Abstract
Both basal metabolic rate (BMR) and maximum lifespan potential (MLSP) vary with body size in mammals and birds and it has been suggested that these are mediated through size-related variation in membrane fatty acid composition. Whereas the physical properties of membrane fatty acids affect the activity of membrane proteins and, indirectly, an animal’s BMR, it is the susceptibility of those fatty acids to peroxidation which influence MLSP. Although there is a correlation between body size and MLSP, there is considerable MLSP variation independent of body size. For example, among bird families, Galliformes (fowl) are relatively short-living and Psittaciformes (parrots) are unusually long-living, with some parrot species reaching maximum lifespans of more than 100 years. We determined BMR and tissue phospholipid fatty acid composition in seven tissues from three species of parrots with an average MLSP of 27 years and from two species of quails with an average MLSP of 5.5 years. We also characterised mitochondrial phospholipids in two of these tissues. Neither BMR nor membrane susceptibility to peroxidation corresponded with differences in MLSP among the birds we measured. We did find that (1) all birds had lower n-3 polyunsaturated fatty acid content in mitochondrial membranes compared to those of the corresponding tissue, and that (2) irrespective of reliance on flight for locomotion, both pectoral and leg muscle had an almost identical membrane fatty acid composition in all birds.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Gudbjarnason et al. (1978) demonstrated a strong positive relationship between the heart rate of different-sized mammals (measured as body mass in mammals ranging from mice to whales) and the docosahexaenoic acid content in their cardiac phospholipids. They offered no explanation for their observation and from their graph describing the relationship it was unclear whether the variation in this most highly polyunsaturated fatty acid was directly related to heart rate or inversely related to body size of the particular mammals plotted. Seventeen years later, a comparison of the fatty acid composition of tissue phospholipids from mammals ranging from mice to cattle confirmed the heart size-related phospholipid relation and further showed this pattern was also present in skeletal muscle, liver and kidney phospholipids (Couture and Hulbert 1995). It was later suggested that this size-related variation in the membrane fatty acid composition of mammals was associated with size-related variation in cellular metabolic rate (Hulbert and Else 1999, 2000) as well as to size-related variation in maximum lifespan of mammals (Pamplona et al. 1998).
While this early work concentrated on mammals, later it was shown that the fatty acid composition of pectoral muscle phospholipids in birds also varied systematically with body size (Hulbert et al. 2002a). Similar size-related variation in birds was reported for fatty acid composition of heart phospholipids (Szabo et al. 2006) and liver, kidney and lung phospholipids (Szabo et al. 2010). These trends are not restricted to total tissue phospholipids of birds as they have also been observed in liver mitochondrial phospholipids (Brand et al. 2003), as well as microsomal phospholipids of the avian kidneys (Turner et al. 2005) and avian hearts (Turner et al. 2006). Unlike most other tissues, the fatty acid composition of brain phospholipids does not vary with body size in birds (Szabo et al. 2010) or in mammals (Hulbert et al. 2002b). Although birds and mammals have size related variation in membrane fatty acid composition, there are significant differences between these groups. For example, the docosahexaenoic acid content of microsomal phospholipids is much lower in birds compared to similar-sized mammals for both kidney (Turner et al. 2005) and heart (Turner et al. 2006).
Both basal metabolic rate (BMR) and maximum lifespan potential (MLSP) vary with body size in both mammals and birds (Hulbert et al. 2007) and it has been suggested that variation in membrane fatty acid composition likely mediates these size-affected traits, but by different mechanisms. For example, it is thought that the molecular activity of membrane-bound proteins is strongly influenced by the physical properties of particular membrane fatty acids, which, in turn, affect cellular metabolic activity. By contrast, their influence on MLSP is mediated by the different susceptibilities of individual fatty acids comprising particular membranes to peroxidative damage (Hulbert 2008). Fatty acids differ greatly is their susceptibility to lipid peroxidation. Only polyunsaturated fatty acids undergo peroxidation (saturates and monounsaturates are peroxidation-resistant), and the more polyunsaturated the fatty acid, the greater its peroxidation susceptibility. Combining the knowledge of the fatty acid composition of membrane lipids with their known susceptibilities to peroxidative damage allows one to calculate a single number, the peroxidation index (PI), which represents the membrane’s susceptibility to lipid peroxidation (Hulbert et al. 2007).
As part of an investigation into the role that oxidative stress plays in determining the longevity of birds, we report here both the BMR and the fatty acid composition of tissue phospholipids of five bird species. We have selected two species of Galliformes (fowl; king and Japanese quail) that are short-living (Arnold 1988) and three species of Psittaciformes (parrots; budgerigar, lovebird, cockatiel) that are unusually long-living within birds (Brouwer et al. 2007). The average body mass of the parrots and fowl we studied is approximately the same while the parrot–fowl average MLSP varies fivefold. Because of their differences in their reliance on flight for locomotion, we have compared both flight muscles (pectoral muscle) and non-flight muscles (leg muscle). For two tissues (pectoral muscle and liver) we have also compared the fatty acid composition of mitochondrial phospholipids with that of total tissue phospholipids. In order to minimise the influence of diet, all species were fed the same diet for 2 months prior to measurements of BMR and phospholipid fatty acid composition.
