Abstract
Namaqua rock mice (Aethomys namaquensis) consume nectar xylose when visiting Protea flowers. Whole-animal metabolism studies suggest that the gastrointestinal microflora plays an important role in xylose metabolism in A. namaquensis. We collected caecal contents under anaerobic conditions, cultured caecal microflora both aerobically and anaerobically, and assessed caecal microbial xylose utilization using a 14C-xylose incubation assay. All four mice sampled hosted culturable caecal micro-organisms that tested positive for xylose utilization. These were classified by 16S rRNA based taxonomy as: Bacillus subtilis, Bacillus pumilus, Bacillus licheniformis, Shigella boydii, Arthrobacter sp. and members of the fungal genera Aspergillus and Penicillium. Cultures of these isolates were then analyzed by gas chromatography to determine the types and quantities of short-chain fatty acids produced by xylose fermentation. These results are discussed in the context of other studies of gut microflora in vertebrates.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In two South African genera of the Proteaceae, Protea and Faurea, xylose constitutes as much as 39% by mass of total nectar sugars, the remainder of which are the more common nectar sugars sucrose, glucose and fructose (Nicolson and van Wyk 1998). Before nectar sugar analyses among the Proteaceae (van Wyk and Nicolson 1995; Nicolson and van Wyk 1998), the pentose sugar xylose had not been reported as a floral nectar sugar and was only known to occur in the extrafloral nectar of grasses (Bowden 1970). Its presence in the nectar of Proteaceae prompted studies of sugar preferences and sugar absorption efficiencies among insect, bird and rodent pollinators of the Proteaceae, reviewed by Jackson and Nicolson (2002). These studies suggested that xylose is of some importance in the diet of the Namaqua rock mouse Aethomys namaquensis, which pollinates certain geoflorous Protea species but is unlike other pollinators of the Proteaceae in that it consumes pure xylose solutions and gains energetic benefit from this sugar (Johnson et al. 1999). A high total proportion (78%) of xylose doses given to rock mice was processed in some way, with only 2% of ingested xylose simply excreted as feces (Johnson et al. 1999, 2006). Of the processed fraction, some 42% is likely metabolized in the gut lumen, and a further 34% absorbed from the intestine into the blood, then filtered and excreted in the urine (Johnson et al. 2006). The fate of the remaining 22% is unknown, but it is more likely to be retained in the blood and slowly excreted rather than efficiently metabolized by the mouse tissues, which probably lack the necessary enzymes for this process (xylose isomerase and xylulokinase). Certain bacteria, yeasts and fungi possess these enzymes and are well known to be efficient xylose utilizers (Demetrakopolous and Amos 1978; Chandrakant and Bisaria 1998; Ho et al. 1999; Jeffries and Shi 1999; Aristidou and Penttilä 2000).
Gastrointestinal microflora are essential components of the digestive system in vertebrates that consume plant material (Stevens and Hume 1995). Plant polysaccharides, such as cellulose and hemicellulose, can only be utilized by vertebrates if the necessary gastrointestinal microbes first degrade the polysaccharides into components that are metabolically useful to the host. The hemicellulose component of plant cell walls, xylan, is a polysaccharide made up of xylose monomers. The degradation of xylan and the microbial utilization of xylose have been well studied in ruminants (e.g. Turner and Roberton 1979; Hespell et al. 1987; Matte et al. 1992; Marounek and Kopečný 1994) and less thoroughly investigated in non-ruminants where, particularly in small herbivores, the major part of microbial plant digestion occurs in the caecum (Stevens and Hume 1995). Microbial degradation and fermentation of plant material in the vertebrate gut produces, predominantly, short-chain fatty acids (SCFA), which the host animal is able to use in oxidative metabolism. The general nutritional contributions of gut microbes to vertebrates, and SCFA production and contributions to the energy requirements of vertebrates have been comprehensively reviewed by Stevens and Hume (1995, 1998).
Xylose utilization in situ by intestinal bacteria of A. namaquensis has been previously confirmed using captive mice fed 14C-labeled xylose first with their natural gut flora intact and then again after antibiotic treatment to reduce gut flora populations (Johnson et al. 2004, 2006). Reduction of gut microbe populations reduced xylose metabolism to 57% of pre-treatment values, highlighting the importance of gut symbionts in complete utilization of this sugar by the rodent. Here, we further investigate this relationship by isolation and identification of some important xylose-utilizing microbes from the cecum of this mouse species. Diet plays an important role in the structure and metabolic activity of intestinal microbial communities; we investigated bacterial xylose utilization in the gut contents of mice in their natural habitat, eating their natural diet. We cultured obligate and facultatively anaerobic microbes isolated from the mouse caecum under strict anaerobic conditions, to simulate conditions in the mouses’ caeca as closely as possible. We also sampled caecal contents anaerobically and assessed bacterial xylose utilization using a 14C-xylose incubation assay. We identified positive xylose utilizers from these cultures to species level by DNA sequencing. This approach highlights a novel role for rodent endosymbionts in the context of plant–pollinator relationships.
Materials and methods
Sample collection and isolation of microflora
Aethomys namaquensis individuals were caught under permit (numbers 180/2003 and 001-201-0002, Western Cape Nature Conservation) in Sherman live traps at Wolfieskop (33°55′S, 19°24′E) and Jonaskop (33°56′S, 19°31′E), near Villiersdorp in the Riviersonderend Mountains, South Africa. These sites were chosen because of the presence of Protea humiflora, a ground-flowering species known to be pollinated by A. namaquensis (Fleming and Nicolson 2002), and in which xylose comprises 5–9% of total nectar sugars (Nicolson and van Wyk 1998). Animals were captured during the flowering season (July–September) when xylose, in the form of P. humiflora nectar, was present in their diet. Ethical approval for animal capture and the protocol below was obtained from Sub-Committee B of the Research Committee of the University of Stellenbosch.
