Abstract
Studies of auditory temporal resolution in birds have traditionally examined processing capabilities by assessing behavioral discrimination of sounds varying in temporal structure. Here, temporal resolution of the brown-headed cowbird (Molothrus ater) was measured using two auditory evoked potential (AEP)-based methods: auditory brainstem responses (ABRs) to paired clicks and envelope following responses (EFRs) to amplitude-modulated tones. The basic patterns observed in cowbirds were similar to those found in other songbird species, suggesting similar temporal processing capabilities. The amplitude of the ABR to the second click was less than that of the first click at inter-click intervals less than 10 ms, and decreased to 30% at an interval of 1 ms. EFR amplitude was generally greatest at modulation frequencies from 335 to 635 Hz and decreased at higher and lower modulation frequencies. Compared to data from terrestrial mammals these results support recent behavioral findings of enhanced temporal resolution in birds. General agreement between these AEP results and behaviorally based studies suggests that AEPs can provide a useful assessment of temporal resolution in wild bird species.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Temporal resolution refers to the precision with which the auditory system can extract time-varying information from acoustic stimuli (Viemeister and Plack 1993). Several recent behavioral studies of model species suggest that birds have greater temporal resolution than terrestrial mammals (Dooling et al. 2002; Lohr et al. 2006). Single-unit physiological studies also point to greater temporal processing capabilities in birds than in mammals (e.g. Klump and Gleich 1991; Gleich and Klump 1995; Köppl 1997).
Temporal resolution of the peripheral auditory system is thought to be limited by two factors. The first is a trade-off between frequency resolution and temporal resolution imposed by the auditory filters (Viemeister and Plack 1993). The second is adaptation of the sensory hair-cell synapse and neural refractoriness (Eggermont and Spoor 1973; Joris et al. 2004). The trade-off imposed by the auditory filters arises because frequency components are more likely to be processed in separate channels when auditory filters have relatively narrow bandwidths, thereby improving the frequency resolution (Moore 1993). However, when auditory filters are wide, carrier frequencies and frequency sidebands are likely to be processed in the same auditory filter, which is expected to improve temporal resolution (Viemeister and Plack 1993).
Here we explored temporal resolution in the brown-headed cowbird (Molothrus ater), a species in which the sexes differ in their auditory filter bandwidths (Gall and Lucas 2010). We had two main goals in this study: (1) determine whether sex-specific differences in auditory filter bandwidth would produce sex differences in temporal resolution and (2) determine whether the species level association between auditory filter bandwidth and temporal resolution found in other songbirds would apply to brown-headed cowbirds. We previously found that male brown-headed cowbirds had broader auditory filters than females (Gall and Lucas 2010). Therefore, if sex-specific auditory filter width is limiting the temporal resolution, males are expected to have greater temporal resolution than females. Auditory filter widths and temporal resolution data are available for four additional songbird species: Carolina chickadees (Poecile carolinensis), house sparrows (Passer domesticus), tufted titmice (Baeolophus bicolor) and white-breasted nuthatches (Sitta carolinensis; Henry and Lucas 2008, 2010; Henry et al. 2011). The auditory filters of brown-headed cowbirds are wider than those of white-breasted nuthatches and tufted titmice, similar to Carolina chickadees, and narrower than house sparrows (Gall and Lucas 2010). Therefore, based on the trade-off between auditory filter width and temporal resolution, we predicted that brown-headed cowbirds would have similar temporal resolution to Carolina chickadees, greater temporal resolution than white-breasted nuthatches and tufted titmice, and poorer temporal resolution than house sparrows.
