Abstract
Temporal cues are important for some forms of auditory processing, such as echolocation. Among odontocetes (toothed whales, dolphins, and porpoises), it has been suggested that porpoises may have temporal processing abilities which differ from other odontocetes because of their relatively narrow auditory filters and longer duration echolocation signals. This study examined auditory temporal resolution in two Yangtze finless porpoises (Neophocaena phocaenoides asiaeorientalis) using auditory evoked potentials (AEPs) to measure: (a) rate following responses and modulation rate transfer function for 100 kHz centered pulse sounds and (b) hearing thresholds and response amplitudes generated by individual pulses of different durations. The animals followed pulses well at modulation rates up to 1,250 Hz, after which response amplitudes declined until extinguished beyond 2,500 Hz. The subjects had significantly better hearing thresholds for longer, narrower-band pulses similar to porpoise echolocation signals compared to brief, broadband sounds resembling dolphin clicks. Results indicate that the Yangtze finless porpoise follows individual acoustic signals at rates similar to other odontocetes tested. Relatively good sensitivity for longer duration, narrow-band signals suggests that finless porpoise hearing is well suited to detect their unique echolocation signals.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Among mammals, odontocetes (toothed whales, dolphins, and porpoises) have relatively rapid auditory temporal processing abilities. This may partially be an adaptation for echolocation, which requires comparatively short processing times, high temporal resolution, to discriminate cues (see Bullock et al. 1968; Au et al. 1988; Au 1993). Higher processing speeds are also associated with high frequency hearing and wide auditory filters. Wide filters (often found at higher frequencies) are considered to have shorter temporal responses, reducing “ringing” after stimulus cessation, and thus allowing less physiological overlap between two stimuli close in time (Viemeister and Plack 1993). In many mammals, wider filters also result in decreased frequency discrimination. Odontocetes are unusual in that they combine high temporal resolution with narrow frequency discrimination (Supin and Popov 1995; Supin et al. 2001). However, it is not clear how temporal processing abilities vary between most odontocetes. Species can differ in filter bandwidths, hearing ranges or echolocation parameters, and these characteristics may affect temporal resolution abilities.
The Yangtze finless porpoise (Neophocaena phocaenoides asiaeorientalis) is a freshwater odontocete that may differ in temporal resolution capabilities. They show a relative narrowing of auditory filters, or a filter quality (the ratio of filter center frequency to its passband width, also referred to as Q) of more than 40 at higher frequencies (Popov et al. 2006). While other odontocetes tested also show a narrowing, it is typically to a lesser extent. For example, the high-frequency Q of the bottlenose dolphin (Tursiops truncatus) is near 20 (Popov et al. 1996). This high Q and filter narrowing for the porpoise would imply improved frequency discrimination but decreased auditory temporal resolution.
Finless porpoise echolocation sounds are distinct from the short, broadband clicks of most delphinids (i.e. family Delphinidae). These signals are typically narrowband (20 ± 4.24 kHz in 3-dB bandwidth) and on average fairly long duration at ~70 μs with some continuing, up to 120 μs with 10 or more sinusoid cycles (Li et al. 2005). With longer duration echolocation pulses, it is possible that the finless porpoise will not require as high temporal processing speeds, or as precise resolution, as the dolphin. Perhaps the porpoise auditory system may show improved detection of longer, narrower-band signals.
The high-frequency limit of finless porpoise hearing is 95 dB re 1 μPa at 152 kHz (Popov et al. 2005). While 95 dB is sensitive for this frequency, and they have not been tested higher, 152 kHz is not exceedingly high for an odontocete. Bottlenose dolphin’s thresholds are 137 dB at 150 kHz, Lagenorhynchus albirostris hear 99 and 120 dB at 152 and 181 kHz, respectively, and Phocoena phocoena thresholds are 106 dB at 180 kHz (Johnson 1966; Kastelein et al. 2002; Nachtigall et al. 2008). If the finless porpoise has a high frequency cut-off similar to other odontocetes, their temporal resolution may also be similar to other odontocetes. To resolve these uncertainties there is a need to evaluate how the finless porpoise temporally processes sounds and whether echolocation processing, filter bandwidth, or high-frequency hearing limits may dominantly affect their temporal resolution abilities.
