Abstract
The environmental physiology of terrestrial Antarctic nematodes is reviewed with an emphasis on their cold-tolerance strategies. These nematodes are living in one of the most extreme environments on Earth and face a variety of stresses, including low temperatures and desiccation. Their diversity is low and declines with latitude. They show resistance adaptation, surviving freezing and desiccation in a dormant state but reproducing when conditions are favourable. At high freezing rates in the surrounding medium the Antarctic nematode Panagrolaimus davidi freezes by inoculative freezing but can survive intracellular freezing. At slow freezing rates this nematode does not freeze but undergoes cryoprotective dehydration. Cold tolerance may be aided by rapid freezing, the production of trehalose and by an ice-active protein that inhibits recrystallisation. P. davidi relies on slow rates of water loss from its habitat, and can survive in a state of anhydrobiosis, perhaps aided by the ability to synthesise trehalose. Teratocephalus tilbrooki and Ditylenchus parcevivens are fast-dehydration strategists. Little is known of the osmoregulatory mechanisms of Antarctic nematodes. Freezing rates are likely to vary with water content in Antarctic soils. Saturated soils may produce slow freezing rates and favour cryoprotective dehydration. As the soil dries freezing rates may become faster, favouring freezing tolerance. When the soil dries completely the nematodes survive anhydrobiotically. Terrestrial Antarctic nematodes thus have a variety of strategies that ensure their survival in a harsh and variable environment. We need to more fully understand the conditions to which they are exposed in Antarctic soils and to apply more natural rates of freezing and desiccation to our studies.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Terrestrial Antarctic nematodes are part of a community of organisms that are living in one of the harshest environments on Earth. Less than 0.5% of continental Antarctica is ever free of snow and ice (Fox and Cooper 1994). Even rarer are the ice-free areas that receive sufficient meltwater in summer to support the growth of visible vegetation (moss, algae and lichens). Antarctic terrestrial communities consist of only a few species, making it feasible to attempt an overall understanding of their ecology (Block 1994). Since they are living at the limits of life on Earth, terrestrial Antarctic organisms may be particularly sensitive indicators of climate change. They are also good models for studying how life survives in extreme environments and they may allow us to discover improved methods of storing biological materials (Wharton 2002a).
There are a number of factors in terrestrial Antarctic environments that may be considered stressful (Convey 1996). Mean and extreme temperatures decrease with increasing latitude. This brings lower temperatures for growth and increased exposure to freezing. Daily temperature fluctuations in the microenvironments inhabited by terrestrial Antarctic organisms can be considerable (25–50°C), depending on snow cover and the absorption of solar radiation, resulting in exposure to freeze/thaw cycles (Convey 1996). Since most water is locked up as snow and ice, the environment is generally extremely dry although water may be locally abundant, with the availability of water varying on a temporal and geographical scale. Light is limited on a seasonal, as well as a daily, basis from 24 h daylight in midsummer to 24 h darkness in midwinter. These factors combine to produce short growing seasons. Other stresses may include low nutrient availability, unstable substrates, ultraviolet radiation, osmotic stress and unpredictable environmental conditions (Convey 1996; Wharton 2002a).
Although conditions in terrestrial Antarctic habitats can generally be considered stressful the degree of stress will vary on both a continental and local scale. Two climatic regions are recognised. The maritime Antarctic comprises parts of the Antarctic Peninsula and nearby islands. This has a cold maritime climate with mean monthly air temperatures above 0°C for several months during summer and rarely falling below –15°C in winter (Block 1994). This encompasses a small proportion of the continent. The remaining areas are part of the continental Antarctic with mean monthly air temperatures rarely above 0°C in summer and winter means as low as –25°C even in coastal areas (Block 1994).
There are three main types of ice-free areas in the continental Antarctic (Walton 1984). There are nunataks, which are isolated mountain tops that project above the ice sheet, there are some large areas permanently free of snow and ice due to local climatic conditions (e.g. the Dry Valleys of Victoria Land), and there are coastal sites where the winter snow cover melts during summer to provide liquid water and ice-free ground. This review summarises our understanding of the environmental physiology of terrestrial Antarctic nematodes found in these areas.
The distribution and ecology of nematodes in terrestrial Antarctic ecosystems
The diversity of the terrestrial Antarctic nematode fauna is low. The most recent survey lists 29 species from the maritime Antarctic and 14 from the continental Antarctic (Andrássy 1998). Although this might seem to be a rather sparse fauna, nematodes are the most diverse and abundant invertebrates in these habitats. Nematode diversity declines with increasing latitude (Fig. 1), a pattern also found over a narrow range of latitudes within the maritime Antarctic (Maslen 1979). This may be related to increased abiotic stress with increasing latitude and a corresponding decrease in the biota.