Four animals (Mouse 1–Mouse 4) caught during the night were taken back to the laboratory and housed individually in standard rodent cages (44 cm L × 28 cm B × 23 cm H) at a constant temperature of 22°C with ad libitum access to water and food pellets (Prestige Products, Crispy Rat Muesli). Within 24 h of capture, animals were killed by CO2 inhalation and placed in an anaerobic chamber (atmosphere: 85% N2, 10% CO2 and 5% H2) (Forma Scientific, OH, USA) where the caecum of each was excised. One 0.5 g sample of caecal contents for each animal was weighed into a sterile tube containing glass beads and 4.5 ml sterile pre-reduced rumen fluid-based liquid medium (Campbell et al. 1997). The sample was vortexed to homogenize the caecal contents and resuspend bacteria from caecal material. Isolation of caecal samples was carried out under strictly anaerobic conditions within the anaerobic chamber.
Cultivation of microflora
Enumeration and growth of culturable caecal microbes was done both aerobically and anaerobically to obtain as many different microbial species in culture as possible. Serial 10-fold dilutions (10−2–10−7) of the single sample from each mouse suspended in liquid medium were plated out in duplicate on a rumen fluid-based medium, [a medium used to specifically culture rat caecal and fecal microflora (Campbell et al. 1997)]. Plates were incubated at 37°C for 48 h. Anaerobic incubation was carried out in anaerobic jars. The number of colony forming units (CFU) per gram of caecal content was calculated for each plate.
Xylose utilization
For each of the four mice, single representatives of all culturable colonies were selected from the plates at the highest dilution that yielded sufficient numbers of colonies (30 < n < 300). We selected representatives on the basis of colony size, color, and shape, and re-streaked these onto rumen fluid-based medium to produce pure cultures (Mouse 1: 10 isolates, 4 aerobic and 6 anaerobic; Mouse 2: 13 isolates, 10 aerobic and 3 anaerobic; Mouse 3: 16 isolates, 7 aerobic and 9 anaerobic; Mouse 4: 6 isolates, 2 aerobic and 4 anaerobic). All these isolated cell lines (n = 45; 23 aerobic and 22 anaerobic isolates) were used for assessment of xylose utilization using a 14C-xylose radio-isotopic incubation assay that we modified from procedures described by Krishnan et al. (1980). The 14C-assay was performed by placing a 2 ml microtube (Quality Scientific Plastics, Porex Bio Products Group, Fairburn, GA, USA) into a 20 ml glass vial. The vials and microtubes were autoclaved and 1.25 ml aliquots of a 1 M NaOH solution were placed into the microtube to trap CO2 released by growing cultures during incubation. NaOH traps CO2 as sodium hydrogen carbonate (NaHCO3) which remains in solution. The assay medium, dispensed in 1 ml aliquots into each vial, contained: 0.05 g xylose, 0.05 g casitone, 40 ml clarified rumen fluid (filtered through cheesecloth, flushed with CO2, autoclaved, stored at 4°C and centrifuged at 10,000×g for 20 min before use in medium), 7.5 ml solution A (g/l: 6.0 K2PO4 and 2.0 Na3C6H5O7·2H2O) and 7.5 ml solution B (g/l: 12.0 NaCl, 12.0 (NH4)2SO4, 6.0 KH2PO4, 1.2 CaCl2, 2.46 MgSO4·7H2O and 20.0 Na3C6H5O7·2H2O) made up to 100 ml with distilled water. Prior to inoculation, 0.1 μCi uniformly labeled 14C-xylose (specific activity: 542 μCi/mg, Amersham Life Science, IL, USA) was added to the assay medium in each vial. Isolated cultures were inoculated into the assay medium in three separate vials, which were sealed with a sterile rubber stopper and metal crimp seal. Vials inoculated with anaerobic cultures were flushed with CO2 before being crimp sealed. Vials were incubated at 37°C oscillating at 70 rpm for 48 h.
After incubation, vial contents were prepared for liquid scintillation counting as follows: all NaOH was removed from the microtube by micro-pipette. The low-retention plastic of the micro-tubes ensured that no liquid was lost during this process. The NaOH solution was dispensed into a glass scintillation vial, to which 14 ml Hionic Fluor scintillation cocktail (Perkin Elmer, MA, USA) was added; 0.25 ml aliquots of each sample’s assay medium were dispensed into four scintillation vials and each mixed with 16 ml Hionic Fluor; 1 ml distilled water was used to rinse the assay vial and was dispensed into a scintillation vial and mixed with 15 ml Ultima Gold scintillation cocktail (Packard, Connecticut, USA). Radioactivity of samples was determined on a Beckman liquid scintillation counter (LS 5000 TD, Beckman Instruments, CA, USA). The protocol counted radioactivity in the full 14C window (0 to 154 keV) to ensure the highest counting efficiency. Counting efficiency was calculated from the C14 quench curve generated for the scintillation counter using manufacturer’s standards. Xylose utilization was assessed by determining the percentage of the original 0.1 μCi 14C-xylose dose converted to 14CO2 and trapped in the NaOH solution.
Microbe identification
Positive bacterial xylose utilizers, identified by the 14C-xylose assay, were processed for identification. DNA was extracted using the Dneasy Tissue Kit (QIAGEN catalogue no. 69504). DNA encoding the 16S rRNA gene was amplified by PCR with two 16S rRNA primers, one forward (F1:S16,1) and one reverse (R5:S16,12) primer. Amplifications were carried out on an Applied Biosystems (CA, USA) 27000 thermal cycler. PCR products were purified using the Wizard SV Gel and PCR Clean-up System (Promega Corporation, WI, USA). Strands were cycle sequenced using BigDye dye termination chemistry (Perkin Elmer, CT, USA) and all unincorporated dye label was removed by Sephadex columns (strip of eight columns, Princeton Separations, NJ, USA) according to the manufacturers’ protocol, before the samples were run on an ABI Prism 3100 sequencer (Applied Biosystems). Nucleotide sequences obtained were analyzed using Sequence Navigator software (ABI, version 1.01). Nucleotide homology searches were performed in the GenBank database with BLAST.