We used two auditory evoked potential (AEP) methods to assess temporal resolution. AEPs are electrical potentials generated by the auditory nerve and brainstem and measured at the scalp. AEPs have been used to estimate temporal resolution in a variety of mammalian species using both auditory brainstem responses (ABRs) to paired-click stimuli (Supin and Popov 1995; Parham et al. 1996; Ohashi et al. 2005) and envelope following responses (EFRs) to sinusoidal amplitude modulated (SAM) tones (Dolphin and Mountain 1992; Mann et al. 2005; Cook et al. 2006; Finneran et al. 2009). However, their application for assessing temporal resolution in passerine birds has been limited (see Henry and Lucas 2008; Henry et al. 2011). First, we assessed the amplitude of ABRs to paired-click stimuli with variable inter-click intervals. These responses, which include a prominent biphasic deflection generated by the auditory nerve in birds (Brittan-Powell et al. 2002), reflect the ability of the peripheral auditory system to detect the sounds separated by a short silent interval. Second, we created modulation rate transfer functions (MRTF) from EFRs to SAM tones with 100% modulation depth. In birds, the amplitude of the EFR is thought to reflect the strength of phase locking to the envelope of the stimulus in the auditory nerve and possibly in the brainstem (Henry and Lucas 2008).
Methods
Subjects and anesthesia
All procedures were approved under Purdue Animal Care and Use Committee (PACUC) protocol #08-132. Brown-headed cowbirds were trapped with baited walk-in traps on Purdue-owned land in West Lafayette, IN. Additional birds were provided by USDA APHIS. We trapped 19 adults (9 males, 10 females) in the 2009 breeding season, which were used in the EFR experiment. We trapped seven adult birds (3 males, 4 females) in the 2010 breeding season, which were used in the paired-click experiment. After capture, individuals were transported to the Purdue University animal facility. Birds were housed individually in 1 m3 steel cages and provided ad libitum with mixed seed and vitamin-treated water. After completion of auditory AEP experiments brown-headed cowbirds were transferred to a different Purdue University animal care and use protocol (# 08-012) for use in visual physiology experiments.
Individuals were anesthetized with a combination of ketamine (40–60 mg/kg) and midazolam (6–8 mg/kg) injected into the breast muscle. Individuals were then positioned at the center of an anechoic sound chamber on a microwavable heating pad wrapped in towels (Pet Supply Imports, South Holland, IL, USA). The temperature, which was monitored with a probe placed under the left wing of the bird, between the body of the bird and the outermost towel, and connected to a digital readout, was maintained at 39 ± 2º by adding or removing layers of towel. We placed a non-inverting needle electrode (Nicolet Biomedical, Fitchburg, WI, USA) just below the scalp at the vertex of the head. An inverting needle was placed below the scalp at the auditory meatus and a ground electrode at the nape of the neck. Impedance was maintained below 7 kΩ.
General methodology
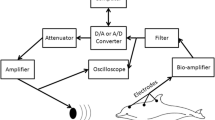
Our methodology was similar to that described in previously studies (Henry and Lucas 2008; Gall et al. 2011; Henry et al. 2011). Briefly, stimulus presentation, data acquisition and data storage were coordinated with a Tucker Davies Technology System II (TDT, Gainesville, FL, USA) and a computer running BioSig32. Stimuli were generated digitally in SigGen32 with an AP2 signal processing card. Stimuli were then converted to analog with a TDT model DA3-4 convertor and equalized across frequencies (Behringer Ultragraph 31-band equalizer, model FBQ6200, Bethel, WA, USA). Stimuli were amplified (Crown D75 amplifier, Elkhart, IN, USA) and presented through a magnetically shielded speaker (RCA model 40–5000; Indianapolis, IN, USA) suspended 30 cm above the bird. Responses were conducted from the subject to a TDT headstage (HS4) and then to a biological amplifier (TDT DB4). Responses were digitized (TDT AD2) and recorded on the computer (sampling rate 40 kHz).
Paired clicks
The paired-click experiment was identical to that of Henry et al. (2011). Briefly, clicks were created in SigGen32 by applying a 0.25 ms Blackman gate to a 0.67 ms 3 kHz tonepip with an amplitude of 60 dB SPL. Presentation of the paired-clicks stimuli were alternated with a reference stimulus consisting of a single click. Paired clicks were presented with inter-click intervals of 0.7, 1, 1.5, 2, 3, 4, 5, 7, 10, and 25 ms. Stimuli were presented in alternating polarity at a rate of 19.1 stimuli (either paired click or reference click) per second.