The diversity of terrestrial Antarctic nematodes decreases with increasing latitude. Data from: 1 Signy Island (Maslen 1981), 2 Adelaide Island (Convey and Worland 2000; N.R. Maslen, personal communication), 3 Alexander Island (Convey and Wynn-Williams 2002), 4 Nunataks Dronning Maud Land (Sohlenius et al. 1996), 5 Ross Island (Sinclair 2001), 6 Dry Valleys (Wharton and Brown 1989)
Terrestrial Antarctic nematodes feed predominantly on bacteria, cyanobacteria and algae. Their habitats, however, often have very low microbial contents. Rates of decomposition are slow, due to low numbers of microbes, low temperatures and restricted water availability. There are a few fungivorous, plant-parasitic and predatory species (Andrássy 1998).
Some species of nematodes are associated with certain habitats. For example, Panagrolaimus davidi is associated with coastal sites (Wharton and Brown 1989) and in particular penguin colonies and ornithogenic soils (Porazinska et al. 2002; Sinclair 2001). Presumably debris from the penguin colonies boosts nutrient availability in these soils. Scottnema lindsayae is associated with dry and saline soils (Treonis et al. 1999) and P. magnivulvatus has been recorded only from nunataks (Sohlenius et al. 1996). Given the different nature of these habitats, we might expect these species to vary in their responses to environmental conditions.
Conditions in the maritime Antarctic support, in places, the growth of extensive moss turfs and carpets and even two species of higher plants (Block 1994). In contrast, visible vegetation in the continental Antarctic is extremely sparse. Nematodes inhabiting the latter are thus likely to be exposed to much harsher abiotic conditions.
Resistance and capacity adaptation in Antarctic terrestrial nematodes
Organisms exposed to extreme environmental conditions show two broad responses (Wharton 2002a, 2002b). Some are adapted to grow and reproduce under conditions that are constantly extreme (extremophiles, displaying capacity adaptation), whilst others survive extreme conditions in a dormant state, only growing and reproducing when conditions are favourable (cryptobiotes, displaying resistance adaptation).
There is evidence for capacity adaptation in some Antarctic nematodes. Plectus antarcticus lays eggs and completes its life cycle at lower temperatures than nematodes from temperate regions (Caldwell 1981). Scottnema lindsayae has a higher fecundity, faster rates of juvenile development and better survival at 10°C than at 15°C (Overhoff et al. 1993). In contrast, P. davidi grows best at 25°C (Brown 1993; Wharton 1997) and thus does not show capacity adaptation to cold. It is, however, resistant to low temperatures and desiccation. The survival strategies of this and probably many other Antarctic nematodes are dominated by resistance adaptation.
Cold tolerance strategies of Antarctic terrestrial nematodes
Antarctic nematodes have to face the problems of exposure to very low temperatures and to freezing events. In the maritime Antarctic (Signy Island) the minimum soil surface temperature was –17.1°C and there were few freeze/thaw events (Davey et al. 1992). In the continental Antarctic (Cape Bird, Ross Island) the minimum soil surface temperature was –31.7°C and there were frequent freeze/thaw events during late spring and summer (Sjursen and Sinclair 2002; Wharton 1997). The moisture status of the soil varies on both a small geographical scale and with time (Campbell et al. 1997; Sinclair and Sjursen 2001a; Wharton 1997). This suggests that Antarctic nematodes face exposure to low temperatures under highly variable conditions.
Cold tolerance strategies have traditionally been divided into freezing tolerance and freeze avoidance (Lee 1991). Freeze avoiding animals maintain their body fluids as a liquid at temperatures below their melting point (they supercool) but die once freezing occurs (at the supercooling point). Freezing-tolerant animals can survive ice forming in their bodies and tend to show little supercooling ability. More recently a third cold tolerance mechanism (cryoprotective dehydration) has been described, where a soil organism surrounded by ice dehydrates rather than freezes (Holmstrup et al. 2002; Holmstrup and Westh 1994).
Our understanding of the cold tolerance strategies of Antarctic nematodes have undergone a number of significant shifts. At first the techniques used for studying the cold-tolerance mechanisms of arthropods were applied to nematodes (Pickup 1990a, 1990b, 1990c). This involved attaching nematodes to thermocouples to record freezing exotherms, after removing surface water and coating with liquid paraffin to prevent desiccation. Under these conditions nematodes supercool and show mainly a freeze-avoiding strategy. Supercooling points vary seasonally and are also related to feeding activity. If the supercooling point was relatively high some nematodes survived, suggesting some freezing tolerance ability (Pickup 1990a, 1990b, 1990c).