Fungal colonies that proved to be xylose utilizers were identified to genus level based on the morphology of the colonies and microscopic analyses of conidiophore morphology (Domsch et al. 1980; Raper and Fennel 1965).
Short-chain fatty acid production
After taxonomic identification, the caecal microflora species isolated as positive xylose utilizers were further investigated by gas chromatography in order to identify the types of SCFA they each produce during xylose metabolism. Colonies from stock tubes were streaked onto rumen fluid-based medium to reproduce cultures. After incubation at 37°C for 24 h, colonies from the plates were used to inoculate pre-culture test tubes containing 5 ml rumen fluid medium. Pre-culture tubes were incubated at 37°C for another 24 h and optical density was measured at 600 nm, in order to determine the volume of pre-culture, with an optical density of 0.1, required to inoculate experimental tubes. For each caecal microflora species, duplicate experimental tubes were prepared for two xylose concentrations that were tested. The medium used to cultivate cultures for gas chromatographic analysis was the same as the assay medium used in the xylose utilization assay described above, except that the concentration of xylose was either 1% or 2% (w:v). Duplicate experimental tubes of each concentration were inoculated with culture from the pre-culture tubes. Control tubes were not inoculated with culture. All tubes were incubated at 37°C for 12 h. After incubation the pH of each tube was determined using a pH meter, for comparison with the initial pH of culture medium (7.2) before incubation. Each tube was then centrifuged at 10,000×g for 15 min at 4°C. The supernatant was filter-sterilized with 0.45 μm filters and stored at −20°C until analysis by gas chromatography. Samples of the rumen fluid used to make up the culture medium were also analyzed by gas chromatography to determine the concentrations of SCFA contributed by rumen fluid to the freshly made medium.
Gas chromatographic analysis
A standard solution of SCFA (acetic, propionic, butyric, iso-butyric, valeric and iso-valeric) was prepared by dissolving 1 ml of each fatty acid and 0.5 ml of n-hexanol in 1 l of formic acid solution (one part 35% formic acid and three parts distilled water). Samples were prepared for analysis by diluting 1 ml of 35% formic acid with 3 ml of aqueous, filtered sample and adding 2 μl of n-hexanol as internal standard. The injection volume was 1 μl. Samples were analyzed using a Varian 3700 gas chromatograph equipped with a flame ionization detector and a 30 m bonded phase Nukol (Supelco, Inc., Belafonte, PA) fused silica capillary column (0.53 mm diameter and 0.50 μm film thickness). The column temperature was initially held at 105°C for 2 min, increased at 10°C min−1 to 190°C and held for 10 min. The injector temperature was set at 150°C, while the detector was set at 300°C. Nitrogen was used as carrier gas at a flow rate of 6.1 ml min−1. SCFA were quantified by means of Borwin Version 1.2 integration software (JMBS Developments, Le Fontanil, France), operating in the internal standard mode. Results were converted to original sample concentration.
Statistics
One-sample t tests were used to compare the total amounts of acid produced in the 1 and 2% xylose culture media after incubation to the total amount of acid in control media before incubation, in order to detect significant increases in acid production. One-sample t tests were also used to compare the final pH values in the 1 and 2% xylose media to the pH of the culture media before incubation to detect significant decreases in the pH as a result of acid production. Finally, we used stepwise multiple regression with pH as the dependent variable and concentrations of each different SCFA by each species as independent variables, to ascertain which SCFA accounted for the greatest proportion of the pH variation between species. These analyses were performed using the Statistica 7 software package (Statsoft Inc., Tulsa, OK, USA).
Results
Isolation and cultivation of microflora
The total number of CFU cultured from caecal samples on rumen fluid-based medium ranged from 5.7 × 108 to 5.2 × 109 per g caecal content (coefficient of variation = 82%) for aerobic cultures, and from 2.6 × 108 to 9.1 × 1010 per g caecal content (coefficient of variation = 89%) for anaerobic cultures.
Xylose utilization
For all 45 isolates assayed in triplicate, total recovery of the label, expressed as a percentage of the original dose, ranged from 60.8 to 99.9% (mean = 87.2% ± 11.7 SE). Of the 45 isolates assayed, 24 showed evidence of xylose utilization as label trapped in the NaOH solution. Of those 24 positive xylose utilizers, 4 were isolated from Mouse 1, 7 from Mouse 2, 10 from Mouse 3 and 4 from Mouse 4 (Table 1). The mean percentage of the label trapped in NaOH for the 24 isolates, each assayed in triplicate, ranged from 6.4 to 60.1% of the original dose. The rest of the isolates all had less than 0.8% of the label trapped in NaOH. Counts obtained from these samples were close to background counts obtained for blank NaOH solutions.
Microbe identification
Of the 24 isolates identified as positive xylose utilizers, 21 were bacterial and three were fungal isolates. The 21 bacterial isolates were made up of 16 isolates belonging to the genus Bacillus (which comprised three different species: Bacillus subtilis, Bacillus pumilus and Bacillus licheniformis), four Shigella boydii isolates and one isolate belonging to the genus Arthrobacter (Table 1). Morphological analyses of the three fungal isolates identified one as an Aspergillus species and two as members of the genus Penicillium. Thus four bacterial isolates were identified to species level and one bacterial and two fungal isolates identified to genus level. These seven isolates are hereafter referred to as the xylose-utilizing isolates. Results are presented and discussed for each xylose-utilizing isolate in turn, rather than as communities within each mouse. A group of possibly pathogenic microbes, which included S. boydii, the Arthrobacter species and Penicillium fungi were all isolated from one mouse, and another pathogenic fungus, Aspergillus, from another mouse. The pathogenicity of these microbes to wild mice is not known.