We sampled responses (ABRs) for 40 ms beginning 1.2 ms before stimulus arrival at the ear. Responses were bandpass filtered from 0.1–3 kHz and amplified 100,000×. Responses to paired clicks and reference clicks were recorded in separate averaging buffers and averaged over 700 paired click or 700 reference click stimulus presentations. We derived the response to the second click using a point-to-point subtraction in which the ABR to a single click was subtracted from the ABR responses to paired-click stimuli (Fig. 1). We measured the amplitude of each ABR from the first positive peak to the first negative trough in PRAAT (Boersma and Weenink 2009; Fig. 1). We then calculated ABR recovery (%) as the amplitude of the ABR to the second click relative to amplitude of the ABR to a single click multiplied by 100.
Auditory brainstem responses (ABRs) to paired clicks with inter-click intervals of 25 ms (left) and 1.5 ms (right) from a single female brown-headed cowbird. When inter-click intervals were short (<3 ms) ABRs to the second click overlapped the ABRs to the second click. ABRs to the second click were derived by a point-to-point subtraction of the response to a single click from the responses to paired-click stimuli
Envelope following responses
Amplitude-modulated stimuli were created in SigGen32 using the formula:
where A is the amplitude of the stimulus, f c is the carrier frequency in hertz, f m is the modulation frequency in hertz (i.e. the rate of amplitude modulation) and t is time in seconds. Each stimulus was 53.3 ms in length and was presented at peak amplitude of 64 dB SPL. We used three different carrier frequencies (2, 3, and 4 kHz). Each carrier frequency was modulated at 17 different rates (f m = 35, 110, 185, 260, 335, 410, 485, 560, 635, 710, 785, 835, 910, 1,010, 1,310, 1,610, and 1,910 Hz). The amplitude modulation stimuli had energy at three frequencies: the carrier frequency (f c), the carrier frequency minus the modulating frequency (f c−f m) and the carrier frequency plus the modulating frequency (f c + f m). The modulation depth was 100%. Note that the stimuli were designed so that no energy was present in the stimuli at the modulating frequency (Fig 2a). Each stimulus had a 3 ms cos2 onset/offset ramp and constant phase.
Examples of the amplitude-modulated stimuli used in this experiment. On the left hand side of each panel the top trace shows the waveform of a 53.3 ms stimulus with a carrier frequency of 2 kHz and a modulation frequency of a 410 Hz or b 1,610 Hz. The middle trace of each panel shows the raw response waveform of a single male cowbird to a 2 kHz tone with a modulation frequency of a 410 Hz and b 1,610 Hz. The response is composed of the auditory brainstem response (ABR), the envelope following response (EFR) and the frequency following response. The ABR is the prominent biphasic deflection beginning ~2 ms after stimulus onset and the EFR begins directly after the ABR. The bottom trace shows response waveforms that have been a low-pass filtered at 1 kHz or b bandpass filtered from 1.4–1.8 kHz to highlight the envelope following response. On the right hand side of each panel the top trace shows the power spectrum of the stimulus, with dotted lines denoting frequencies of interest. Note that there is energy present in the stimulus at the carrier frequency (F c) and each of the side bands (F c + F m, F c−F m) but not at the modulation frequency (F m). The middle trace is the power spectrum of the raw response waveform. The dotted lines indicate peaks in the power spectrum at the modulation frequency, carrier frequency and sidebands. The bottom trace shows the power spectrum for response waveforms that have been filtered to highlight the EFR
Responses were sampled for 60 ms. Each EFR was the averaged responses of 500 stimulus presentations with 2 replicates each. Responses were amplified 200,000× and bandpass filtered from 10 Hz to 10 kHz. EFRs recorded to stimuli presented in a single phase can contain both neural responses and a cochlear microphonic (hair-cell related potentials). Our response seem to reflect primarily neural activity as phase-locked potentials were relatively minimal prior to the ABR (see also Henry and Lucas 2008). We also presented stimuli in alternating polarity to a single bird to be sure that the EFR was not influenced by a cochlear microphonic (see Henry and Lucas 2008). The alternating polarity results are presented in the electronic supplementary material (ESM). We generated a power spectrum (Fig. 2) from the AEP waveform and then extracted the amplitude of the EFR (dB re:V) in PRAAT Ver 5.0.33. We also extracted the noise floor in 25 Hz bins at ± 100 Hz from the peak of interest. We discarded any responses where the EFR was less than 3 dB above the noise floor.