Nematodes are essentially aquatic animals, requiring at least a film of water around soil particles for movement, growth and reproduction (Wharton 1986). They are thus much more likely to be exposed to inoculative freezing (ice from the surrounding water travelling through a body opening, or across the cuticle, to cause the freezing of the animal) than are the fully-terrestrial arthropods (Wharton 1995). Since nematodes are transparent, the formation of ice in their bodies can be observed on a cold microscope stage. P. davidi, isolated from Cape Bird Ross Island, has been the main model for these studies. When frozen in water, under conditions where there is a relatively rapid spread of ice through the sample, the ice enters the nematode's body via body openings—in particular the excretory pore (Wharton and Ferns 1995). The ice then spreads rapidly throughout the body and eventually all compartments freeze (Fig. 2). Freezing is not confined to the extracellular spaces and the nematode survives intracellular freezing (Wharton and Ferns 1995; Wharton et al. 2003). P. davidi is the only animal known to survive extensive intracellular freezing. It has survived exposure to –40°C (Wharton and Block 1997) and to –80°C (Wharton and Brown 1991) with high levels of survival. Freezing tolerance appears to be widespread amongst Antarctic nematodes (Convey and Worland 2000; Wharton and Block 1993). Freezing tolerance occurs in larval and adult nematodes but the eggs of P. davidi can survive by freeze avoidance, since the eggshell prevents inoculative freezing (Wharton 1994).
Freezing of P. davidi starts near the posterior bulb of the oesophagus (arrow) and spreads throughout the body until all compartments, including intracellular compartments, freeze. From Wharton and Ferns (1995), reproduced with the permission of the Company of Biologists (scale bar=100 μm)
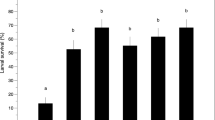
There is a clear relationship between the survival of P. davidi and the freezing rate of the sample, which in turn is dependant upon the sample volume and temperature (Wharton et al. 2002). Survival is favoured by slow freezing rates. At fast freezing rates a proportion of nematodes appear to be physically damaged by the growth of ice crystals in the surrounding medium, with body contents protruding through breaks in the cuticle. Similar damage after freezing has been observed in other Antarctic nematodes (Convey and Worland 2000). Slow freezing rates (in samples nucleated at high subzero temperatures) tend to inhibit inoculative freezing and enhance survival (Fig. 3). In samples frozen at –1°C no inoculative freezing occurred; instead the nematodes became visibly dehydrated (Wharton et al. 2003) and survived by cryoprotective dehydration (Holmstrup and Westh 1994). The dehydration is driven by the difference in vapour pressure between supercooled water and ice at the same temperature (Fig. 4). In samples frozen at lower temperatures, the nematodes freeze by inoculative freezing but a proportion survive (Fig. 3), confirming that they are freezing tolerant (Wharton et al. 2003). P. davidi thus appears to have a variety of strategies for surviving low temperatures. Using a freeze-substitution technique and transmission electron microscopy to visualise ice at an ultrastructural level it can be seen that increasing freezing rate produces a shift from cryoprotective dehydration to extracellular freezing to intracellular freezing (D.A. Wharton and Downes, unpublished results).
The effect of temperature on the freezing (filled circles) and survival (open circles) of P. davidi. Samples were nucleated and held at the test temperature for 30 min. Samples at 0°C were unfrozen controls. From Wharton et al. (2003), reproduced with the permission of the Company of Biologists
The difference in the vapour pressure of supercooled water and ice at various temperatures (solid line) and the relative humidities generated (dotted line). Data from Weast (1989)
Cold tolerance mechanisms of Antarctic terrestrial nematodes
Studies on cold tolerance mechanisms have focused on P. davidi, given its ease of culture. After inoculative freezing of this nematode the freezing process can be completed extremely rapidly (0.2 s), facilitated by the small size of the animal (Wharton and Ferns 1995). This is much faster than in other freezing-tolerant animals and may aid survival since it would prevent the osmotic stress that would result if different body compartments froze at different times. During freezing 82% of body water is converted into ice, about the limit of freezable water in biological systems (Wharton and Block 1997). Nematodes grown at 20°C and then acclimated to lower temperatures show an increase in the production of the disaccharide trehalose and increased survival (Wharton et al. 2000). Trehalose is known to act as a cryoprotectant in some animals and may also provide protection against the effects of dehydration. A number of nematode species have been shown to synthesise trehalose in response to desiccation and/or low temperatures (Wharton 2002b). In some annelids and frogs cryoprotectants are synthesised during the freezing process itself, rather than as a response to low-temperature acclimation (Storey and Storey 1992; Holmstrup and Sjursen 2001). In nematodes the freezing process is probably too rapid to allow this to occur.