Methodology
Keeping conditions
All experiments were approved by the University of Wollongong Animal Ethics Committee and were conducted in conformity with the NHMRC Australian Code of Practice for the Care and Use of Animals for Scientific Purposes. Six male budgerigars (Melopsittacus undulatus) (MLSP = 21 years; average mass = 26.0 g) derived from wild-type native budgerigars were purchased from a bird breeder in Queensland, Australia. Ten Japanese quails (Coturnix japonica) (MLSP = 6 years; average mass = 221.2 g) were purchased from Kyeema Organics, Windellama, Australia; and were of mixed sex. Eight lovebirds (Agapornis roseicollis) (MLSP = 25 years; average mass = 48.9 g), nine cockatiels (Nymphicus hollandicus) (MLSP = 35 years; average mass = 83.2 g), and eight king quails (Coturnix chinensis) (MLSP = 5 years; average mass = 45.4 g) were from a local breeder (Andrew’s Pet Shop, Smithfield, Australia) and were of mixed sex. For cockatiels, MLSP was obtained from http://www.eol.org/pages/1177924, while for king quails MLSP was from http://www.cyberquail.com/faq.html and for all other species from the AnAge database (http://genomics.senescence.info/species). All birds used in this study were young adults, being approximately 1 year old.
All birds had free access to water and to the same pellet diet (see Table 1 for composition) for approximately 2 months prior to measurement. This diet was palatable to both parrots and quails and was formulated to provide adequate intake of n-3 and n-6 polyunsaturated fatty acids. This common diet was used to ensure that all measured differences were not attributable to differences in diet. The body mass of all birds was monitored during the 2-month period. Budgerigars were the first species to be measured, and for this species BMR was not measured nor were liver mitochondria isolated. The BMR value for budgerigars was taken from a previous study (Weathers and Schoenbaechler 1976). All other species were housed and measured in pairs of similar-sized species (king quail–lovebird; Japanese quail–cockatiel) and for these species-pairs BMR was measured and liver and pectoral muscle mitochondria were isolated. Both parrots and quails were housed in outdoor aviaries a minimum of 2 months prior to sampling.
BMR was measured and the following day birds were euthanized. Aliquots of pectoral and leg muscle, liver, heart, kidney and brain were taken and immediately frozen in liquid nitrogen for subsequent analyses. Blood was collected into heparinised vials following cardiac puncture and centrifuged to extract erythrocytes, which then were stored in liquid nitrogen for later analyses.
Chemicals
All solvents used for phospholipid fatty acid extraction were from Crown Scientific, Moorebank, Australia. All other chemicals were from Sigma, Castle Hill, Australia.
Whole animal metabolic rate
Whole animal oxygen consumption was determined at least 4 h after the birds had last fed, in the dark, during the birds’ rest phase, in 2- or 5-l respirometer chambers following procedures used by Buttemer et al. (2008). Chambers were fitted with a perch for parrots or provided with paper towels on the lower surface for quail. The respirometers were placed in an incubator at a constant temperature of 28–30°C, a range that is thermoneutral for these species. Air was provided to the respirometers at a flow rate of 500–750 ml/min, depending on the size of birds being sampled, and was controlled with mass-flow controllers (Tylan Model FC-280S). The exhaust air from respirometers passed through water and carbon dioxide absorbents (Drierite and soda lime, respectively) and the oxygen content of inlet and outlet air was analysed with an oxygen analyser (Oxzilla, Sable Systems International, Las Vegas, USA). The output signals from the mass flow controller and the oxygen analyser were recorded on a computer that was equipped with an A-to-D-converter and custom-designed software (Warthog Systems, University of California, Riverside, USA). The minimum metabolic rate was selected as the minimum continuous oxygen consumption over a 5-min period.
Isolation of mitochondria
Pectoral muscle and liver mitochondria were isolated by differential centrifugation as described previously (Trzcionka et al. 2008).
Briefly, for pectoral muscle and heart mitochondria, the tissue was finely diced in CP-1 medium (100 mM KCl, 50 mM Tris/HCl, pH 7.4, and 2 mM EGTA), digested on ice for 3 min in CP-2 medium [CP-1, to which was added 0.5% (w/v) BSA, 5 mM MgCl2, 1 mM ATP and 2.45 units/ml Protease Type VIII (Sigma P 5380)] and homogenized using a dounce homogenizer. The homogenate was transferred to a temperature-controlled centrifuge and spun for 10 min at 500g and 4°C. The resulting supernatant was subjected to a high-speed spin cycle (10,600g, 10 min, 4°C) and the pellet was resuspended in CP-1. The high-speed spin cycle was repeated and the resuspension finally centrifuged for 10 min at 3,800g and 4°C. The final pellet was resuspended in a minimum volume of CP-1 buffer.