Statistical analyses
We used SAS 9.2 for all statistical analyses. All data were checked for normality and heteroscedasticity. EFR strength was not normally distributed and we used Proc Transreg to choose a Box–Cox transformation (λ = −2). Data were analyzed with repeated measures ANOVAs in Proc Mixed. The dependent variable in the paired-click model was relative amplitude and the independent variables were inter-click interval, sex and their interaction. The dependent variable in the EFR model was EFR strength and the independent variables were carrier frequency, modulation frequency, sex and their interactions. We calculated the degrees of freedom using the Kenward–Roger algorithm. We chose a first-order autoregressive covariance structure for the paired-click model and the EFR model, as they produced the lowest AIC values. However, the covariance structure did not qualitatively affect the results. Significant effects were investigated post-hoc with the LSMEANS procedure and the DIFF option (main effects) or the SLICE option (interactions effects) and the p-values were adjusted for multiple comparisons using the Tukey–Kramer method. LSMEANS ± S.E (back transformed when appropriate) are reported throughout.
Results
Paired clicks
There was a significant effect of inter-click interval on ABR recovery (F 9,99 = 25.6, p < 0.001). Response recovery decreased from 90–100% at intervals greater than 10 ms to approximately 30% at an interval of 1 ms (Fig. 3a). ABR recovery differs neither between inter-click intervals of 1.5 and 2 ms (t 188 = 1.46, p = 0.15), nor between inter-click intervals of 5 and 7 ms (t 118 = 0.52, p = 0.6). Response recovery was significantly different between all other time points (t 116 > 2.1, p < 0.04). There was neither significant difference in ABR recovery between the sexes (F 1,7 = 6.9, p = 0.34; Fig. 3b), nor was there a significant interaction of sex and inter-click interval on ABR recovery (F 9,99 = 0.79, p = 0.63).
Envelope following responses
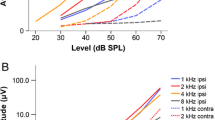
There were significant main effects of carrier frequency (F 2,875 = 33.3, p < 0.001) and modulation frequency (F 16,875 = 86.2, p < 0.001) but not sex (F 1,17 = 0.27, p = 0.61) on EFR strength (Fig. 4). EFR strength was significantly stronger when the carrier was 3 kHz than when carrier frequency was 2 kHz (t 875 = 7.7, p < 0.001) or 4 kHz (t 875 = 6.3, p < 0.001). There was no significant difference in EFR strength between carrier frequencies of 2 and 4 kHz (t 875 = 1.4, p = 0.16). At all of the carrier frequencies, EFR strength peaked at modulation frequencies of approximately 500 Hz, and decreased at higher and lower modulation frequencies.
There was also significant sex × modulation frequency (Fig. 4a; F 16,875 = 2.6, p < 0.001) and carrier frequency × modulation frequency (Fig. 4b; F 32,875 = 6.3, p < 0.001) interactions. Females had greater EFR strength at a modulation frequency of 260 Hz (F 1,37 = 8.1, p = 0.007) and there was a weak sex effect at 1,310 Hz (F 1,37 = 3.8, p = 0.05), but not at any other modulation frequency (F 1,37 < 1.9, p > 0.18). Comparing the effect of modulation frequency across carrier frequencies, the peak in EFR strength extended to higher modulation frequencies by approximately 150 Hz when the carrier frequency was 3 kHz than when the carrier frequency was 2 kHz or 4 kHz. EFR strength varied significantly among carrier frequencies at modulation frequencies of 260 (F 2,875 = 12.7, p < 0.001), 335 (F 2,875 = 3.7, p = 0.02), 560 (F 2,875 = 14.5, p < 0.001), 635 (F 2,875 = 38.3, p < 0.001), 710 (F 2,875 = 12.9, p < 0.001), 860 (F 2,875 = 4.2, p = 0.014), 1,310 (F 2,875 = 28.0, p < 0.001), 1,610 (F 2,875 = 52, p = 0.005) and 1,910 (F 2,875 = 33.3, p = 0.003). At most modulation frequencies EFR strength was strongest when the carrier frequency was 3 kHz. At 1,310 Hz, however, EFR strength was strongest when the carrier frequency was 4 kHz.