There also appears to be a protein component to the cold tolerance mechanisms of this nematode. Proteins that interact with ice (ice-active proteins, IAPs) can be divided into three classes. Ice-nucleating proteins (INPs) trigger ice formation, antifreeze proteins (AFPs) inhibit ice nucleation, whilst recrystallisation-inhibiting proteins (RIPs) have neither of these properties (or have them only weakly) but affect the stability of ice crystals after they have formed. INPs appear to be absent in P. davidi and this is to be expected in a freezing-tolerant animal that can rely on inoculative freezing (Wharton and Worland 1998). There is little or no thermal hysteresis activity (a difference between the melting and freezing point in the presence of an ice crystal), suggesting the absence of AFPs (D.A. Wharton and H. Ramløv, unpublished observations). In contrast, there is evidence that P. davidi produces a protein that inhibits the activity of organic ice nucleators, a property of AFPs (Olsen and Duman 1997a, 1997b; Wharton and Worland 1998). Antifreeze activity is not likely to be of advantage to a freezing-tolerant organism but ice nucleation inhibition could assist cryoprotective dehydration by hindering inoculative freezing.
Recrystallisation occurs in ice held at a constant (or varying) subzero temperature. This involves a change in the size distribution of ice crystals as larger crystals grow at the expense of small crystals. This could be damaging to a frozen organism (Knight and Duman 1986; Knight et al. 1988, 1995). Recrystallisation inhibition may be the major function of antifreeze proteins in freezing-tolerant organisms, where antifreeze activity would not assist their survival (Duman 2001). P. davidi produces a protein (a RIP) that inhibits recrystallisation (Ramløv et al. 1996). The P. davidi RIP has now been isolated, purified and sequenced (G. Goodall, D.A. Wharton and C.J. Marshall, unpublished results). It has no sequence homology to any other IAP. It does, however, belong to a family of nematode-specific proteins of widespread distribution but with no assigned function. The role that this protein plays in freezing tolerance and/or cryoprotective dehydration has yet to be established.
Anhydrobiosis in Antarctic terrestrial nematodes
Although the absolute amount of freshwater in Antarctica is huge, most of it is unavailable to organisms—locked up as ice or attached to soil components (e.g. clay, organic matter). The availability of liquid water is thus a major determinant of the distribution of terrestrial Antarctic organisms and desiccation is a significant challenge to them (Kennedy 1993). The degree of desiccation stress varies in different sites. Some wet moss habitats in the maritime Antarctic are permanently saturated with water, whilst lichen-encrusted bryophyte communities are drier (Kennedy 1993). Communities supported by meltwater from snowbanks may become desiccated when the source of water becomes exhausted (Sinclair and Sjursen 2001a). The dry soil habitats of the Dry Valleys region support populations of nematodes, which must be able to survive desiccation (Treonis et al. 2000; Virginia and Wall 1999).
Some species of nematodes are capable of anhydrobiosis, losing all their body water and surviving in an ametabolic, dormant state (Wharton 2002b). Two broad groups of anhydrobiotic nematodes are recognised (Womersley 1987). Slow-dehydration strategists need to be dried slowly before they can survive exposure to extreme desiccation. They rely on their moss or soil habitat drying slowly to provide the slow rate of water loss necessary for anhydrobiotic survival. Fast-dehydration strategists themselves have the adaptations necessary to slow the rate of water loss and can survive immediate exposure to low relative humidity (Wharton 2002b).
P. davidi is a slow-dehydration strategist and only survives exposure to 0% relative humidity (RH) if it is first dried at 99% or 76% RH (Wharton and Barclay 1993). Drying on a model substrate (agar), that might more closely mimic the drying characteristics of its environment, did not enhance its survival. Such a treatment is essential for some slow-dehydration strategists to survive desiccation even at 97% RH (Womersley and Ching 1989), suggesting that P. davidi can survive faster rates of water loss than these. Slow drying produces coiling, which may reduce the rate of water loss by reducing the surface area of cuticle directly exposed to air. The time taken for nematodes to recover upon rehydration is related to the severity of the stress experienced during desiccation (Wharton 2002b; Wharton and Barclay 1993). Trehalose is produced by P. davidi (Wharton et al. 2000) and is important for anhydrobiotic survival (Crowe et al. 1992). Antarctic nematodes are often found in a coiled state and this may indicate that they are in a state of anhydrobiosis (Treonis et al. 2000).