For the isolation of liver mitochondria, the liver was immediately placed in ice-cold STE buffer (250 mM sucrose, 5 mM Tris/HCl, pH 7.4, and 2 mM EGTA), minced with scissors and disrupted with a dounce homogenizer. The homogenate was spun for 3 min at 1,000g and 4°C, and the supernatant centrifuged for 10 min at 10,600g and 4°C. The high-speed spin cycle was repeated twice and the final pellet resuspended in a minimal volume of isolation medium.
The protein concentration of mitochondrial suspensions was determined by the Biuret method using BSA as standard (Gornall et al. 1949).
Fatty acid extraction
Lipids were extracted, phospholipids were isolated and the fatty acid composition of those phospholipids was determined by gas chromatography as described elsewhere (Abbott et al. 2010). Briefly, all solvents used were HPLC grade and contained 0.01% (w/v) butylated hydroxytoluene. Tissues (0.1–0.2 g) and mitochondria were homogenised in 4–5 ml of a 2:1 (v/v) chloroform–methanol mixture using a glass/glass homogeniser, and rotated at 4°C overnight. Sulphuric acid (2 ml of 1 M solution) was added, the samples spun for 10 min at 1,000 rpm, and the chloroform layer transferred into new tubes. This step was repeated a second time, followed by the addition of sodium hydrosulphite (to remove any water) and a filtration step using a Pasteur pipette containing silane treated glass wool pushed down into the stem of the pipette. The extracted lipids were dried down under nitrogen following an addition of 5 ml Hexane. Phospholipids were separated from total lipids using Sep-Pak Classic Silica Cartridges (Waters, Rydalmere, Australia). This separation step included trapping of the triglycerides and phospholipids on the column, elution of the triglycerides using ethyl acetate, and the final elution of phospholipids using methanol (3 times 5 ml). Methanol was removed by a drying down step under nitrogen, followed by the addition 2 ml of a 4:1 (v/v) methanol–toluene mixture and a transmethylation step using 200 μl acetyl chloride. The samples were heated to 100°C for 1 h, cooled on ice and 5 ml of a 6% K2CO3 solution was added. The tubes were vortexed and centrifuged for 10 min at 3,000 rpm. The upper toluene phase containing the phospholipids was transferred into GC vials.
The extracted fatty acid methyl esters were measured by gas chromatography (Shimadzu GC-17A, Rydalmere, Australia) using a Varian WCOT fused silica column (50 m × 0.25 mm internal diameter, CP7419, Sydney, Australia) with the following temperature program: 150°C initial temperature; 17.5°C/min to 170°C; 0.5°C/min to 178°C; 15°C/min to 222°C; 2°C/min to 232°C. Fatty acid composition of tissues and mitochondria was identified by comparison with an external standard (FAME Mix C4–C24; Sigma Aldrich, Sydney, Australia) and expressed as mole percentage of total fatty acids. The PI is a measure of the calculated susceptibility of the phospholipid fatty acids to peroxidative damage and was calculated as PI = (0.025 × % monoenoics) + (1 × % dienoics) + (2 × % trienoics) + (4 × % tetraenoics) + (6 × % pentaenoics) + (8 × % hexaenoics) (Hulbert et al. 2008).
Statistical analysis
Data analysis was performed using JMP 5.1 (Statistical Analysis System Institute Inc., Cary, NC, USA). All results are expressed as means with their standard errors with P = 0.05 set as the level of significance. Data were tested for normality using the Shapiro–Wilk W test and homogeneity of variance using the O’Brien and Brown-Forsythe tests. Data not normally distributed were compared using non-parametric Wilcoxon/Kruskal–Wallis tests and any data showing unequal variance were compared using a Welch ANOVA. A one-way ANOVA was completed with either each single bird species or with short versus long lifespan as the independent variable and membrane fatty acid composition parameters as dependent variables. Means were then compared using the Tukey–Kramer honestly significant difference test. There was no effect of gender on any of the measurements so we assume that the effect of gender is negligible for all animals used in this study.
Results
Maximum lifespan and basal metabolic rate in birds
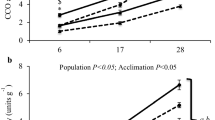
The maximum lifespan and basal metabolic rate (BMR) of parrots and quails is presented in Fig. 1. The maximum lifespan for the parrots ranged from 21 years (budgerigar) to 35 years (cockatiel) with an average MLSP of 27 years, while the average for the quail species was 5.5 years, which represents an approximately fivefold longevity difference. Contrasted to this large longevity difference there was no parrot–quail difference in BMR. The budgerigars have the highest mass-specific BMR (data from Weathers and Schoenbaechler (1976) due to technical problems with our measurements), but this is expected as they have the smallest mass. Apart from the budgerigar, we found no statistical difference in BMR of the species we measured.