Discussion
General patterns
When plotted as a function of click rate (i.e. 1 over the time interval between the first and second click; Fig. 5), ABR recovery functions in brown-headed cowbirds were low-pass in shape and rolled off from near 100% recovery at a click rate of 100 Hz to 30% at 1,000 Hz. MRTFs, in contrast, were bandpass with maximum EFR amplitude at modulation frequencies from 335–635 Hz. The difference in shape at low modulation frequencies can be explained in part by the properties of the stimuli used and corresponding effects on neural synchrony. For ABR recovery experiments, the click stimuli have the same, rapid onset ramp at all click rates. For the EFR experiment, in contrast, the onset ramp of stimulus amplitude modulation cycles becomes progressively faster with increasing modulation rate. This should increase neural synchrony and consequently increase the peak amplitude of the population response to each cycle. Previous work also suggest that shorter duration or pulsed stimuli are more likely to result in bandpass temporal modulation transfer functions due to phasic onset responses, while continuous stimuli produce a temporal modulation transfer function with low-pass characteristics (Gleich and Klump 1995).
Click-response recovery as a function of click rate (i.e. 1 over the interval between paired clicks) in brown-headed cowbirds and other species. ABR recovery functions of Carolina chickadees, house sparrows, and white-breasted nuthatches are from Henry et al. (2011). The ABR recovery function of dolphins is from Supin and Popov (1995). The recovery function of cats is from Parham et al. (1996) and based on (1) compound action potentials for click rates of 500 Hz and below and (2) single-unit data for the click rate of 1,000 Hz
EFR-based MRTF in cowbirds were similar to functions measured in other songbirds. Indeed, at a carrier frequency of 2.75 kHz, MRTFs of tufted titmice, house sparrows, and white-breasted nuthatches are also bandpass with maximum EFR amplitude at modulation frequencies from approximately 350–710 Hz (Henry and Lucas 2008). Furthermore, the paired-click ABR recovery function of cowbirds was remarkably similar to ABR recovery functions of Carolina chickadees and white-breasted nuthatches, and to a lesser extent, of house sparrows (Fig. 5; Henry et al. 2011). The ABR recovery function also closely mirrors the ability of bullfinches, greenfinches and pigeons to behaviorally discriminate between paired and single clicks in a generalization paradigm (Wilkinson and Howse 1975). These patterns suggest that broadly speaking, cowbirds have similar temporal processing capabilities to other songbirds. This agrees with behavioral reports, which also tend to show similar temporal capabilities across model species. For example, minimum gap detection thresholds as a function of sensation level and MRTFs are similar across a variety of avian model species including budgerigars, starlings, zebra finches, and barn owls (reviewed in Dooling et al. 2000). Note that sensitivity to the temporal fine structure of harmonic tone complexes and Schroeder waveforms, on the other hand, appears to vary more across species (Dooling et al. 2002; Lohr et al. 2006).
Comparison to mammals
EFR-based MRTFs and ABR recovery functions in cowbirds and other songbirds deviate from patterns observed in most mammals. In humans and gerbils, for example, EFR-based MRTFs are low-pass in shape and roll off above modulation frequencies of 55 and 100 Hz, respectively (Kuwada et al. 1986; Dolphin and Mountain 1992). However, gerbils do show some recovery at modulation rates between 300–500 Hz. EFR-based MRTFs are probably not directly comparable between birds and mammals, however, because EFRs in birds likely arise from the auditory nerve or brainstem while mammalian EFRs arise from the cortex and midbrain (Dolphin and Mountain 1992; Gleich and Klump 1995; Henry and Lucas 2008). Single-unit studies of cats and gerbils at the level of the auditory nerve suggest that temporal coding of AM tones rolls off above modulation frequencies of 300–400 Hz for fibers with characteristic frequencies near the carrier frequency of our AM stimuli (i.e. 2–4 kHz; Frisina et al. 1990; Joris et al. 2004). These values are more similar but still slightly lower than the corner frequency observed in the present study (i.e. 630 Hz). Additionally, in studies of paired-click recovery in mammals (Fig. 5), percent recovery of the compound action potential in cats rolls off from 100% at a click rate of 60 Hz to 30% at 500 Hz (Parham et al. 1996), and in dolphins, ABR recovery rolls off from 100% at 200 Hz to 30% at 2,000 Hz (Supin and Popov 1995). Taken together, these studies suggest that the songbirds have slightly greater temporal resolution than some terrestrial mammals, but lower temporal resolution than dolphins.