Pickup and Rothery (1991) compared the rates of water loss and anhydrobiotic survival of Ditylenchus sp. B (=Ditylenchus parcevivens, Andrássy 1998) and Teratocephalus tilbrooki at Signy Island. D. parcevivens inhabits erect branching thalli of a fructiose lichen (Usnea spp.) and hence might be expected to be exposed to high rates of water loss, whilst T. tilbrooki inhabits moss carpets (Andreaea spp.) that would produce slow rates of water loss when exposed to desiccation. Rates of water loss of T. tilbrooki at 60% RH/5°C were high but nevertheless the nematodes could survive desiccation for many days. Survival of 0% RH was not reported for this nematode. D. parcevivens has much slower rates of water loss and could survive direct exposure to 0% RH (Pickup and Rothery 1991). It thus has the characteristics of a fast-dehydration strategist. The desiccation responses of T. tilbrooki are unusual. It does not have the characteristics of a slow-dehydration strategist, despite its habitat, since it will survive high rates of water loss. The only other nematode reported with these characteristics is a Plectus sp. isolated from moss growing on a flat roof (Hendriksen 1982).
Osmotic stress and Antarctic terrestrial nematodes
Some Antarctic soils are highly saline (Virginia and Wall 1999) and inputs of water, from precipitation or melting snow and ice, may subject nematodes to sudden variations in osmotic stress. Soil nematodes in general may have to tolerate three- to fivefold variations in external osmotic pressure as a result of rainy or drought conditions (Thompson and Geary 2002). Since nematodes are reliant upon a high internal turgor pressure within the pseudocoel for coordinated movement to occur (Wharton 1986), the ability to osmoregulate in the highly variable osmotic environment provided by Antarctic soils is likely to be of great importance. Osmoregulation in nematodes may involve the permeability of the cuticle and epidermis, the production of organic osmolytes (such as trehalose, glycerol, amino acids and urea) to balance hyperosmotic stress and the removal of excess water (via the excretory system or the intestine) during hypoosmotic stress (Thompson and Geary 2002). None of these mechanisms have been investigated in Antarctic nematodes, although P. davidi is known to synthesise trehalose and glycerol (Wharton et al. 2000) and these could be acting as osmolytes.
P. davidi shrinks during exposure to single salt solutions (0.1–0.4 mol l−1 NaCl). There is no recovery in length in hyperosmotic solutions, suggesting an inability to osmoregulate (Viglierchio 1974). Single salt solutions may not, however, give an accurate picture since other ions may be required for osmoregulation to occur (Wright and Newall 1976, 1980). P. davidi appears to be isotonic to <0.05 mol l−1 NaCl (Viglierchio 1974). The survival of P. davidi declines with increasing osmolality. It can survive exposure to distilled water for at least 24 h (Wharton and To 1996), which may indicate an ability to osmoregulate in hypoosmotic solutions.
Interacting/changing stresses and Antarctic terrestrial nematodes
Although temperature, water and osmotic stress have been considered separately so far in this review they clearly have important interactions. When a solution starts to freeze the osmotic concentration of the unfrozen portion is raised as salts are excluded from the growing ice crystals (Shepard et al. 1976). This freeze concentration effect could dehydrate nematodes as a result of hyperosmotic stress. This may be sufficient to prevent the freezing of nematodes (Forge and MacGuidwin 1992). Inoculative freezing of P. davidi can still occur, however, in solutions of osmolalities up to 1130 mosmol l−1 and if salt concentrations are raised by a factor of 120 (Wharton and To 1996). Cryoprotective dehydration occurs in distilled water, in which a freeze concentration effect is not possible (Wharton et al. 2003) and may be the dominant effect during the freezing of soil water. There may, however, be an interaction between osmotic stress and cold tolerance. P. davidi survives a freezing stress better at intermediate, rather than high or low osmolalities (Wharton and To 1996). Water loss from soil through dehydration will also raise the osmotic concentration of soil water.
In P. davidi cryoprotective dehydration is favoured by high nucleation temperatures and slow cooling rates producing slow rates of freezing (Wharton et al. 2003). Cooling rates are likely to be slow in Antarctic soils. Sinclair and Sjursen (2001b) recorded maximum cooling rates of 0.021°C min−1 at Keble Valley, Cape Bird. In soil saturated with water the large number of nucleators present and slow cooling rates are likely to produce high nucleation temperatures and slow rates of freezing. This will favour cryoprotective dehydration. As the soil dries out, however, the nematodes will be in decreasing volumes of water and this will produce lower nucleation temperatures and faster freezing rates. Since the nematodes have only a restricted ability to resist inoculative freezing (Wharton et al. 2003) this will favour freezing tolerance. Eventually the soil will dry out completely and the nematodes survive anhydrobiotically (Wharton and Barclay 1993). There may thus be a shift from cryoprotective dehydration to freezing tolerance and then to anhydrobiosis as the soil loses water (Fig. 5).