Auditory evoked potential (AEPs) are frequently used to examine odontocete temporal processing (Nachtigall et al. 2007). These evoked potential methods allow rapid non-invasive data collection with thresholds comparable to those obtained by behavioral methods (Yuen et al. 2005; Finneran and Houser 2006). Temporal resolution is estimated using AEPs by presenting brief stimuli (either broadband clicks or sinusoidally amplitude modulated tones) at specified rates (Vermeister 1979; Supin and Popov 1995). At lower presentation rates, AEP responses are linked to each input, yielding a rate or envelope following response (RFR or EFR). Rate specific RFR amplitudes determine the modulation rate transfer function (MRTF) which resembles a low-pass filter function; response amplitudes are higher for rates for which individual pulses are distinguished better. Response amplitudes decrease at faster repetition rates because a subject’s AEPs no longer follow individual pulses and responses are similar to the detection of a continuous sound. The corner, high-frequency cutoff of the MRTF reflects the limit of an auditory system’s ability to follow stimuli presented close in time and can be applied to estimate maximum auditory temporal resolution (Vermeister 1979; Supin and Popov 1995).
AEP temporal resolution measures have been obtained for several odontocete species (Supin and Popov 1995; Szymanski et al. 1998; Cook et al. 2006; Mooney et al. 2006; Finneran et al. 2007; Mooney et al. 2009), but not to date for any porpoise species. In this study, we measured auditory temporal resolution of the Yangtze finless porpoise using MRTFs and examined finless porpoise hearing capabilities of simulated echolocation pulses varying in duration. Consequent pulses mirrored short duration dolphin clicks and longer duration porpoise signals. The goal was to investigate how finless porpoises detect brief sounds. The results are viewed in light of their echolocation, hearing capabilities, and the evolution of auditory temporal processing.
Methods
The hearing of two Yangtze finless porpoise (one male and one female) was tested in April 2010 at the Institute of Hydrobiology, Chinese Academy of Sciences, Wuhan, China where they have been maintained for the last 6 and 14 years, respectively. At the time of the experiments, the male (Abao) was estimated to be 15 years old, weighed 47.8 kg, and was 145 cm in length. The female (Ying Ying) weighed 41.8 kg, was 142 cm long and was considered a similar age. These animals were among six porpoises housed at the facilities. All animals were maintained in large cement pools which received filtered water from the Yangtze River.
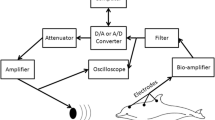
Hearing test experiments were conducted on the platform of a smaller pool which was fitted with a soft, foam mat (Fig. 1). The animal rested on this mat, out of the water, for the course of a hearing test session (~60 min). Afterwards, the animal was gently lowered back to the shallow pool water and the pool was refilled. The basic audiogram of the female was collected in-water several years prior (Popov et al. 2005). Subsequent probe hearing tests at 8, 54, and 120 kHz showed both animals had sensitive hearing abilities (Table 1).
Experimental setup. During the hearing tests, the respective porpoise was situated out of the water on several foam mats and kept moist using wet towels and continuous watering via a hose. Sound was presented using a suction cup jawphone (1). Responses were recorded using a three electrode configuration with the non-inverting electrode (2) just behind the blowhole, the ground on the animals back (3) and the inverting electrode on the fluke (4)
Acoustic stimuli
Sound stimuli were broadband pulses digitally created using a custom LabView (National Instruments, Austin, TX, USA) program operating in a laptop computer. The signal was converted from digital to analog with a National Instruments PCMCIA-6062E data acquisition card using an update rate of 512 kHz. The pulse was then sent to a HP 350D attenuator (Palo Alto, CA, USA) which could control sound levels in 1 dB (re 1 μPa) increments. From the attenuator, the signal was monitored on a digital oscilloscope (Tektronix TPS 2014; Beaverton, OR, USA) and played to the animal using a ‘jawphone’ transducer. The jawphone consisted of a Reson 4013 transducer (Slangerup, Denmark) implanted in a custom silicone suction-cup (KE1300T, Shin-Etsu, Tokyo, Japan). This cup could be non-invasively attached to the lower jaw of the animal and sounds were presented directly to the porpoise. This location was predetermined as a region of acoustic sensitivity. The jawphone was attached to the animal using conductive gel (Signagel, Parker Laboratories, Fairfield, NJ, USA) which eliminated reflective air gaps between the cup and the animal’s skin.