Short-chain fatty acid production
The concentrations of SCFA in the autoclaved rumen fluid were comparable to the concentrations of SCFA present in freshly made media and in the control tubes after the incubation period. Thus, the initial concentrations of SCFA in the culture medium are due to the use of rumen fluid in the medium, and higher concentrations of SCFA in experimental tubes after incubation indicated SCFA production. Each of the seven xylose-utilizing isolates produced the SCFA that are typically end-products of microbial fermentation, namely acetic, propionic and butyric acids. Low, but measurable concentrations of iso-butyric, valeric and iso-valeric acids were also detected. After incubation all acids were present in the culture medium at concentrations greater than those present in the controls. Consequently, for each acid we subtracted the quantities of SCFA measured in the controls from those measured in experimental tubes to estimate the amount of acid produced by each species during incubation. The production of fatty acids by the five bacterial species was generally greater than that by the two fungal species (Table 2).
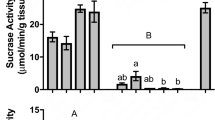
The amounts of acid produced depended on substrate concentration but not consistently in the expected direction of more substrate yielding more acid. The three Bacillus species produced between two and six times as much acetic acid when grown in the 2% xylose medium compared to 1% xylose. The Arthrobacter species, as well as the Penicillium isolate, also produced more acetic acid in the 2% medium. Bacillus pumilus, S. boydii and the Penicillium isolate produced as much as twice the amount of propionic acid in the 2% medium. S. boydii also produced more butyric acid in the 2% medium. Species that produced higher concentrations of acid in the 1% xylose medium were S. boydii and the Aspergillus species, which produced more acetic acid, and B. subtilus, Arthrobacter and Aspergillus species which produced more propionic acid. The Arthrobacter species also produced more butyric acid in the 1% medium relative to the 2% xylose medium. The remaining acids, iso-butyric, valeric and iso-valeric, were produced in much lower quantities than the other three acids, except for B. pumilus and B. licheniformis, which produced 4.41 and 19.61 μM/12 h, respectively, in the 2% medium. The production of iso-valeric acid by B. licheniformis at 2% exceeded production of this acid by all other isolates by an order of magnitude, but we are unable to explain this result. Across all the acids only B. licheniformis was consistent in producing more acid in response to more xylose in the medium. B. pumilus did so for five out of the six acids, S. boydii, for four out of the six, the Penicillium isolate for three, B. subtilus for two and the Arthrobacter species for one out of the six. In both media, the total amount of acid produced was significantly greater than the initial amount of acid in the medium (P < 0.05), a difference largely due to the production of acetic acid. Mean molar ratios of acetate:propionate:butyrate across all isolates in the 1% medium were 52:35:13, with acetate ranging from 26 to 71% of total acid, proprionate from 22 to 58%, and butyrate from 7 to 23%. In the 2% medium, mean molar ratios of these acids, respectively, were 62:29:9, with acetate ranging from 38 to 79% of total acid, propionate from 16 to 62%, and butyrate from 0 to 19%.
The production of acid during incubation was also reflected in the decrease in pH from the initial medium pH of 7.2. Final pH values in the 1% medium ranged from 5.57 to 7.12, and in the 2% medium from 5.47 to 6.06 (Table 2). In both media the final pH differed significantly from the initial pH of 7.2 (P < 0.05). The stronger production of acids in the 2% medium was also reflected in the pH readings, with a highly significant decrease in the 2% medium (P < 0.00001). Stepwise multiple regressions revealed that across all seven isolate species, the molar concentrations of four SCFA emerged as significant predictors of pH. For 1% xylose medium, acetic and iso-valeric acids accounted for more than 97% of all pH variance (R = 0.973, adjusted R² = 0.8934, F (3,3) = 17.766, P < 0.021; for acetic acid, P < 0.037576, and for iso-valeric acid, P < 0.006532). For 2% xylose medium, propionic and butyric acid concentrations together accounted for 95.8% of pH variance (R = 0.9583, adjusted R² = 0.8366, F (3,3) = 11.239, P < 0.03866; for propionic, P < 0.036, and for butyric acid, P < 0.014).
Discussion
We have shown here how gut symbionts enable Namaqua rock mice to obtain energy from xylose, an unusual nectar sugar whose presence in Proteacea nectar is still enigmatic (Jackson and Nicolson 2002). In addition to nectar, rock mice eat plant material (floral structures and seeds), and like other rodents, have a caecal fermentation chamber where cellulose in this material is broken down by gut bacteria. Gut morphology and fermentative capacity may thus be a pre-adaptation sensu Shelley (1999), allowing mice that consume xylose-rich nectar to access the energy in this sugar.
In birds, the other vertebrate pollinators in the Cape Floristic Kingdom (“fynbos”), large populations of intestinal bacteria and specialized caecal bacteria enhance the efficiency of cellulose digestion (Mead 1989; Lan et al. 2005). Caeca therefore tend to be large in herbivorous and omnivorous birds, and small, vestigal or absent in carnivorous, insectivorous, piscivorous, granivorous and nectarivorous species (Clench and Mathias 1995; Preest et al. 2003). Since the presence of xylose-fermenting microbes in host animal’s digestive systems appears to be linked to a more widespread fermentative capacity for long-chain carbohydrates, it is not unexpected that nectarivorous bird pollinators of the Proteaceae lack xylose-fermenting gut microflora. The third group of pollinators of the fynbos, insects such as the green protea beetle (Trichostetha fasicularis), may well possess xylose-utilizing endosymbionts (Johnson and Nicolson 2001): xylose-fermenting gut yeasts of the Pichia stipitis clade are associated with wood-ingesting beetles (Nardon and Grenier 1989; Suh et al. 2003).