Maximum lifespan potential (MLSP) and basal metabolic rate (BMR) of long-living parrots and short-living quails. The dotted lines represent the average of the three parrot species (parrot average) and two quail species (quail average) for both MLSP and BMR. For BMR, shown are mean ± SEM, n = 8 for king quails and lovebirds, n = 9 for cockatiels, and n = 10 for Japanese quails. The budgerigar BMR value was taken from Weathers and Schoenbaechler (1976). Bars without a common letter are significantly different (P < 0.05)
Tissue phospholipid fatty acid composition
The fatty acid composition data have been divided into two groups. Figure 2 presents the data for those tissues (heart, leg muscle, kidney, brain and erythrocytes) in which only total tissue phospholipids were analysed. Figure 3 presents the data for pectoral muscle and liver in which both total tissue and mitochondrial phospholipids (from the same individual birds) were analysed separately.
Tissue phospholipid fatty acid composition (left-hand graphs) and the peroxidation index (PI) (right-hand graphs) of heart, leg muscle, kidney, brain and erythrocytes of long-living parrots and short-living quails. The fatty acid composition (mol %) is shown as saturated fatty acids (SFA), monounsaturated fatty acids (MUFA), n-6 polyunsaturated fatty acids (n-6 PUFA) and n-3 polyunsaturates (n-3 PUFA). Significant differences in fatty acid sub-types between parrots and quails are shown in between the left-hand and right-hand graphs, with *P < 0.05, **P < 0.01 and ***P < 0.001. No longevity patterns are present in PI. For PI, bars without a common letter are significantly different (P < 0.05). Shown are mean ± SEM, n = 6 for budgerigars, n = 8 for king quails and lovebirds, n = 9 for cockatiels, and n = 10 for Japanese quails
Tissue and mitochondrial phospholipid fatty acid composition (left-hand graphs) and the peroxidation index (PI) of total tissue and mitochondrial phospholipids (right-hand graphs) of pectoral muscle and liver of long-living parrots and short-living quails. The fatty acid composition (mol %) is shown as saturated fatty acids (SFA), monounsaturated fatty acids (MUFA), n-6 polyunsaturated fatty acids (n-6 PUFA) and n-3 polyunsaturates (n-3 PUFA). Significant differences in fatty acid sub-types between parrots and quails are shown in between the left-hand and right-hand graphs, with *P < 0.05, **P < 0.01 and ***P < 0.001. No longevity patterns are present in PI. For PI, bars without a common letter are significantly different (P < 0.05). Shown are mean ± SEM, n = 6 for budgerigars, n = 8 for king quails and lovebirds, n = 9 for cockatiels, and n = 10 for Japanese quails
The membrane fatty acid composition (percent of total fatty acids) is presented as saturated (SFA), monounsaturated (MUFA), n-6 polyunsaturates (n-6 PUFA) and n-3 polyunsaturates (n-3 PUFA) in the left-hand graphs. The corresponding PI values for these membrane fatty acids are presented in the right-hand graphs in the same figures. Statistically significant differences in fatty acid composition between long-living parrots and short-living quails in fatty acid composition are shown between the left-hand and right-hand panels of these figures. The specific fatty acid composition for each tissue and bird species is presented in supplementary tables.
In general, there were minimal differences in membrane fatty acids between parrots and quails. Most tissues had similar proportions of fatty acid subtypes, except the brain phospholipids, which contained a relatively low n-6 PUFA and high n-3 PUFA content compared to other tissues in all species (Fig. 2). To determine if there were any fatty acid differences related to longevity, values for parrots as a whole were statistically compared to those for quails. This analysis revealed that parrot tissues had a higher MUFA content (in leg muscle, kidney and erythrocytes) and a lower total PUFA content (in heart, leg muscle and kidney) in comparison to the short-living quails. In all three tissues, the low total PUFA content was due to a low concentration of n-6 PUFA, and in the kidney also because of a low n-3 PUFA content. Interestingly, the hearts from parrots had a high n-3 PUFA content in contrast to their other tissues. The parrot brain exhibited a low MUFA, a high n-6 PUFA and low n-3 PUFA content, with no difference in total polyunsaturates.
The relative absence of differences in the fatty acid composition comparing long-living parrots to short-living quails is also apparent in the comparison of peroxidation indices. The PI values were lowest in erythrocytes (average value = 101), and highest in the brain (average value = 192) (Fig. 2). This is primarily related to differences in the phospholipid content of the most highly polyunsaturated fatty acid (docosahexaenoic acid; DHA) (see supplement).
Comparison of tissue and mitochondrial phospholipid composition
Similar to the phospholipids from the other tissues, there was little difference between parrots and quails in fatty acid sub-types of pectoral muscle and liver total phospholipids (Fig. 3), nor was there any substantial difference of fatty acid composition in mitochondrial phospholipids. The PI values were very similar in parrots and quails, both for total phospholipids and for mitochondrial phospholipids. A statistical comparison regarding longevity-related patterns in fatty acid sub-types revealed that parrot liver had a higher SFA content, more n-6 PUFA and less n-3 PUFA than quail liver. This PUFA distribution was also observed in mitochondrial phospholipids. Whereas longevity-related patterns were absent in pectoral muscle tissue, pectoral muscle mitochondria had a higher MUFA and lower n-6 PUFA content in the parrots in comparison to quail pectoral muscle mitochondria.