The stimulus pulses were centered at 100 kHz. For the MRTFs, pulses were generated using 2 cycles of a 100 kHz sine wave resulting in a 30 μs stimulus. These were produced in 20 ms bouts with the pulses presented at varying rates. Rates were varied from 125 to 4,000 Hz (125, 250, 375, 500, 625, 750, 875, 1,000, 1,125, 1,250, 1,375, 1,500, 1,750, 2,000, 2,500, 3,000, 3,500, and 4,000 Hz). 1,000 bouts were repeated for each rate. For evoked potential response measurements with varied pulse duration, the 100 kHz centered pulses were presented with the number cycles varying from 1 to 10. The designed duration of these pulses was from 10 to 100 μs (see calibrations below). This was to evaluate relative response differences between short, broadband pulses, similar to dolphin clicks, and longer duration, narrower pulses with more carrier frequency cycles, which reflect porpoise echolocation signals.
Jawphone stimuli were calibrated in the water at the test facility before the experiment using the same sounds as in the hearing tests. While calibration measurements were in the free- and far-fields, it is acknowledged that jawphone presented stimuli were not received by the animal in this manner. However, this calibration allows for some comparisons with how sounds may be received in the far-field while recognizing the differences between free-field and contact transducer measurements (Cook et al. 2006; Finneran and Houser 2006). Received measurements were made using a Reson 4013 transducer. The jawphone projector and receiver were placed 1 m apart at 1 m depth. The received signals were viewed on an oscilloscope (Tektronix TPS 2014, Beaverton, OR, USA) and the peak-to-peak voltages (V p-p) were measured. These values were then calculated into sound pressure levels (SPL, dBp-p re 1 μPa) as is standard to measure odontocete click intensities due to the inherent brevity of the signals (Au 1993). The stimuli were also recorded using the custom data acquisition program. Sound records were sampled at a rate of 512 kHz and stored as a mean of 10 stimuli. From these recorded files and the dBp-p it was possible to calculate and compare the energy flux density of the pulses (dB re 1 uPa2 s), a valuable metric of short signals which vary in duration (Madsen 2005). Fast Fourier transforms of the recorded files allowed us to view the spectra of the pulses. From these, we confirmed the center frequency of each pulse type (following Au 1993; Madsen and Wahlberg 2007). Pulses were centered at 100 ± 3 kHz, with the exception of the 3 cycle pulse, which was centered at 93 kHz. Pulse durations were also measured from the recorded files and characterized as time between two points at which the wave oscillations rose from and descended into the background noise (Au 1993; Li et al. 2005). Durations for pulses generated with 1 through 10 cycles were 28, 30, 41, 43, 57, 59, 65, 78, 84, and 91 μs, respectively (Fig. 2). Analyses were conducted using EXCEL, MiniTab and MatLab software.
