heat (Buss and Estes 1971; Hiley 1975; Phillips and Heath 1992; Willmer et al. 2005; Wright 1984, Weissenböck et al. 2010a, 2010b). Further heat loss was most likely assisted by ear flapping which (although not quantified) indeed appeared to be much more frequent in elephants at the Thai study site. Irrespective of the mechanism, either facilitated heat dissipation and/or decreased endogenous heat production, our animals did not only return to levels of T b as observed at low T a, but also to further lower the daily minimum T b as T a increased (Figs. 2, 3). Thus, elephants entered the day with a thermal reserve for additional heat storage, very similar to arid-zone heterotherms (Louw and Seely 1982; Willmer et al. 2005).
Theoretically, the fact that elephants in the Thai group were chained at night could have prevented them from fully employing the enhanced metabolic heat production of locomotion, as a defence against body cooling at night, or from positioning themselves relative to other objects, including other elephants, to manipulate radiant heat transfer. However, if those mechanisms had played an important role, minimum T b at the end of the night in elephants of the Thai would be expected to increase with mean T a. As illustrated in Fig. 4, the opposite was the case (see also Table 2). Also, since it seems likely that, during night, all elephants were at thermoneutrality, no additional mechanism such as enhanced heat production would be required. However, the ability of Asian elephants to dissipate heat during nocturnal cooling might be impaired by high humidity in their natural habitats, in which RH at high environmental temperature may routinely exceed 90% (Oliver 2005; Spinage 1994). Even at the moderate mean RH of 70.5% at our Thai study site, average water vapour pressure was 3.05 kPa, compared with 1.65 kPa at the German site (given the mean T a at both sites). Hence, the potential for evaporative cooling was almost twice as high at the German site. However, while high humidity will hamper evaporative heat loss, this pathway of heat dissipation via respiration and diffusion through the skin apparently accounts for no more than approximately 7% (N = 1) in elephants (Hiley 1975). Heat loss via convection, however, which—next to radiation—appears to be a major mechanism of nocturnal cooling in elephants, will be actually facilitated by increased RH, due to increased specific heat capacity of humid air. Thus, even the high humidity of typical habitats of Asian elephants should not have counteracted the evolution of heterothermy in this species.
While daily cycles of heat storage and release provide a very effective mechanism to cope with warm and hot environments, there are, of course, important additional effects of behavioural thermoregulation. In elephants, coping with heat load involves seeking shade, covering the skin with dust or mud, wallowing, or spraying themselves with water (Hiley 1975; McKay 1973). Previously, it had been suggested that observations of heterothermy may be an artefact, caused by preventing adequate behavioural thermoregulation in captive animals, or by prior dehydration (Fuller et al. 2005; Mitchell et al. 2002). Meanwhile, it has been shown in three studies, however, that heterothermy occurs in free-living animals (e.g. Hetem et al. 2010; Ostrowski et al. 2003; Ostrowski and Williams 2006), which refutes this general criticism. While water limitation may be the primary driver of heterothermy in desert animals (Hetem et al. 2010), in our study animals, which had free daily access to water, dehydration certainly played no role in inducing heterothermy. Also, our analysis indicates that thermoregulation in our Thai animals was unaffected by their daily routine in the elephant park. The animals were able to seek shade or water during the vast majority of the day, and were exposed to direct solar radiation only for short periods. Most importantly, however, the elephants in our study responded to increasing T a by a continuous drop in nocturnal minimum T b, that is, increased capacity for subsequent heat storage. Clearly, this type of fine-tuned, predictive thermoregulatory ability could not have been caused by artificial behavioural constraints but indicates that heterothermy in Asian elephants results from natural selection.
Taken together, our data suggest that adaptive heterothermy is an integral part of thermoregulation in Asian elephants. We are aware, however, that further studies are needed to clarify the underlying mechanisms (i.e. measurements of heat production and thermal conductance) and the frequency, magnitude, and role of heterothermy for free-living elephants. It would particularly be interesting to study the thermoregulation in both Asian and African elephants under the conditions where the same individuals are exposed to a much larger range of environmental temperatures, and higher heat load, than could be examined in this and previous studies on elephants.