In examining the fatty acid composition and PI of mitochondrial phospholipids with tissue phospholipids, we note that both pectoral muscle and liver mitochondria have more n-6 PUFA and less n-3 PUFA than the respective total tissue phospholipids across all birds we studied. This relative exclusion of n-3 PUFA in pectoral muscle mitochondrial membranes compared to those from whole tissue was statistically significant in king quail, Japanese quail and cockatiels and in liver mitochondria of king quail and lovebirds, and was also observed in cockatiels (P = 0.05 for n-6 PUFA and P = 0.08 for n-3 PUFA). In liver phospholipids, mitochondria also exhibited a higher MUFA content in comparison to whole tissue, in all birds as a whole as well as in king quail and lovebirds.
As parrots and quails have different extents of reliance on flight, the comparison of the pectoral muscle phospholipid composition as a choice of the birds’ skeletal muscle tissue might seem inappropriate. However, a comparison of the tissue membrane fatty acid composition of pectoral muscle (Fig. 3) and leg muscle (Fig. 2) and of the peroxidation indices of those two tissues (see Fig. 4) revealed that both skeletal muscle tissues are very similar in these characteristics.
Relationship of skeletal muscle tissue (a) and liver mitochondrial (b) peroxidation index (PI) and maximum lifespan potential (MLSP) in mammals and birds. a Regression line is added for mammal hind limb muscle. Pectoral muscle PI values of previous bird studies, as well as pectoral muscle and leg muscle PI values from the parrots and quails in this study are superimposed. b Regression line is added for mammalian liver mitochondria. Liver mitochondrial PI values of previous bird studies, as well as liver mitochondrial PI values from the parrots and quails in this study are superimposed. For source of previous mammal and bird studies (see Hulbert 2010). For skeletal muscle, western sandpiper data (MLSP 9.2 years; PI 227) are taken from Guglielmo et al. (2002)
Discussion
Birds, in general, live twice as long as similar-sized mammals, which was first shown by Lindstedt and Calder (1976). Holmes et al. (2001) later pointed out that differences in metabolic rate between birds and mammals could not explain the observed longevity differences. We compared similar-sized parrots and fowl, which exhibit, on average, a fivefold difference in maximum lifespan. Similar to the bird–mammal longevity differences, our results show that the large longevity difference between the parrots and quails is not related to differences in BMR. Birds and mammals have a similar inverse relationship between MLSP and BMR, but the elevation of the bird relationship is higher (Hulbert et al. 2007). Using the regression equation describing this relationship in birds, both quail species are predicted to have a lifespan of 13 years based on their BMR, while parrots are predicted to have a maximum lifespan of 11–12 years. Thus, the quail live approximately half as long while parrots live two to three times longer than predicted from the relationship between bird BMR and MLSP.
One of the main goals of this study was to determine whether membrane fatty acid composition varied in a manner that might help explain differences in MLSP among the birds we studied. An advantage of our experimental design is that fatty acid composition was measured for both total tissue phospholipids and for phospholipids from mitochondria isolated from the same individuals. Previous comparisons relating membrane fatty acid composition to species longevity have depended solely on either skeletal muscle total tissue phospholipids or on liver mitochondrial phospholipids. Thus, we can examine membrane fatty acid composition in the context of different organs in the same individuals, in tissues and mitochondria of the same organ, and across species with different MLSP.
Our measurements of PI phospholipids collected from the seven tissues reveal a wide range of values, from a low average value of 101 in erythrocytes to an average value of 192 in brain tissue. The PUFA in erythrocyte membranes are predominantly n-6 PUFA (82% of total PUFA) with the highly polyunsaturated n-3 PUFA docosahexaenoic acid (DHA) being only 2.8% of total fatty acids. In contrast, the brain forms the other extreme with 46% of total PUFA being n-6 PUFA and a DHA content of 16% of total fatty acids. The great variation in membrane composition between tissues reflects differences in membrane fatty acid regulation, which likely relate to different performance requirements of cells in these tissues. Although the high omega-3 PUFA and DHA levels in the brain increase the risk of oxidative damage, DHA is known to have an important function in brain development, learning ability, and visual acuity (Picq et al. 2010). On the other side, the pulmonary design of birds means their erythrocytes are constantly surrounded by high concentrations of oxygen and iron associated with haemoglobin, two of the major peroxidation catalysts (Clemens and Waller 1987). To protect erythrocytes against this high peroxidation potential, birds have evolved membranes with a very low susceptibility to peroxidation by selecting fatty acid types, which balance peroxidation risk against the other functional requirements of that specific tissue.