Evoked potential hearing tests
Hearing tests were conducted using auditory evoked potential methodology. Evoked responses were collected using three 1-cm diameter gold-plated electroencephalogram electrodes (Grass Technologies, Astro-Med, Warwick, RI, USA) implanted in custom built silicone suction cups. The active (or non-inverting) electrode was placed several centimeters behind the blowhole, along the midline of the animal. A ground electrode was attached to the posterior peduncle and a reference (inverting) electrode was placed on the tail fin of the porpoise. The cups were attached using the aforementioned conductive gel. The incoming electrophysiological signals were amplified 10,000× and filtered with a biological amplifier (Grass Technologies CP511, Warwick, RI, USA). The filter bandpass settings were typically 300–3,000 Hz, but were increased to 100–3,000 or 300–10,000 Hz depending on the stimulus presentation rate. Responses were then conducted to the data acquisition card and custom program where they were sampled at 16 kHz, and then recorded and stored on the laptop computer. The responses were collected in 30 ms records that began coincident with the stimulus presentation. There was a 20 ms break before the stimulus/AEP recording began again; 1,000 responses were collected for each trial. These records were averaged and stored as the mean response. This step served to reduce unwanted electrophysiological noise. Unaveraged responses were monitored on the oscilloscope during the experimental sessions.
Recorded response waveforms were first viewed relative to time (Fig. 3). By taking a fast Fourier transform (FFT) of the response waveforms, response energy was also examined in the frequency domain (Fig. 4). In these records, a 16-ms portion of the evoked potential waveform, from 5 to 21 ms, was used for the FFT. If a following response was detectable, a peak in the spectrum occurred at the corresponding stimulus rate. To obtain the MRTF for both porpoises, the spectra amplitude value at the presentation rate was plotted relative to the corresponding presentation rate. However, this may weigh following responses at different rates unevenly because at lower presentation rates fewer pulses are presented per 20 ms bout. To compensate for this, “weighted” MRTFs were calculated by (a) the sum of values from the presentation rate peak and its harmonics and (b) the square root of the sum of the squares of the fundamental and the harmonics (Fig. 5). These methods follow previous work and allow for comparisons to other studies (e.g., Supin and Popov 1995; Szymanski et al. 1998; Mooney et al. 2006).
Porpoise rate following responses (male subject) to pulses played at rates of 250, 1,250, 2,000 and 3,500 Hz. Note the individual waveforms for each pulse at 250 Hz, indicated by a box. At 1,250 Hz, the RFR is similar to a sine wave. At 2,000 Hz, individual following responses are clear but the dominant response is the stimulus onset. At 3,500 Hz, there is no indication of following individual pulses, but the stimulus onset response is notable
Spectra of the responses viewed by fast Fourier transforms of the RFRs in Fig. 3. Peak amplitudes at the repetition frequency indicate RFR magnitude. There are peaks at the repetition frequency and harmonics of that frequency for 250 and 1,250 Hz. A peak is visible at 2,000 Hz, indicating following of that repetition rate. There is no clear peak in the 3,500 Hz spectrum indicating the animal did not follow individual pulses at that rate
a Male (black) and female (grey) finless porpoise MRTFs calculated using FFT peak values at the presentation rate (thin lines) and the square root of the summed squares of the fundamental and harmonic peaks (bold lines). The “Sum” method data were similar to the peak data. b Mean MRTFs (both animals) used to estimate auditory temporal resolution calculated using FFT peak values at the presentation rate (Peak), the summation of the fundamental and harmonic peaks (Sum) and the square root of the summed squares of the fundamental and harmonic peaks (Square). Corner frequencies were 1,125–1,250 Hz
The probe hearing tests were conducted in the same manner but sinusoidally amplitude modulated tones of 8, 54, and 120 kHz were presented. Thresholds were determined by FFT of the following responses, plotting the peaks of those FFTed responses, matching a regression to fit those peak values, and determining the threshold as the SPL that the response is zero. These methods have been well described elsewhere (Nachtigall et al. 2007).
Results
Rate following responses
Results were similar for both animals and have been summarized together (unless otherwise stated). At lower stimulus presentation rates, distinct AEP responses were detectable for each individual pulse (Fig. 3). Individual evoked responses consisted of 3–4 waves occurring 2–5 ms after stimulus onset. As stimulus rate increased, the individual evoked potential responses blended to a sinusoidal following response which was clearly distinguishable at mid-frequency rates of 1,000–1,250 Hz. At 1,500–2,000 Hz, following responses were detectable but diminished; the onset response to the pulse train was easily identifiable. Beyond 2,500 Hz, the following responses were not visible and only the onset response to the stimulus was easily seen.