The response of a soil nematode to freezing temperatures will depend upon the temperature of ice nucleation and the volume of soil water. If the water content is high the nucleation temperature that results in soil freezing will be high (given the large number of soil ice nucleators and the large volume of solution) and the freezing rate will be slow, favouring a cryoprotective dehydration mechanism. As the soil dries out the nematode will be in smaller volumes of fluid with lower nucleation temperatures. Freezing rates will thus be high, favouring inoculative freezing and freezing tolerance. As the soil continues to dry the nematode will become free of surface water, desiccate and survive in a state of anhydrobiosis. The form of the relationship between freezing rate and soil water content is hypothetical
Conclusions
Terrestrial Antarctic nematodes live in an extremely harsh and variable environment. Their repertoire of survival mechanisms allows them to persist in the face of extreme physical challenges but the relative importance of these (including freezing tolerance, cryoprotective dehydration, anhydrobiosis) is unclear. We need to apply techniques that can more directly assess the conditions that nematodes are exposed to in Antarctic soils. This may be difficult since nematodes have to be removed from their environment in order to study them, a process that changes their physiological state (Wharton 1995). More natural rates of change of temperature and water content need to be used in our experiments.
We have much to learn of the mechanisms underlying the survival mechanisms of nematodes. Such an understanding may have important implications for the storage of biological materials and our interpretation of the ecology of terrestrial Antarctic organisms.
References
Andrássy I (1998) Nematodes in the sixth continent. J Nem Morphol Syst 1:107–186
Block W (1994) Terrestrial ecosystems: Antarctica. Polar Biol 14:293–300
Brown IM (1993) The influence of low temperature on the Antarctic nematode Panagrolaimus davidi. PhD thesis, University of Otago, New Zealand
Caldwell JR (1981) The Signy Island terrestrial reference sites: XIII. Population dynamics of the nematode fauna. Br Antarct Surv Bull 54:33–46
Campbell IB, Claridge GGC, Balks MR, Campbell DI (1997) Moisture content in soils of the McMurdo Sound and Dry Valleys region of Antarctica. In: Lyons WB, Howard-Williams C, Hawes I (eds) Ecosystem processes in Antarctic ice-free landscapes. Balkema, Rotterdam, pp 61–76
Convey P (1996) The influence of environmental characteristics on life history attributes of Antarctic terrestrial biota. Biol Rev 71:191–225
Convey P, Worland MR (2000) Survival of freezing by free-living Antarctic soil nematodes. CryoLetters 21:327–332
Convey P, Wynn-Williams DD (2002) Antarctic soil nematode response to artificial climate amelioration. Eur J Soil Biol 38:255–259
Crowe JH, Hoekstra F, Crowe LM (1992) Anhydrobiosis. Annu Rev Physiol 54:579–599
Davey MC, Pickup J, Block W (1992) Temperature variation and its biological significance in fellfield habitats on a maritime Antarctic island. Antarct Sci 4:383–388
Duman JG (2001) Antifreeze and ice nucleator proteins in terrestrial arthropods. Annu Rev Physiol 63:327–357
Forge TA, MacGuidwin AE (1992) Effects of water potential and temperature on survival of the nematode Meloidogyne hapla in frozen soil. Can J Zool 70:1553–1560
Fox AJ, Cooper PR (1994) Measured properties of the Antarctic ice sheet derived from the SCAR Antarctic digital database. Polar Rec 30:201–206
Hendriksen NB (1982) Anhydrobiosis in nematodes: studies on Plectus sp. In: Lebrun P, André HM, De Medts A, Grégoire-Wibo C, Wauthy G (eds) New trends in soil biology. Dieu-Brichart, Louvain-la-Neurve, pp 387–394
Holmstrup M, Sjursen H (2001) Freeze induced glucose accumulation in the enchytraeid, Fredericia ratzeli, from Greenland. CryoLetters 22:273–276
Holmstrup M, Westh P (1994) Dehydration of earthworm cocoons exposed to cold: a novel cold hardiness mechanism. J Comp Physiol B 164:312–315