Behavioral studies also point to sensitive temporal processing capabilities in birds. Zebra finches and budgerigars can detect changes in the temporal fine structure of harmonic tone complexes and Schroeder waveforms occurring within time intervals as short as 1 ms, while human listeners require time intervals greater than 3–4 ms for accurate discrimination (Dooling et al. 2002; Lohr et al. 2006). Furthermore, birds are more sensitive to gaps in noise, particularly at low sensation levels, and show a slower decrease in sensitivity to AM with increasing modulation frequency (reviewed in Dooling et al. 2000). Single-unit studies also provide some support for greater temporal resolution in birds. In mammals, temporal coding of fine structure and envelope modulations decreases progressively from the level of the auditory nerve to the cortex (Langner 1992). In starlings, in contrast, temporal coding of silent gaps in noise is remarkably similar between auditory nerve fibers and primary-like neurons of the auditory forebrain (Klump and Gleich 1991). Finally, neurons of the cochlear nucleus in several bird species phase-lock accurately to clicks at presentation rates as high as 1,000 Hz under some conditions (Konishi 1969).
Sex differences and auditory filters
The sex difference in EFR amplitude observed at modulation frequencies of 260 and 1,310 Hz suggests that female cowbirds may have slightly greater temporal resolution than males. Moreover, while auditory filter bandwidth is greatest at 4 kHz, EFR amplitude was greatest at 3 kHz. These results are at odds with theoretical predictions based on auditory filter bandwidth. Previously, we found that females have narrower auditory filters than males (Gall and Lucas 2010). Narrower filters, in turn, should decrease the maximum modulation frequency that can pass through the cochlea based on linear systems theory, and hence, should limit temporal resolution (Ruggero 1994; de Boer 1996). Other studies of birds have also provided mixed support for this hypothesis. Another study based on AEPs, for instance, noted the expected negative relationship between auditory filter bandwidth and temporal resolution across individual Carolina chickadees, but differences across species were consistent in some cases but not others (Henry et al. 2011). Similarly, a behavioral study of silent gap detection found the predicted relationship between auditory filter bandwidth and temporal resolution across stimulus frequencies in zebra finches but not budgerigars (Okanoya and Dooling 1990). Finally, turning to single-unit data, a study of starlings found no relationship between tuning curve bandwidth and sensitivity to silent gaps across auditory nerve fibers (Klump and Gleich 1991). The mixed results probably reflect factors other than auditory filter bandwidth that also limit temporal processing to varying degrees. In addition to bandwidth, the shape of the auditory filter is likely to affect temporal processing with higher order filters constraining temporal resolution to a greater degree than lower order filters at a given bandwidth (Klump and Gleich 1991). Neural refractoriness can influence responsiveness to sequential sounds separated by up to 20–30 ms, while adaptation of the hair-cell synapse can operate over even longer time frames under some conditions (Eggermont and Spoor 1973). The sensation level of stimuli may also affect estimates of temporal resolution. Studies of gross-potentials evoked by paired-click stimuli have generally found an increase in temporal resolution at lower sensation levels (i.e. response recovery at short inter-click intervals is greater; Parham et al. 1996). In a single unit study of starlings, however, the sensitivity of auditory nerve fibers to silent gaps in noise increased at higher sensation levels (Klump and Gleich 1991).
In addition to the difference in temporal resolution found here, female cowbirds have also been shown to have greater frequency resolution and greater auditory sensitivity than males (Gall and Lucas 2010; Gall et al. 2011). The mechanisms underlying these differences are unclear, but may relate in part to effects of sex hormones on auditory function. Estrogen receptors have been identified within the avian cochlea (Noirot et al. 2009), and may account for auditory differences between sexes and seasons in cowbirds and other songbird species (Lucas et al. 2002, 2007; Henry and Lucas 2009, 2010; Caras et al. 2010). The functional significance of these sex differences also remains an open question. Heightened auditory processing capabilities in females could have evolved, for example, under selection for accurate localization of host nest sites. As brood parasites, female reproductive success should be closely tied to the ability to find suitable heterospecific nest sites using visual and acoustic cues.