At lower modulation rates, FFT peaks occurred at both the fundamental stimulus rate as well as its harmonic intervals (Fig. 4). This was evident at the 250 Hz pulse rate, where FFT peaks occurred not only at 250, but 500, 750, 1,000, and so on up to ~3,000 Hz. Similarly, at 1,250 Hz pulse rate, FFT peaks were seen at 1,250 and 2,500 Hz. With a lack of following responses at the higher modulation rates such as 3,500 Hz, no clear peak was distinguishable from the background noise.
There was some variability between the porpoises’ MRTF values at the lower modulation rates (Fig. 5). Because they incorporate the harmonic peaks, weighted MRTFs impacted the responses to lower frequency modulation rates more dramatically. This was reflected in the high MRTF values at 125 and 250 Hz for the summed MRTF and elevated lower frequency values for the squared MRTF. Above 250 Hz, the MRTFs were generally the shape of low-pass filters, i.e., good responses up to a corner frequency, after which responses dropped off steeply. This shape suggests that individual pulses can be followed well up to, or near, the corner frequency. The unweighted following rate maximums were 1,125 Hz for the male and 1,000 Hz for the female; the maximum mean value, or corner frequency, was 1250 Hz. Secondary, lower-frequency peaks were evident at 625 Hz. Minima were at 375 and 875 Hz. Temporal resolution limit is often calculated as −20 dB of the maximum corner frequency (Mulsow and Reichmuth 2007); −20 dB here was just over 2,000 Hz for both the male and female porpoise. The weighted MRTFs reflected similar trends.
The response spectra of AEP waveforms to single pulses were calculated using a 256 point FFT of the mean of ten evoked potential responses (Fig. 6). Similar to the MRTF, peaks were evident at lower (250, 675 Hz) and higher frequencies (1,125, 1,375, 1,625 Hz). Minima were also evident at 375 and 875 Hz. After 1,625 Hz, energy in the responses decreased rapidly towards 2,000 Hz. However, energy in the waveforms was still evident out to 2,500 Hz where a small peak was found.
The mean spectrum of ten AEP responses (256 point FFT; ordinate axis is linear scale). Two representative AEP waveforms are inset for reference. Note the spectral peaks near 1,500 Hz and similar, but slightly higher spectrum than the MRTF results (Fig. 5)
Varying pulse duration
Thresholds to pulse stimuli of 1, 2, 5 and 10 cycles (28, 30, 57 and 91 μs) were calculated using cross-correlation of evoked potential waveforms. Responses were cross-correlated with a standard response (Fig. 7). The peak values of the cross-correlation function were then plotted relative to the corresponding sound level which produced the AEP responses. A regression was fitted to these values and the SPL at which the regression line crossed zero response amplitude was taken as the estimated threshold. Hearing thresholds were lower for longer pulses with a greater number of cycles (pooled data for the male and female using a two sample t test; p < 0.01; df = 6; Fig. 8). Thresholds were not significantly different across pulse durations when stimuli were calculated using energy flux density (Fig. 8b).
Threshold calculations of AEP waveforms using cross correlation and regression analyses. a the AEP waveforms cross-correlated with a standard (the top trace), b the cross-correlation functions, and c the peak values of those functions (black dots) relative to the corresponding sound level which generated the response. A best-fit regression (open squares) is fitted to the peak values and used to estimate the sound level at which theoretically no response occurs
Thresholds of responses generated with pulses of various cycles presented SPL (a) and energy flux density (b). Five and ten cycle (57 and 91 μs) pulses generated significantly lower thresholds for both porpoises (male and female data are pooled and examined using a two sample t test; p < 0.01; df = 6)
AEPp-p response amplitudes were also evaluated at constant SPLs (both 95 and 105 dB) but using a varied duration (Fig. 9). The resulting AEPp-p response amplitudes varied considerably. The shortest and longest duration pulses (28, 84 and 91 μs; 1, 9 and 10 cycles) had lower response amplitudes. Pulses of 30–57 μs (2–5 cycles) produced greater AEPp-p responses and values generally declined from 43 to 91 μs (4–10 cycles). Other waveform attributes (e.g., latency, number of waves) did not substantially change. While the AEPp-p changed with pulse duration, response amplitudes at certain super-threshold levels did not necessarily correlate with hearing thresholds, i.e., lower AEPp-p responses did not mean higher thresholds and vice-versa.