Xylose utilization by rodent gut microflora
Removal of the caecum under anaerobic conditions and exposure of caecal contents in an anaerobic environment optimized survival and culture of obligate anaerobic microflora. Moreover, our use of a rumen-fluid based medium, previously used to culture rat caecal and fecal microflora (Campbell et al. 1997) created growth conditions favorable for positively identified xylose utilizers: Bacillus subtilis, B. pumilus, B. licheniformis, S. boydii, an Arthrobacter species and fungal isolates belonging to the genera Aspergillus and Penicillium. We acknowledge the potential bias introduced when experimental strains are selected using a synthetic medium and grown in isolation. In our study, this approach may have favored Bacillus species, common to caecal samples from all four mice. However, to enable replication of experiments and because of the potential for shifts of microbial community composition over time, we chose to work with dominant community members in pure culture as a first step to characterizing this particular microbe community.
The bacteria identified here probably contribute modestly to the nutrition of A. namaquensis, particularly during the austral winter and spring: the community of xylose utilizers in the digestive tract of Namaqua rock mice is enriched when xylose is included in the diet (Johnson et al. 2006). This community is likely not unique among mammals: domestic pigs are known to possess gut bacteria that ferment xylose (e.g. Kiriyama et al. 1992). It is unique in terms of its occurrence in a herbivore rather than an omnivore, and in that the context for this occurrence is the remarkable Cape Floristic Kingdom (“fynbos”), the biodiversity of which is mirrored by a diversity of plant–pollinator relations that are still being explored.
The Bacillus species isolated from Namaqua rock mice in this study have also been isolated from the gastrointestinal tract of broiler chickens (Barbosa et al. 2005). Mouse gut B. subtilis and B. licheniformis grew under both aerobic and anaerobic conditions in the laboratory. B. pumilus isolates grew only aerobically, but are most likely capable of anaerobic growth as well, since they were isolated from the anaerobic environment of the gut, although the isolation and assaying process revealed two of the aerobic cultures as positive xylose utilizers. More than 90% of B. subtilis, B. pumilus and B. licheniformis strains are capable of producing acid from xylose (Sneath et al. 1986). Xylose utilization, expressed as the percentage of the dose recovered in NaOH during the radio-isotopic incubation assay, ranged from 8.5 to 36.1% for the seven B. subtilis isolates and from 6.5 to 48.1% for the seven B. licheniformis isolates. The two B. pumilus isolates showed very low xylose utilization (6.4 and 6.7%).
The pathogenic bacterium S. boydii was isolated under anaerobic conditions from one of the four mice used in this study. The genus Shigella comprises Gram-negative rods that are facultatively anaerobic (Sneath et al. 1986). Within the genus 10% or less of the strains are positive for acid production from xylose, and xylose fermentation among different S. boydii strains is variable (Sneath et al. 1986). The four S. boydii isolates cultured here all grew only anaerobically and showed xylose utilization ranging from 14.7 to 37.7% of the original dose.
A single isolate identified as Arthrobacter sp. was not identified to species level since it matched most significantly with an unidentified Arthrobacter species in the GenBank database. Species within the genus Arthrobacter are Gram-negative rod shaped bacteria in young cultures and Gram-positive cocci in older cultures (Sneath et al. 1986; Eschbach et al. 2003). Most species of Arthrobacter are obligate aerobes and make up an important fraction of the indigenous bacterial flora of soils; however, some species (Arthrobacter globiformis and Arthrobacter nicotianae) have shown an adaptive capacity to grow anaerobically (Eschbach et al. 2003). Ninety percent or more strains of Arthrobacter nicotianae utilize xylose (Sneath et al. 1986). Some Arthrobacter species are regarded as opportunistic pathogens (Funke et al. 1996). Recently a number of new Arthrobacter species, isolated from human and other animal sources, have been described: A. luteolus and A. albus from human clinical specimens (Wauters et al. 2000); A. rhombi, from organs of Greenland halibut (Reinhardtius hippoglossoides) (Osorio et al. 1999); A. nasiphocae from the nasal cavities of the common seal (Phoca vitulina) (Collins et al. 2002), and A. gandavensis from mastitic milk and the uterus of dairy cows (Storms et al. 2003). Of all of these new Arthrobacter species, only A. luteolus utilized xylose (Wauters et al. 2000). The single Arthrobacter species that we isolated was not a strong xylose utilizer (only 6.8% of the 14C-xylose dose was recovered).
Gastrointestinal fungi also play an important role degrading plant fiber during digestion in herbivores. However, the two fungal isolates that we cultured from caecal samples are opportunistic pathogens rather than belonging to the known group of beneficial fungi found in the gut. Aspergillus species and Penicillium species are commonly found in the environment worldwide. They grow on plant material and may contaminate cereal grains and animal feeds by secreting mycotoxins (Yiannikouris and Jouany 2002). Aspergillus species in the gastrointestinal tracts of ruminants are associated with hemorrhagic bowel syndrome, in which hemorrhagic lesions develop along the intestinal tract (Jensen and Shoenheyder 1989; Jensen et al. 1989, 1991). Penicillium species also cause infections and intestinal mycoses in humans (Fantry 2001). Although the fungal genera isolated in our study are not known to be beneficial intestinal microbes, they utilize xylose. Both Aspergillus and Penicillium species have xylan-degrading enzyme systems (Filho et al. 1991; Kormelink et al. 1993; McCrae et al. 1994), the control of which has been studied in Aspergillus to improve fermentation yields (Prathumpai et al. 2003). Xylose utilization, as the percentage 14C-labeled xylose recovered during the incubation assay, was 24.0% for the Aspergillus isolate and 34.4 and 60.1% for the two Penicillium isolates.