Another aspect of the PI characteristics of different tissues was the very similar PI of different locomotor muscles. For our parrot–quail comparison, the PI values for both pectoral and leg muscle are almost indistinguishable (Fig. 4a), despite parrots relying mainly on flight and quail mainly on ambulatory locomotion. Examination of locomotor muscle PI in relation to maximum lifespan shows that parrots correspond with the relationship describing PI and MLSP in skeletal muscle of mammals (Fig. 4a) and overlap with PI values reported for other birds with a similar MLSP (Hulbert et al. 2002a). Furthermore, among the three parrot species, skeletal muscle PI is inversely related to MLSP. In the liver mitochondria graph (Fig. 4b), the PI values for the two parrot species lie within the PI values reported for birds with similar longevity. In contrast, it appears that the quails have exceptionally lower PI values than expected for their MLSP (Fig. 4a). The king and Japanese quails are the shortest-living birds for which the fatty acid composition of skeletal muscle phospholipids has been reported to date. Apart from the quails, there are data for ruby-throated hummingbirds (Infante et al. 2001) and western sandpipers (Guglielmo et al. 2002), which both live approximately 9 years. As can be seen from Fig. 4a, both these species have much higher peroxidation indices than those for quails. This suggests that the two quail species may be unusual in the fatty acid composition of their skeletal membrane lipids. Whether the quail species are unusual in regard to their membrane fatty acid composition, it will be necessary to determine the membrane fatty acid composition of other non-galliform bird species that live only 5 years. Another possibility that cannot be ruled out is that the maximum lifespan of quails is underestimated and that those species are in reality able to reach greater longevities than so far recorded.
Our characterisation of phospholipid composition of both total tissue and mitochondrial membranes in pectoral muscle and liver of the same individuals permits scrutiny of the extent of shared characteristics. Although, in general, the fatty acid composition of cell and mitochondrial membranes is quite similar, mitochondria have a statistically lower n-3 PUFA content in their membranes. This difference is mainly a result of reduction in the membrane content of the most highly polyunsaturated n-3 fatty acid, DHA (see supplement). Irrespective of MLSP, the mitochondrial membranes of birds contain significantly lower amounts of DHA in comparison to cell membranes. Given that DHA has one of the highest peroxidation potentials of fatty acids associated with animal membranes (Holman 1954), this would serve well to protect mitochondria from the deleterious effects of reactive oxygen species (ROS), which are primarily derived from superoxide production within the mitochondria.
A similar exclusion of n-3 PUFA in mitochondrial phospholipids relative to tissues surrounding them was observed previously in mammals and birds. For example, whereas human total liver phospholipids are reported to contain n-3 polyunsaturates, they are absent in liver mitochondrial membranes (Hulbert et al. 2010). However, data for humans are scarce and only limited to the liver. A similar finding was observed in rat skeletal muscle where mitochondrial phospholipids are less polyunsaturated than whole tissue phospholipids (Tsalouhidou et al. 2006), and in liver and heart of quails where mitochondrial membranes exhibit a low DHA content (Gutierrez et al. 2006).
The PI values were very similar in parrots and quails, in all tissues and mitochondria examined, highlighting a similar susceptibility to peroxidation. However, a similar membrane composition does not necessarily mean a similar amount of lipoxidation-derived damage and oxidative stress, as other components of the oxidative stress theory such as differences in ROS production, antioxidant defences and repair/removal rates of damaged molecules cannot be excluded and those might determine differences in longevity (Pamplona et al. 2002), although this seems unlikely for the current comparison (Montgomery et al., ms in review).
In general, the membrane fatty acid composition for all tissues differed little between parrots and quails. To minimise possible dietary influences on membrane fatty acid composition all birds were fed the same diet for at least 2 months. The small differences in fatty acid sub-types are therefore species-specific differences and not diet-induced differences. Although little is known about the mechanisms by which membrane fatty acid composition is regulated, research in mammals strongly suggests that membrane fatty acid composition is genetically determined and not strongly influenced by diet (as long as the diet is not deficient in essential fatty acids). For example, three strains of mice that differed in longevity, but were fed the same diet and kept under identical conditions for their lifetime (Miller et al. 2002), differed in membrane fatty acid composition (Hulbert et al. 2006a). As well, a recent study where rats were fed 12 different diets that varied widely in fatty acid composition showed that membrane fatty acid composition was relatively constant and the effect of diet was minimal (Abbott et al. 2010). In this respect, membrane lipids differ from storage lipids where fatty acid composition is strongly influenced by diet. The fatty acid composition values we report here are not dissimilar to published values for tissues from Japanese quail (Szabo et al. 2006, 2010) and for budgerigar brains (Pamplona et al. 2005). Whereas the budgerigars were male, all other bird species were of mixed sex. Membrane fatty acid composition was very similar in male and female birds of each species (no statistical differences), leaving us with the impression that membrane composition and membrane susceptibility to oxidative damage are independent of sex. In this regard, this finding agrees with previous studies showing that sex has no effect on membrane phospholipid composition in bird tissues (e.g. Price and Guglielmo 2009; Baeza et al. 2010).