Holmstrup M, Bayley M, Ramløv H (2002) Supercool or dehydrate? An experimental analysis of overwintering strategies in small permeable arctic invertebrates. Proc Natl Acad Sci USA 99:5716–5720
Kennedy AD (1993) Water as a limiting factor in the Antarctic terrestrial environment: a biogeographical synthesis. Arctic Alpine Res 25:308–315
Knight CA, Duman JG (1986) Inhibition of recrystallization of ice by insect thermal hysteresis proteins: a possible cryoprotective role. Cryobiology 23:256–262
Knight CA, Hallett J, DeVries AL (1988) Solute effects on ice recrystallisation: an assessment technique. Cryobiology 25:55–60
Knight CA, Wen D, Laursen RA (1995) Nonequilibrium antifreeze peptides and the recrystallization of ice. Cryobiology 32:23–34
Lee RE (1991) Principles of insect low temperature tolerance. In: Lee RE, Denlinger DL (eds) Insects at low temperatures. Chapman and Hall, New York, pp 17–46
Maslen NR (1979) Additions to the nematode fauna of the Antarctic region with keys to taxa. Br Antarct Surv Bull 49:207–229
Maslen NR (1981) The Signy Island terrestrial reference sites. XII. Population ecology of nematodes with additions to the fauna. Br Antarct Surv Bull 53:57–75
Olsen TM, Duman JG (1997a) Maintenance of the supercooled state in overwintering pyrochroid beetle larvae, Dendroides canadensis: role of hemolymph ice nucleators and antifreeze proteins. J Comp Physiol B 167:105–113
Olsen TM, Duman JG (1997b) Maintenance of the supercooled state in the gut of overwintering pyrochroid beetle larvae, Dendroides canadensis: role of gut ice nucleators and antifreeze proteins. J Comp Physiol B 167:114–122
Overhoff A, Freckman DW, Virginia RA (1993) Life cycle of the microbivorous Antarctic Dry Valley nematode Scottnema lindsayae (Timm 1971). Polar Biol 13:151–156
Pickup J (1990a) Seasonal variation in the cold hardiness of three species of free-living Antarctic nematodes. Funct Ecol 4:257–264
Pickup J (1990b) Seasonal variation in the cold-hardiness of a free-living predatory nematode, Coomansus gerlachei (Mononchidae). Polar Biol 10:307–315
Pickup J (1990c) Strategies of cold-hardiness in three species of Antarctic dorylaimid nematodes. J Comp Physiol B 160:167–173
Pickup J, Rothery P (1991) Water-loss and anhydrobiotic survival in nematodes of Antarctic fellfields. Oikos 61:379–388
Porazinska DL, Wall DH, Virginia RA (2002) Invertebrates in ornithogenic soils on Ross Island, Antarctica. Polar Biol 25:569–574
Ramløv H, Wharton DA, Wilson PW (1996) Recrystallization in a freezing-tolerant Antarctic nematode, Panagrolaimus davidi, and an alpine weta, Hemideina maori (Orthoptera, Stenopelmatidae). Cryobiology 33:607–613
Shepard ML, Goldston CS, Cocks FH (1976) The H2O-NaCl-glycerol phase diagram and its application in cryobiology. Cryobiology 13:9–23
Sinclair BJ (2001) On the distribution of terrestrial invertebrates at Cape Bird, Ross Island, Antarctica. Polar Biol 24:394–400
Sinclair BJ, Sjursen H (2001a) Terrestrial invertebrate abundance across a habitat transect in Keble Valley, Ross Island, Antarctica. Pedobiologia 45:134–145
Sinclair BJ, Sjursen H (2001b) Cold tolerance of the Antarctic springtail Gomphiocephalus hodgsoni (Collembola, Hypogastruridae). Antarct Sci 13:271–279
Sjursen H, Sinclair BJ (2002) On the cold hardiness of Stereotydeus mollis (Acari: Prostigmata) from Ross Island, Antarctica. Pedobiologia 46:188–195
Sohlenius B, Boström S, Hisrchfelder A (1996) Distribution patterns of microfauna (nematodes, rotifers and tardigrades) on nunataks in Dronning Maud Land, East Antarctica. Polar Biol 16:191–200
Storey KB, Storey JM (1992) Natural freeze tolerance in ectothermic vertebrates. Annu Rev Physiol 54:619–637
Thompson DP, Geary TG (2002) Excretion/secretion, ionic and osmotic regulation. In: Lee DL (ed) The biology of nematodes. Taylor & Francis, London, pp 291–320
Treonis AM, Wall DH, Virginia RA (1999) Invertebrate biodiversity in Antarctic Dry Valley soils and sediments. Ecosystems 2:482–492
Treonis AM, Wall DH, Virginia RA (2000) The use of anhydrobiosis by soil nematodes in the Antarctic Dry Valleys. Funct Ecol 14:460–467