Short-chain fatty acid production
Mammals do not possess the enzyme xylanase which is required to hydrolyze xylan, but bacteria, yeasts and fungi do. Xylans represent a major energy source for microbial fermentation within ruminants and non-ruminants (Hespell et al. 1987). Xylose released during xylan degradation in the gut lumen is utilized inside the microbial cell and the predominant end-products, SCFA, are absorbed by the host, providing energy for body functions (Stevens and Hume 1995, Johnson et al. 2006). Other carbon sinks resulting from colonic microbial fermentation are the microbes themselves, plus CO2 and CH4. The radio-isotopic assay used here assesses the utilization of a labeled substrate, 14C-xylose, by recovery of the label in CO2 released during fermentation. Label not recovered as CO2 remained in the assay culture as SCFA, other by-products of fermentation, and unutilized 14C-xylose and was recovered and quantified as label in the culture. Of this fraction, it is the SCFA that are the major contributors to the energy requirements of the host animal, analogous to but not as significant as the contribution made by the fermentation of long-chain carbohydrates to the nutrition of herbivorous mammal species consuming cellulose (Bergman 1990). In ruminants, SCFA contribute 70% to the total energy requirements (Stevens and Hume 1998). In non-ruminants, such as the rock hyrax (Procavia habessinica) and naked mole-rat (Hetercephalus glaber), contributions to total energy requirements are 44 and 22%, respectively (Rübsamen et al. 1982; Buffenstein and Yahav 1991).
The main types of SCFA produced by microbial fermentation of carbohydrates are acetic acid, propionic acid and butyric acid, and each is utilized by different tissues in the host’s body (Bergman 1990). Pathways of SCFA metabolism differ slightly between species. The types and proportions of SCFA produced from long-chain carbohydrate (largely cellulose) fermentation are dependent on the site of fermentation (fore-gut or hind-gut), the bacterial species inhabiting the gastrointestinal tract and on the host’s diet, and in mammals vary from 75:15:10 to 40:40:20 when expressed as molar proportions of acetate:propionate:butyrate (Bergman 1990). Our results for SCFA production from xylose by rock mouse gut symbionts fall within this range, with acetic acid dominating as it does across all mammal species studied to date (see Bergman, 1990 for a review of mammals). Acetic acid constitutes as much as 98% of all SCFA in the rock hyrax stomach (Rübsamen et al. 1982), and 70 and 80% respectively of fecal fatty acid concentrations of free-living marine and land iguanas, hind-gut fermenters (Mackie et al. 2004). Tropical marine fish fed on microalgae reflect the same trend (Stevens and Hume 1998). Propionic acid, the next most important SCFA we report, is released particularly during hind-gut fermentation, including that of sugars such as the methylpentose l-rhamnose by humans (Vogt et al. 2004).
We have shown that xylose utilization by the bacterial and fungal species isolated from A. namaquensis caecum results in the production of SCFA, particularly acetic, propionic and butyric acids, but also iso-butyric, valeric and iso-valeric acids. Also, molar concentrations of the first three and the last of these acids together explain more than 95% of all inter-specific (inter-isolate) pH variation under the culture conditions that we used. Bacterial gut symbionts of A. namaquensis appear to be stronger fatty acid producers than are the fungal species. The proportions of fatty acids produced vary between microbial species studied, with acetic acid predominating. The fatty acid concentrations we report are for in vitro cultures of individually grown bacterial species. In the gut lumen, these bacteria would most likely experience interspecific competition that might affect the proportions of SCFA produced by each. Other carbon sources in the rumen fluid medium such as peptones may have contributed to fatty acid production, but our use of a single batch of rumen fluid ensured that differences in fatty acid production between the two media solely reflected different concentrations of xylose in the two media, rather than variation in concentration of other carbon sources. Although medium pH decreased after incubation as a result of acid production, the magnitude of this change in most cases indicates only weak acid production (Holdeman et al. 1977). It is important to note that the culture medium used in this experiment was used to maximize colony growth and detection of xylose use rather than acid production. An alternative medium coupled with manipulation of growth conditions might result in stronger acid production. Nonetheless, we have shown that the caecal microflora of rock mice, and even possibly its pathogenic elements, produce the SCFA typical of gut microbial fermentation when given xylose as a substrate.
Protea nectar may be merely a dietary supplement for rock mice (Wiens et al. 1983; Fleming and Nicolson 2002), but as it is available in winter and early spring when abundance of other resources such as seeds may be low, xylose is likely a “junk food” of some energetic value to these rodents. The breeding season of rock mice coincides with the end of the P. humiflora flowering season and continues for approximately 2 months afterwards. Rock mice may make use of nectar and pollen to improve their body condition in preparation for breeding (Fleming and Nicolson 2002). In this context, the symbiotic fermentation of xylose we describe permits utilization of a carbon source that otherwise would be entirely lost to the mice, given their tissues’ apparently inefficient metabolism of this sugar. Although A. namaquensis makes metabolic use of xylose (Johnson et al. 2006), this remains its least preferred sugar (Johnson et al. 1999). The rodent caecum, with its resident microflora capable of fermenting xylose to SCFA, may be a pre-adaptation that has enabled Namaqua rock mice to benefit from the energy in this intriguing nectar sugar as they pollinate the Proteaceae.