Comparison of oxidative stress parameters in species that differ in MLSP can be approached in two ways: (1) animals can be chosen to have the same biological age, meaning that the parrots would be approximately five-times older than the quails, or (2) animals can be chosen to have the same chronological age. We used the second approach and selected birds that were all approximately 1 year old, with the prediction that if oxidative stress plays a role in longevity the quails (that reached already 20% of their lifespan whereas the parrots reached only 5% of their MLSP) would show more pronounced levels of oxidative damage. If membrane fatty acid composition is important in bird longevity, quail membranes at their age of 1 year would be expected to show a higher susceptibility to damage. Although we do not know whether membrane fatty acid composition changes with age in these bird species, the evidence to date suggest membrane fatty acid composition is relatively constant throughout a species lifespan. For example, in naked mole rats membrane composition is essentially the same in individuals that are <1, 7 and ~80% of their MLSP (Hulbert et al. 2006b).
Membrane fatty acid composition is believed to affect MLSP because of the different susceptibilities of different fatty acids to peroxidative damage. These differences are encapsulated in the PI which, for two sources of phospholipids (skeletal muscle total phospholipids and liver mitochondrial phospholipids), has been shown to be inversely related to MLSP in mammals and birds (see Fig. 4). The PI values we measured for quails depart from this general pattern (especially for skeletal muscle). As can be seen from Fig. 4, there is less membrane fatty acid data available for birds than for mammals. Although in this contribution we have added extra bird data, the existing data set is still not definite enough to ascertain whether birds and mammals show the same MLSP-PI relationship. Such a conclusion awaits more bird data, both from other tissues and also from bird species with a wide range of maximum lifespans, especially from short-living non-galliform bird species.
References
Abbott SK, Else PL, Hulbert AJ (2010) Membrane fatty acid composition of rat skeletal muscle is most responsive to the balance of dietary n-3 and n-6 PUFA. Br J Nutr 103:522–529
Arnold TW (1988) Life histories of North American game birds: a reanalysis. Can J Zool 66:1906–1912
Baeza E, Chartrin P, Meteau K, Bordeau T, Juin H, Le Bihan-Duval E, Lessire M, Berri C (2010) Effect of sex and genotype on carcase composition and nutritional characteristics of chicken meat. Br Poult Sci 51:344–353
Brand MD, Turner N, Ocloo A, Else PL, Hulbert AJ (2003) Proton conductance and fatty acyl composition of liver mitochondria correlates with body mass in birds. Biochem J 376:741–748
Brouwer K, Jones ML, King CE, Schifter H (2007) Longevity records for Psittaciformes in captivity. Int Zoo Yearb 37:299–316
Buttemer WA, Warne S, Bech C, Astheimer LB (2008) Testosterone effects on avian basal metabolic rate and aerobic performance: facts and artefacts. Comp Biochem Physiol A Mol Integr Physiol 150:204–210
Clemens MR, Waller HD (1987) Lipid peroxidation in erythrocytes. Chem Phys Lipids 45:251–268
Couture P, Hulbert AJ (1995) Membrane fatty acid composition of tissues is related to body mass of mammals. J Membr Biol 148:27–39
Gornall AG, Bardawill CJ, David MM (1949) Determination of serum proteins by means of the biuret reaction. J Biol Chem 177:751–766
Gudbjarnason S, Doell B, Oskarsdottir G (1978) Docosahexaenoic acid in cardiac metabolism and function. Acta Biol Med Ger 37:777–784
Guglielmo CG, Williams TD, Zwingelstein G, Brichon G, Weber JM (2002) Plasma and muscle phospholipids are involved in the metabolic response to long-distance migration in a shorebird. J Comp Physiol B Biochem Syst Environ Physiol 172:409–417
Gutierrez AM, Reboredo GR, Mosca SM, Catala A (2006) A low degree of fatty acid unsaturation leads to high resistance to lipid peroxidation in mitochondria and microsomes of different organs of quail (Coturnix coturnix japonica). Mol Cell Biochem 282:109–115
Holman RT (1954) Autoxidation of fats and related substances. Pergamon Press, London
Holmes DJ, Fluckiger R, Austad SN (2001) Comparative biology of aging in birds: an update. Exp Gerontol 36:869–883
Hulbert AJ (2008) The links between membrane composition, metabolic rate and lifespan. Comp Biochem Physiol A Mol Integr Physiol 150:196–203
Hulbert AJ (2010) Metabolism and longevity: is there a role for membrane fatty acids? Integr Comp Biol 50:808–817
Hulbert AJ, Beard LA, Grigg GC (2008) The exceptional longevity of an egg-laying mammal, the short-beaked echidna (Tachyglossus aculeatus) is associated with peroxidation-resistant membrane composition. Exp Gerontol 43:729–733
Hulbert AJ, Else PL (1999) Membranes as possible pacemakers of metabolism. J Theor Biol 199:257–274
Hulbert AJ, Else PL (2000) Mechanisms underlying the cost of living in animals. Annu Rev Physiol 62:207–235