a Peak-to-peak amplitude of AEP responses (waves 2, 3, 4) for corresponding pulse cycles. Sound levels were kept constant at 95 dB re: 1 μPap-p (mean of both animals). Stimuli energy flux density is plotted in blue dots (right axis). b Waveforms of representative responses to various pulses (one animal). Note waveform shape is constant, although p-p amplitudes changes
Discussion
Temporal resolution
The finless porpoise MRTFs calculated here were a low-pass filter shape, similar to other odontocete species examined (Supin and Popov 1995; Szymanski et al. 1998; Cook et al. 2006; Mooney et al. 2006; Finneran et al. 2007; Mooney et al. 2009) suggesting some evolutionary conservation of auditory temporal resolution among odontocetes. High frequency MRTF peaks were visible at 1,000, 1,125 and 1,250 Hz. The “corner” frequency was approximately 1,250 Hz, indicating the porpoises followed pulse rates well up to that frequency. Beyond 1,250 Hz, the following responses, FFT peaks and the corresponding MRTF values decreased. However, rate following responses were detectable up to 2,000–2,500 Hz suggesting at least some degree of auditory temporal resolution at these very high pulse rates (Fig. 5).
The MRTF corner frequency is typically used as the modulation rate of amplitude modulated tones in measurement of AEP audiograms (Nachtigall et al. 2007). There was slight variability between the porpoises as the corner frequency was easily distinguished for the female and not as obvious in the male MRTF. A 1,250 Hz corner frequency is on par with bottlenose dolphin MRTFs but slightly higher than most other odontocete species (Supin and Popov 1995; Mooney et al. 2009). For example, corner frequencies include 800 Hz for Orcinus orca, 1,000 Hz for Grampus griseus, 1,000 Hz for Delphinapterus leucas and 1,125 Hz for Lagenorhynchus albirostris (Szymanski et al. 1998; Klishin et al. 2000; Mooney et al. 2006; Mooney et al. 2009). While there might be a slight improvement, finless porpoise temporal resolution is not substantially higher than other odontocetes. Considering the MRTF similarities among odontocetes, it is likely these data are representative for other porpoise species. Yet, the Yangtze finless porpoise is confined to freshwater and sound speeds are slightly slower than salt water (Urick 1983). It is suggested that comparable temporal resolution data should be acquired for species such as the harbor (Phocoena phocoena) and Dall’s porpoise (Phocoenoides dalli) to more completely assess porpoise sound processing speeds.
Similar to other odontocetes, the spectrum of an AEP waveform to a single pulse was generally reflective of the shape and frequency response of the MRTF. Due to these similarities in troughs and peaks, it has been suggested that the AEP response characteristics (produced by several anatomical generators with differing response latencies) may influence MRTF shape (Supin and Popov 1995). The different peaks in the click AEP likely result from populations of neurons in different brain centers firing synchronously. The power spectrum of a click AEP represents the speed of AEP signals moving between response generators. In this sense, it may be reflective of temporal resolution but it is likely more representative of the speed of signal transmission in the brain. Thus, the similarities between AEP spectra and MRTF do not support using AEP spectra to gauge temporal resolution abilities. At lower frequencies, the spectral minima (e.g., 375 and 875 Hz) do not indicate decreased temporal processing abilities because the animal’s waveforms reflect clear responses. Odontocete temporal processing abilities at these rates is also supported by psychophysical data from gap detection and masking studies (Moore et al. 1984; Au et al. 1988). The porpoise AEP spectrum had substantial energy at 1,500 Hz and a second peak at 2,500 Hz, suggesting it does not constrain the MRTF maximum following response. The decreasing MRTF values at higher rates likely reflect the capability of the neuronal structures to follow the rate presented (Supin and Popov 1995). Estimates of temporal resolution based on evoked potentials can differ from perceptual temporal resolution results, although direct comparisons are limited because the methodologies, species, and questions addressed are very different (Moore et al. 1984; Au et al. 1988). But by generally comparing these data it can be determined that maximal perceptual temporal processing rates are not less than the MRTF upper frequency (i.e., the limits set by the peripheral auditory evoked potential generators).