Abbreviations
- CFU:
-
Colony forming units
- SCFA:
-
Short-chain fatty acids
References
Aristidou A, Penttilä M (2000) Metabolic engineering applications to renewable resource utilisation. Curr Opin Biotechnol 11:187–198
Barbosa TM, Serra CR, La Ragione RM, Woodward MJ, Henriques AO (2005) Screening for Bacillus isolates in the broiler gastrointestinal tract. Appl Environ Microbiol 71:968–978
Bergman EN (1990) Energy contributions of volatile fatty acids from the gastrointestinal tract in various species. Physiol Rev 70:567–590
Bowden BN (1970) The sugars in the extrafloral nectar of Adropogan gayanus var. bisquamulatus. Phytochemistry 9:2315–2318
Buffenstein R, Yahav S (1991) The effect of diet on microfaunal population and function in the caecum of a subterranean naked mole-rat, Heterocephalus glaber. Br J Nutr 65:249–258
Campbell JM, Fahey GC Jr, Wolf BW (1997) Selected indigestible oligosaccharides affect large bowel mass, caecal and fecal short-chain fatty acids, pH and microflora in rats. J Nutr 127:130–136
Chandrakant P, Bisaria VS (1998) Simultaneous bioconversion of cellulose and hemicellulose to ethanol. Crit Rev Biotech 18:295–331
Clench MH, Mathias JR (1995) The avian caecum: a review. Wilson Bull 107:93–121
Collins MD, Hoyles L, Foster G, Falsen E, Weiss N (2002) Arthrobacter nasiphocae sp. nov., from the common seal (Phoca vitulina). Int J Syst Evol Microbiol 52:569–571
Demetrakopolous GE, Amos H (1978) Xylose and xylitol: metabolism, physiology and nutritional value. World Rev Nutr Diet 32:96–122
Domsch KH, Gams W, Anderson T-H (1980) Compendium of soil fungi, vol 1. Academic, London
Eschbach M, Mobitz H, Rompf A, Jahn D (2003) Members of the genus Arthrobacter grow anaerobically using nitrate ammonification and fermentative processes: anaerobic adaptation of aerobic bacteria abundant in soil. FEMS Microbiol Lett 223:227–230
Fantry L (2001) Gastrointestinal infections in the immunocompromised host. Curr Opin Gastroenterol 17:40–45
Filho EX, Tuohy MG, Puls J, Coughlan MP (1991) The xylan-degrading enzyme systems of Penicillim capsulatum and Talaromyces emersonii. Biochem Soc Trans 19:25S
Fleming PA, Nicolson SW (2002) How important is the relationship between Protea humiflora (Proteaceae) and its non-flying mammal pollinators? Oecologia 132:361–368
Funke G, Hutson RA, Bernard KA, Pfyffer GE, Wauters G, Collins MD (1996) Isolation of Arthrobacter spp. from clinical specimens and decription of Arthrobacter cumminsii sp. nov. and Arthrobacter woluwensis sp. nov. J Clin Microbiol 34:2356–2363
Hespell RB, Wolf R, Bothast RJ (1987) Fermentation of xylans by Butyrivibrio fibrisolvens and other ruminal bacteria. Appl Environ Microbiol 53:2849–2853
Ho NW, Chen Z, Brainard AP, Sedlak M (1999) Successful design and development of genetically engineered Saccharomyces yeasts for effective cofermentation of glucose and xylose from cellulosic biomass to fuel ethanol. Adv Biochem Eng Biotechnol 65:163–192
Holdeman V, Cato EP, Moore WEC (1977) Anaerobe Laboratory Manual, 4th edn. Anaerobe Laboratory, Virginia Polytechnic Institute and State University, Blacksburg, Virginia 24061
Jackson S, Nicolson SW (2002) Xylose as a nectar sugar: from biochemistry to ecology. Comp Biochem Physiol B 131:613–620
Jeffries TW, Shi NQ (1999) Genetic engineering for improved xylose fermentation by yeasts. Adv Biochem Eng Biotechnol 65:117–161
Jensen H, Shoenheyder H (1989) Immunofluorescence staining of hyphae in the histopathological diagnosis of mycoses in cattle. J Med Vet Mycol 27:33–44
Jensen H, Basse A, Aalbaek B (1989) Mycosis in the stomach compartments of cattle. Acta Vet Scand 30:409–423
Jensen H, Shoenheyder H, Basse A (1991) Acute disseminated aspergillosis in a cow with special reference to penetration and spread. J Comp Pathol 104:411–417
Johnson SA, Nicolson SW (2001) Pollen digestion in flower-feeding Scarabaeidae: protea beetles (Cetoniini) and monkey beetles (Hopliini). J Insect Physiol 47:725–733
Johnson SA, van Tets IG, Nicolson SW (1999) Sugar preferences and xylose metabolism of a mammal pollinator, the Namaqua rock mouse (Aethomys namaquensis). Physiol Biochem Zool 72:438–444
Johnson SA, Nicolson SW, Jackson S (2004) The effect of different oral antibiotics on the gastrointestinal microflora of a wild rodent (Aethomys namaquensis). Comp Biochem Physiol A 138:475–483
Johnson SA, Nicolson SW, Jackson S (2006) Nectar xylose metabolism in a rodent pollinator (Aethomys namaquensis): defining the role of gastrointestinal microflora using 14C-labelled xylose. Physiol Biochem Zool 79:159–168
Kiriyama H, Hariu Y, Sakata T (1992) Comparison of in vitro productivities of short-chain fatty acids and gases from aldoses and the corresponding alcohols by pig caecal bacteria. J Nutr Biochem 3:447–451
Kormelink FJ, Gruppen H, Vietor RJ, Voragen AG (1993) Mode of action of the xylan-degrading enzymes from Aspergillus awamori on alkali-extractable cereal arabinoxylans. Carbohydr Res 249:355–367
Krishnan R, James HM, Bais R, Rofe AM, Edwards JB, Conyers RAJ (1980) Some biochemical studies on the adaptation associated with xylitol ingestion in rats. Aust J Exp Bio Med Sci 58:627–638