In conclusion, the results of the current study suggest that cowbirds generally have similar temporal resolution to other songbirds. Furthermore, cowbirds and other songbirds appear to have greater temporal processing capabilities than humans and some terrestrial mammals. These conclusions agree with other studies based on behavioral discrimination and single-unit physiological responses, suggesting that AEPs can provide a useful assessment of temporal resolution in wild-caught animal subjects. Finally, we found some evidence of greater temporal processing capabilities in female cowbirds than in males. Further exploration of this and other auditory sex difference in this species may be a fruitful avenue for additional research.
Abbreviations
- ABR:
-
Auditory brainstem response
- AEP:
-
Auditory evoked potential
- EFR:
-
Envelope following response
- MRTF:
-
Modulation rate transfer function
- SAM:
-
Sinusoidal amplitude modulated
References
Boersma P, Weenink D (2009) Praat: doing phonetics by computer, Version 5107 Computer program retrieved May 7, 2009, from http://wwwpraatorg/
Brittan-Powell EF, Dooling RJ, Gleich O (2002) Auditory brainstem responses (ABR) in adult budgerigars (Melopsittacus undulates). J Acoust Soc Am 112:999–1008
Caras ML, Brenowitz E, Rubel EW (2010) Peripheral auditory processing changes seasonally in Gambel’s white-crowned sparrow. J Comp Physiol A 196:581–599
Cook MLH, Varela RA, Goldstein JD, McCulloch SD, Bossart GD, Finneran JJ, Houser D, Mann DA (2006) Beaked whale auditory evoked potential hearing measurements. J Comp Physiol A 192:489–495
de Boer E (1996) Mechanics of the cochlea: modeling efforts. In: Dallos P, Popper AN, Fay RR (eds) The cochlea. Springer, New York, pp 258–317
Dolphin WF, Mountain DC (1992) The envelope following response: scalp potentials elicited in the Mongolian gerbil using sinusoidally AM acoustic signals. Hear Res 58:70–78
Dooling RJ, Lohr B, Dent ML (2000) Hearing in birds and reptiles. In: Dooling RJ, Popper AN, Fay RR (eds) Comparative hearing: birds and reptiles. Springer-Verlag, New York, pp 308–359
Dooling RJ, Leek MR, Gleich O, Dent ML (2002) Auditory temporal resolution in birds: discrimination of harmonic complexes. J Acoust Soc Am 112:748–759
Eggermont JJ, Spoor A (1973) Masking of action potentials in the guinea pig cochlea, its relation to adaptation. Audiol 12:221–241
Finneran JJ, Houser DS, Mase-Guthrie B, Ewing RY, Lingenfelser RG (2009) Auditory evoked potentials in a stranded Gervais’ beaked whale (Mesolplodon europaeus). J Acoust Soc Am 126:484–490
Frisina RD, Smith RL, Chamberlain SC (1990) Encoding of amplitude-modulation in the gerbil cochlear nucleus 1 A hierarchy of enhancement. Hear Res 44:99–122
Gall MD, Lucas JR (2010) Sex differences in auditory filters of brown-headed cowbirds (Molothrus ater). J Comp Physiol A 196:559–567
Gall MD, Brierley LE, Lucas JR (2011) Species and sex effects on auditory processing in brown-headed cowbirds and red-winged blackbirds Anim Behav 81:973–982
Gleich O, Klump GM (1995) Temporal-modulation transfer-functions in the European starling (Sturnus vulgaris): II responses of auditory-nerve fibres. Hear Res 82:81–92
Henry KS, Lucas JR (2008) Coevolution of auditory sensitivity and temporal resolution with acoustic signal space in three songbirds. Anim Behav 76:1659–1671
Henry KS, Lucas JR (2009) Vocally correlated seasonal auditory variation in the house sparrow (Passer domesticus). J Exp Biol 212:3817–3822
Henry KS, Lucas JR (2010) Auditory sensitivity and the frequency selectivity of auditory filters in the Carolina chickadee (Poecile carolinensis). Anim Behav 80:497–507