References
Ahmed MMM, El Kheir IM (2004) Thermoregulation and water balance as affected by water and food restrictions in Sudanese desert goats fed good-quality and poor-quality diets. Trop Anim Health Pro 36:191–204
Aschoff J (1982) The circadian rhythm of body temperature as a function of body size. In: Taylor CR, Johansen K, Bolis L (eds) A Companion to Animal Physiology. Cambridge University Press, Cambridge, London, New York, New Rochelle, Melbourne, Sydney, pp 173–188
Barton K (2010) MuMIn: Multi-model inference. R package version 0.13.17. http://CRAN.R-project.org/package=MuMIn
Burnham KP, Anderson DR (2002) Model selection and multi-model inference: a practical information-theoretic approach, 2nd edn. Springer-Verlag, New York
Buss IO, Estes JA (1971) The functional significance of movements and positions of the pinnae of the African elephant (Loxodonta africana). J Mammal 52:21–27
Elder W, Rodgers D (1975) Body temperature in African elephant as related to ambient temperature. Mammalia 39:395–399
Fuller A, Moss DG, Skinner JD, Jessen PT, Mitchell G, Mitchell D (1999) Brain, abdominal and arterial blood temperatures of free-ranging eland in their natural habitat. Eur J Physiol 438:671–680
Fuller A, Kamerman PR, Maloney SK, Matthee A, Mitchell G, Mitchell D (2005) A year in the thermal life of a free-ranging herd of springbok Antidorcas marsupialis. J Exp Biol 208:2855–2864
Hetem RS, Strauss WM, Fick LG, Maloney SK, Meyer LCR, Shobrak M, Fuller A, Mitchell D (2010) Variation in the daily rhythm of body temperature of free-living Arabian oryx (Oryx leucoryx): does water limitation drive heterothermy? J Comp Physiol B 180:1111–1119
Hiley P (1975) How the elephant keeps its cool. Nat Hist 84:34–40
IUPS Thermal Commission (1987) Glossary of terms for thermal physiology. Pflügers Arch 410:567–587
IUPS Thermal Commission (2003) Glossary of terms for thermal physiology. J Therm Biol 28:75–106
Kinahan AA, Inge-moller R, Bateman PW, Kotze A, Scantlebury M (2007) Body temperature daily rhythm adaptations in African savannah elephants (Loxodonta africana). Physiol Behav 92:560–565
Langman VA, Maloiy GMO (1989) Passive obligatory heterothermy of the giraffe. J Physiol 415:89
Louw GN, Seely MK (1982) Ecology of desert organisms. Longman, London, New York
Mariappa D (1986) Anatomy and histology of the Indian elephant. Indira Publishing House, Oak Park
McKay GM (1973) Behavior and ecology of the Asiatic elephant in South-eastern Ceylon. Smithson Contr Zool 125:1–113
Mitchell D, Maloney SK, Laburn HP, Knight MH, Jessen C (1997) Activity, blood temperature and brain temperature of free-ranging springbok. J Comp Physiol B 167:335–343
Mitchell D, Maloney SK, Jessen C, Laburn HP, Kamerman PR, Mitchell G, Fuller A (2002) Adaptive heterothermy and selective brain cooling in arid-zone mammals. Comp Biochem Physiol B 131:571–585
Mortola JP, Lanthier C (2004) Scaling the amplitudes of the circadian pattern of resting oxygen consumption, body temperature and heart rate in mammals. Comp Biochem Physiol A 139:83–95
Oliver JE (2005) Encyclopedia of world climatology. Springer, Dordrecht, Berlin, Heidelberg, New York
Ostrowski S, Williams JB (2006) Heterothermy of free-living Arabian sand garzelles (Gazella subgutturosa marica) in desert environment. J Exp Biol 209:1421–1429
Ostrowski S, Williams JB, Ismael K (2003) Heterothermy and the water economy of free-living Arabian oryx (Oryx leucoryx). J Exp Biol 206:1471–1478
Phillips PK, Heath JE (1992) Heat exchange by the pinna of the African elephant (Loxodonta africana). Comp Biochem Physiol A 101:693–699
Phillips PK, Heath JE (1995) Dependency of surface temperature regulation on the body size in terrestrial mammals. J Therm Biol 20:281–289
Pinheiro JC, Bates DM (2000) Mixed effects models in S and S-plus. Springer, Berlin
Pinheiro J, Bates D, DebRoy S, Sarkar D (2009) Lme: linear and nonlinear mixed effects models. R package version 3.1-92. http://www.R-project.org
R Development Core Team (2011) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. http://www.R-project.org
Refinetti R (2010) The circadian rhythm of body temperature. Front Biosci 15:564–594
Robertshaw D (2006) Mechanisms for the control of respiratory evaporative heat loss in panting animals. J Appl Physiol 101:664–668
Schmidt-Nielsen K, Schmidt-Nielsen B, Jarnum SA, Houpt TR (1956) Body temperature of the camel and its relation to water economy. Am J Physiol 188:103–112
Spinage CA (1994) Elephants. T & AD Poyser Ltd., London
Taylor CR (1969) The Eland and the Oryx. Sci Am 220:88–97
Taylor CR (1970) Strategies of temperature regulation: effect on evaporation in East African ungulates. Am J Physiol 219:1131–1135
Taylor CR, Lyman CP (1967) A comparative study of the environmental physiology of an East African antelope, the eland, and the Hereford steer. Physiol Zool 40:280–295
Toscano MJ, Friend TH, Nevill CH (2001) Environmental conditions and body temperature of circus elephants transported during relatively high and low temperature conditions. J Elephant Manag Assoc 12:116–149
Weissenböck NM, Schober F, Fluch G, Weiss C, Paumann T, Schwarz C, Arnold W (2010a) Reusable biotelemetric capsules: a convenient and reliable method for measuring core body temperature in large mammals during gut passage. J Therm Biol 35:147–153
Weissenböck NM, Weiss CM, Schwammer HM, Kratochvil H (2010b) Thermal windows on the body surface of African elephants (Loxodonta africana) studied by infrared thermography. J Therm Biol 35:182–188
Willmer P, Stone G, Johnson I (2005) Environmental physiology of animals, 2nd edn. Blackwell Science, Magden
Wright PG (1984) Why Do Elephants Flap Their Ears? S Afr J Zool 19:266–269
Wright PG, Luck CP (1984) Do elephants need to sweat? S Afr J Zool 19:270–274
Acknowledgments
N.M.W. was sponsored by a DOC-fFORTE scholarship from the Austrian Academy of Science. Further financial aid was provided by the Tiergarten Schönbrunn, Vienna and the Tierpark Hellabrunn, Munich. We thank P. Ratanakorn from the Mahidol University Thailand and the management from the Samphran Elephant Ground and Zoo as well as the Zoo Hellabrunn for providing access to the elephants. We thank C. Weiss and the mahouts from the Samphran Elephant Ground and Zoo for their assistance with data collection. Thanks also to G. Fluch for the production of the temperature data loggers and for technical support.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by G. Heldmaier.
Rights and permissions
About this article
Cite this article
Weissenböck, N.M., Arnold, W. & Ruf, T. Taking the heat: thermoregulation in Asian elephants under different climatic conditions. J Comp Physiol B 182, 311–319 (2012). https://doi.org/10.1007/s00360-011-0609-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00360-011-0609-8