Hulbert AJ, Faulks S, Buttemer WA, Else PL (2002a) Acyl composition of muscle membranes varies with body size in birds. J Exp Biol 205:3561–3569
Hulbert AJ, Faulks SC, Harper JM, Miller RA, Buffenstein R (2006a) Extended longevity of wild-derived mice is associated with peroxidation-resistant membranes. Mech Ageing Dev 127:653–657
Hulbert AJ, Faulks SC, Buffenstein R (2006b) Oxidation-resistant membrane phospholipids can explain longevity differences among the longest-living rodents and similarly-sized mice. J Gerontol A Biol Sci Med Sci 61:1009–1018
Hulbert AJ, Pamplona R, Buffenstein R, Buttemer WA (2007) Life and death: metabolic rate, membrane composition, and life span of animals. Physiol Rev 87:1175–1213
Hulbert AJ, Rana T, Couture P (2002b) The acyl composition of mammalian phospholipids: an allometric analysis. Comp Biochem Physiol B Biochem Mol Biol 132:515–527
Hulbert AJ, Trzcionka M, Buttemer WA (2010) Membrane fatty acid composition and longevity of mammals and birds. In: Pamplona R, Barja G (eds) Longevity, mitochondria and oxygen free radicals. Research Signpost, Kerala, pp 79–93
Infante JP, Kirwan RC, Brenna JT (2001) High levels of docosahexaenoic acid (22:6n–3)-containing phospholipids in high-frequency contraction muscles of hummingbirds and rattlesnakes. Comp Biochem Physiol B Biochem Mol Biol 130:291–298
Lindstedt SL, Calder WA (1976) Body size and longevity in birds. Condor 78:91–94
Miller RA, Harper JM, Dysko RC, Durkee SJ, Austad SN (2002) Longer life spans and delayed maturation in wild-derived mice. Exp Biol Med (Maywood) 227:500–508
Pamplona R, Portero-Otin M, Riba D, Ruiz C, Prat J, Bellmunt MJ, Barja G (1998) Mitochondrial membrane peroxidizability index is inversely related to maximum life span in mammals. J Lipid Res 39:1989–1994
Pamplona R, Barja G, Portero-Otin M (2002) Membrane fatty acid unsaturation, protection against oxidative stress, and maximum life span: a homeoviscous-longevity adaptation? Ann N Y Acad Sci 959:475–490
Pamplona R, Portero-Otin M, Sanz A, Ayala V, Vasileva E, Barja G (2005) Protein and lipid oxidative damage and complex I content are lower in the brain of budgerigar and canaries than in mice. Relation to aging rate. Age (Dordr) 27:267–280
Picq M, Chen P, Perez M, Michaud M, Vericel E, Guichardant M, Lagarde M (2010) DHA metabolism: targeting the brain and lipoxygenation. Mol Neurobiol 42:48–51
Price ER, Guglielmo CG (2009) The effect of muscle phospholipid fatty acid composition on exercise performance: a direct test in the migratory white-throated sparrow (Zonotrichia albicollis). Am J Physiol Regul Integr Comp Physiol 297:R775–R782
Szabo A, Febel H, Mezes M, Balogh K, Horn P, Romvari R (2006) Body size related adaptations of the avian myocardial phospholipid fatty acyl chain composition. Comp Biochem Physiol B Biochem Mol Biol 144:496–502
Szabo A, Mezes M, Romvari R, Febel H (2010) Allometric scaling of fatty acyl chains in fowl liver, lung and kidney, but not in brain phospholipids. Comp Biochem Physiol B Biochem Mol Biol 155(3):301–308
Trzcionka M, Withers KW, Klingenspor M, Jastroch M (2008) The effects of fasting and cold exposure on metabolic rate and mitochondrial proton leak in liver and skeletal muscle of an amphibian, the cane toad Bufo marinus. J Exp Biol 211:1911–1918
Tsalouhidou S, Argyrou C, Theofilidis G, Karaoglanidis D, Orfanidou E, Nikolaidis MG, Petridou A, Mougios V (2006) Mitochondrial phospholipids of rat skeletal muscle are less polyunsaturated than whole tissue phospholipids: implications for protection against oxidative stress. J Anim Sci 84:2818–2825
Turner N, Haga KL, Else PL, Hulbert AJ (2006) Scaling of Na+, K+-ATPase molecular activity and membrane fatty acid composition in mammalian and avian hearts. Physiol Biochem Zool 79:522–533
Turner N, Haga KL, Hulbert AJ, Else PL (2005) Relationship between body size, Na+-K+-ATPase activity, and membrane lipid composition in mammal and bird kidney. Am J Physiol Regul Integr Comp Physiol 288:R301–R310
Weathers WW, Schoenbaechler DC (1976) Regulation of body temperature in the budgerygah, Melopsittacus undulatus. Aust J Zool 24:39–47
Acknowledgments
We gratefully acknowledge the assistance on diet formulation provided by Kirk. C. Klasing and Ron Newman. This research was supported by funding from the Australian Research Council (DP8079261). The study was conceived and planned by AJH, WAB and MKM; the experiments were carried out by MKM and the manuscript was written by MKM, AJH, and WAB.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by I.D. Hume.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Montgomery, M.K., Hulbert, A.J. & Buttemer, W.A. Metabolic rate and membrane fatty acid composition in birds: a comparison between long-living parrots and short-living fowl. J Comp Physiol B 182, 127–137 (2012). https://doi.org/10.1007/s00360-011-0603-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00360-011-0603-1