Henry KS, Gall MD, Lucas JR (2011) Songbirds trade off auditory frequency resolution and temporal resolution. J Comp Physiol A 197:351–359
Joris PX, Schreiner CE, Rees A (2004) Neural processing of amplitude-modulated sounds. Physiol Rev 84:541–577
Klump GM, Gleich O (1991) Gap detection in the European starling (Sturnus rulgaris): III processing in the peripheral auditory system. J Comp Physiol A 16:469–476
Konishi M (1969) Time resolution by single auditory neurones in birds. Nature 222:566–567
Köppl C (1997) Phase locking to high frequencies in the auditory nerve and cochlear nucleus magnocellularis of the barn owl, Tyto alba. J Neurosci 17:3312–3321
Kuwada S, Batra R, Maher VL (1986) Scalp potentials of normal and hearing-impaired subjects in response to sinusoidally amplitude-modulated tone. Hear Res 21:179–192
Langner G (1992) Periodicity coding in the auditory system. Hear Res 60:115–142
Lohr B, Dooling RJ, Bartone S (2006) The discrimination of temporal fine structure in call-like harmonic sounds by birds. J Comp Psychol 120:239–251
Lucas JR, Freeberg TM, Krishnan A, Long GR (2002) A comparative study of avian auditory brainstem responses: correlations with phylogeny and vocal complexity, and seasonal effects. J Comp Physiol A188:981–992
Lucas JR, Freeberg TM, Long GR, Krishnan A (2007) Seasonal variation in avian auditory evoked responses to tones: a comparative analysis of Carolina chickadees, tufted titmice, and white-breasted nuthatches. J Comp Physiol A 192:201–215
Mann DA, Colbert DE, Gaspard JC, Casper BM, Cook MLH, Reep RL, Bauer GB (2005) Temporal resolution of the Florida manatee (Trichechus manatus latirostris) auditory system. J Comp Physiol A 191:903–908
Moore BCJ (1993) Frequency analysis and pitch perception. In: Yost WA, Popper AN, Fay RR (eds) Human psychophysics. Springer, New York, pp 58–89
Noirot IC, Adler HJ, Cornil CA, Harada N, Dooling RJ, Balthazart J, Ball GF (2009) Presence of aromatase and estrogen receptor alpha in the inner ear of zebra finches. Hear Res 252:49–55
Ohashi T, Ochi K, Nishino H, Kenmochi M, Yoshida K (2005) Recovery of human compound action potential using a paired-click to stimulus paradigm. Hear Res 203:192–200
Okanoya K, Dooling RJ (1990) Minimum detectable gap in noise as a function of intensity and frequency for two avian species, budgerigars (Melopsittacus undulates) and zebra finches (Poephila guttata). Hear Res 50:185–192
Parham K, Zhao HB, Kim DO (1996) Responses of auditory nerve fibers of the unanesthetized decerebrate cat to click pairs as simulated echoes. J Neurophysiol 76:17–29
Ruggero MA (1994) Cochlear delays and traveling waves: comments on ‘Experimental look at cochlear mechanics’. Audiol 33:131–142
Supin AY, Popov VV (1995) Temporal resolution in the dolphin’s auditory system revealed by double-click evoked potential study. J Acoust Soc Am 97:2586–2593
Viemeister NF, Plack CJ (1993) Time analysis. In: Yost WA, Popper AN, Fay RR (eds) Human psychophysics. Springer, New York, pp 116–154
Wilkinson R, Howse PE (1975) Time resolution of acoustics signals by birds. Nature 258:320–321
Acknowledgments
We would like to thank E. Fernández-Juricic and P. Baumhardt for assistance in acquiring birds, and N. Robinson, L. Brierley, K. Ronald, J. Losier, J. Randolet, and B. Moore for their feedback on this manuscript. All methods were approved by the Purdue Animal Care and Use Committee under protocol number 08–132.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Gall, M.D., Henry, K.S. & Lucas, J.R. Two measures of temporal resolution in brown-headed cowbirds (Molothrus ater). J Comp Physiol A 198, 61–68 (2012). https://doi.org/10.1007/s00359-011-0687-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00359-011-0687-9