Lan Y, Verstegen MWA, Tamminga S, Williams BA (2005) The role of commensal gut microbial community in broiler chickens. World Poult Sci J 61:95–104
Mackie RI, Rycyk M, Ruemmler RL, Aminov RI, Wikelski M (2004) Biochemical and microbiological evidence for fermentative digestion in free-living land iguanas (Conolophus pallidus) and marine iguanas (Amblyrhynchus cristatus) on the Galápagos Archipelago. Physiol Biochem Zool 77:127–138
Marounek M, Kopečný J (1994) Utilisation of glucose and xylose in ruminal strains of Butyrivibrio fibrisolvens. Appl Environ Microbiol 60:738–739
Matte A, Forsberg CW, Gibbons AN (1992) Enzymes associated with metabolism of xylose and other pentoses by Prevotella (Bacteroides) ruminicola strains, Selenomonas ruminantium D and Fibrobacter succinogenes S85. Can J Microbiol 38:370–376
McCrae SI, Leith KM, Gordon AH, Wood TM (1994) Xylan-degrading enzyme system produced by the fungus Aspergillus awamori. Enzyme Microbial Technol 16:826–834
Mead GC (1989) Microbes of the avian caecum: types present and substrates utilised. J Exp Zool 3 Suppl:48–54
Nardon P, Grenier AM (1989) Endosymbiosis in Coleoptera: biological, biochemical, and genetic aspects. In: Schwemmler W, Gassner G (eds) Insect endocytobiosis: morphology, physiology, genetics and evolution. CRC Press, Boca Raton, pp 175–216
Nicolson SW, van Wyk B-E (1998) Nectar sugars in Proteaceae: patterns and processes. Aust J Bot 46:489–504
Osorio CR, Barja JL, Hutson RA, Collins MD (1999) Arthrobacter rhombi sp. nov., isolated from Greenland halibut (Reinhardtius hippoglossoides). Int J Syst Bacteriol 49:1217–1220
Prathumpai W, Gabelgaard JB, Wanchanthuek P, van de Vondervoort PJI, de Groot MJL, McIntyre M, Nielsen J (2003) Metabolic control analysis of xylose catabolism in Aspergillus. Biotechnol Prog 19:1136–1141
Preest MR, Folk DG, Beuchat CA (2003) Decomposition of nitrogenous compounds by intestinal bacteria in hummingbirds. Auk 120:1091–1101
Raper KB, Fennel DI (1965) The genus Aspergillus. Williams & Wilkins, Baltimore
Rübsamen K, Hume ID, Engelhardt W (1982) Physiology of the rock hyrax. Comp Biochem Physiol A 72:271–277
Shelley C (1999) Preadaptation and the explanation of human evolution. Biol Philos 14:65–82
Sneath PHA, Mair NS, Sharpe ME, Holt JG (1986) Bergey’s Manual of Systematic Bacteriology, vol 2. Williams and Wilkins, Baltimore
Stevens CE, Hume ID (1995) Microbial fermentation and synthesis of nutrients and the absorption of end products. In: Comparative physiology of the vertebrate digestive system. Press Syndicate, New York, pp 188–228
Stevens CE, Hume ID (1998) Contributions of microbes in vertebrate gastrointestinal tract to production and conservation of nutrients. Physiol Rev 78:393–427
Storms V, Devriese LA, Coopman R, Schumann P, Vyncke F, Gillis M (2003) Arthrobacter gandavensis sp. nov., for strains of veterinary origin. Int J Syst Evol Microbiol 53:1881–1884
Suh S, Marshall CJ, McHugh JV, Blackwell M (2003) Wood ingestion by passalid beetles in the presence of xylose-fermenting gut yeasts. Mol Ecol 12:3137–3145
Turner KW, Roberton AM (1979) Xylose, arabinose and rhamnose fermentation by Bacteroides ruminicola. Appl Environ Microbiol 38:7–12
Van Wyk B-E, Nicolson SW (1995) Xylose as a major sugar in Protea and Faurea. S Afr J Sci 91:151–153
Vogt JA, Pencharz PB, Wolever TMS (2004) l-Rhamnose increases serum propionate in humans. Am J Clin Nutr 80:89–94
Wauters G, Charlier J, Janssens M, Delmée M (2000) Identification of Arthrobacter oxydans, Arthrobacter, luteolus sp. nov., and Arthrbacter albus sp. nov., isolated from human clinical specimens. J Clin Microbiol 38:2412–2415
Wiens D, Rourke JP, Casper BB, Rickart EA, LaPine TR, Peterson CJ, Channing A (1983) Nonflying mammal pollination of southern African Proteas: a non-coevolved system. Ann Mo Bot Gard 70:1–31
Yiannikouris A, Jouany J (2002) Mycotoxins in feeds and their fate in animals: a review. Anim Res 51:81–99
Acknowledgements
Elanna Bester and Jeanette Cilliers (Microbiology Department), Bettine Jansen van Vuuren (Molecular Genomics Laboratory, Botany and Zoology Department), Ulrike Damm (Plant Pathology Department), Candice Ockert (Institute for Wine Biotechnology) and Gunnar Sigge and Armelle Ntsame-Affane (Food Science Department), all at the University of Stellenbosch, helped generously with laboratory work. HPLC analyses for the faecal microflora study were carried out at the Institute for Plant Biotechnology, University of Stellenbosch. This work was supported by NRF Grant GUN 2053621 to S.W. Nicolson, NRF GUN 2039528 to S. Jackson and by a grant from SubCommittee B of the Research Committee of the University of Stellenbosch to S. Jackson. The experiments described herein comply with the current laws of South Africa.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by I.D. Hume
Rights and permissions
About this article
Cite this article
Johnson, S.A., Jackson, S., Abratt, V.R. et al. Xylose utilization and short-chain fatty acid production by selected components of the intestinal microflora of a rodent pollinator (Aethomys namaquensis). J Comp Physiol B 176, 631–641 (2006). https://doi.org/10.1007/s00360-006-0086-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00360-006-0086-7