Viglierchio DR (1974) Osmoregulation and electrolyte uptake in Antarctic nematodes. Trans Am Microsc Soc 93:325–338
Virginia RA, Wall DH (1999) How soils structure communities in the Antarctic dry valleys. BioScience 49:973–983
Walton DWH (1984) The terrestrial environment. In: Laws RM (ed) Antarctic ecology. Academic Press, London, pp 1–60
Weast RC (1989) Handbook of Chemistry and Physics. CRC Press, Cleveland
Wharton DA (1986) A functional biology of nematodes. Croom Helm, London
Wharton DA (1994) Freezing avoidance in the eggs of the Antarctic nematode Panagrolaimus davidi. Fundam Appl Nematol 17:239–243
Wharton DA (1995) Cold tolerance strategies in nematodes. Biol Rev 70:161–185
Wharton DA (1997) Survival of low temperatures by the Antarctic nematode Panagrolaimus davidi. In: Lyons WB, Howard-Williams C, Hawes I (eds) Ecosystem processes in Antarctic ice-free landscapes. Balkema, Rotterdam, pp 57–60
Wharton DA (2002a) Life at the limits: organisms in extreme environments. Cambridge University Press, Cambridge
Wharton DA (2002b) Survival strategies. In: Lee DL (ed) The biology of nematodes. Taylor & Francis, London, pp 389–411
Wharton DA, Barclay S (1993) Anhydrobiosis in the free-living Antarctic nematode Panagrolaimus davidi. Fundam Appl Nematol 16:17–22
Wharton DA, Block W (1993) Freezing tolerance of some Antarctic nematodes. Funct Ecol 7:578–584
Wharton DA, Block W (1997) Differential scanning calorimetry studies on an Antarctic nematode (Panagrolaimus davidi) which survives intracellular freezing. Cryobiology 34:114–121
Wharton DA, Brown IM (1989) A survey of terrestrial nematodes from the McMurdo Sound region, Antarctica. N Z J Zool 16:467–470
Wharton DA, Brown IM (1991) Cold tolerance mechanisms of the Antarctic nematode Panagrolaimus davidi. J Exp Biol 155:629–641
Wharton DA, Ferns DJ (1995) Survival of intracellular freezing by the Antarctic nematode Panagrolaimus davidi. J Exp Biol 198:1381–1387
Wharton DA, To NB (1996) Osmotic stress effects on the freezing tolerance of the Antarctic nematode Panagrolaimus davidi. J Comp Physiol B 166:344–349
Wharton DA, Worland MR (1998) Ice nucleation activity in the freezing-tolerant Antarctic nematode Panagrolaimus davidi. Cryobiology 36:279–286
Wharton DA, Judge KF, Worland MR (2000) Cold acclimation and cryoprotectants in a freeze-tolerant Antarctic nematode, Panagrolaimus davidi. J Comp Physiol B 170:321–327
Wharton DA, Goodall G, Marshall CJ (2002) Freezing rate affects the survival of a short-term freezing stress in Panagrolaimus davidi, an Antarctic nematode that survives intracellular freezing. CryoLetters 23:5–10
Wharton DA, Goodall G, Marshall CJ (2003) Freezing survival and cryoprotective dehydration as cold tolerance mechanisms in the Antarctic nematode Panagrolaimus davidi. J Exp Biol 206:215–221
Womersley C (1987) A reevaluation of strategies employed by nematode anhydrobiotes in relation to their natural environment. In: Veech JA, Dickson DW (eds) Vistas on nematology. Society of Nematologists, Hyattsville, Maryland, pp 165–173
Womersley C, Ching C (1989) Natural dehydration regimes as a prerequisite for the successful induction of anhydrobiosis in the nematode Rotylenchus reniformis. J Exp Biol 143:359–372
Wright DJ, Newall DR (1976) Nitrogen excretion, osmotic and ionic regulation in nematodes. In: Croll NA (ed) The organisation of nematodes. Academic Press, New York, pp 163–210
Wright DJ, Newall DR (1980) Osmotic and ionic regulation in nematodes. In: Zuckerman BM (ed) Nematodes as biological models. Academic Press, New York, pp 143–164
Acknowledgements
I am grateful to Antarctica New Zealand who have supported my visits to Antarctica. Our current work on the ice active proteins of Antarctic nematodes is funded by the Marsden Fund of the Royal Society of New Zealand.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by: I.D. Hume
Rights and permissions
About this article
Cite this article
Wharton, D.A. The environmental physiology of Antarctic terrestrial nematodes: a review. J Comp Physiol B 173, 621–628 (2003). https://doi.org/10.1007/s00360-003-0378-0
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00360-003-0378-0