Pulse duration and AEP responses
Pulsed stimuli of varied duration but constant SPL affected evoked potential response characteristics in several ways. First, hearing thresholds were 12 dB SPL lower for the longer duration pulses (Fig. 8). Most likely, this has to do with the energy in the pulse and perhaps the pulse bandwidth. For a longer duration pulse there is substantially more energy to detect. When considering brief sounds, longer durations substantially improve signal detection (Johnson 1968; Au et al. 1988). The 10 cycle, 91 μs, pulses had an energy flux density (dB re 1 μPa2 s) 8–10 dB higher than the shorter pulses (1 or 2 cycles; 28, 30 μs). Consequently, hearing thresholds for longer pulses were only slightly more sensitive than short pulse thresholds when stimuli were calculated using energy flux density. Because energy is important for hearing, providing thresholds in energy flux density may yield more biologically accurate thresholds for brief sounds (Fig. 8b). For a porpoise echolocating in the wild, longer duration pulses and echoes may improve signal detection and be an important adaptation for animals producing pulses with lower peak-to-peak source levels (Li et al. 2006).
The shorter pulses also had much broader bandwidth including frequencies for which the porpoise is not as sensitive. The 1 and 2 cycle pulses (28 and 30 μs) of 105 dB had −10 dB bandwidths of near 80 kHz whereas 9 and 10 cycle pulses (84 and 91 us) were 26–30 kHz. For our longer, higher-frequency pulses, more energy was concentrated in regions of best sensitivity (near 100 kHz) and consequently evoked potential results demonstrated lower hearing thresholds. Conversely, these porpoises may not be well adapted to hear broadband pulses with significant lower-frequency energy (as in some dolphin clicks) (Au 1993). Specializing to primarily detect high-frequency pulses suggests that typical mammalian high-frequency hearing loss may be particularly detrimental to finless porpoise hearing and echolocation abilities. Population level hearing measurements would be valuable to evaluate natural hearing loss trends and better understand finless porpoise auditory biology.
Finally, amplitudes of evoked potential waveforms differed for the various pulse durations (Fig. 9). Lower AEPp-p amplitudes at certain supra-threshold sound levels did not coincide with higher thresholds. While the amplitude variation may be random variation, they might also reflect actual differences based on pulse characteristics. It could be that the slopes of the AEP amplitude-versus-sound levels are different among the pulses with different cycles and duration. In terrestrial mammals, stimulus duration contributes to AEP amplitudes up to a certain saturation point (Skinner and Jones 1968; Ostroff et al. 2003). This may have been the case here for the shortest pulses, as amplitude increased then leveled off. For the longer pulses, amplitudes actually decreased with duration. This may be a reflection of the narrowing of the pulse bandwidth. In dolphins, narrower band stimuli produce decreased AEP amplitude responses, especially at higher frequencies (Popov and Supin 2001).
The finless porpoise hearing range does not provide them substantial advancements in auditory temporal processing compared to other odontocetes. Their high frequency hearing is sensitive but comparable to other odontocetes, perhaps resulting in similar temporal resolution abilities. In addition, odontocete high frequency filters may be broad enough that bandwidth does not constrain temporal resolution and other factors such as neural refractory periods and adaption limit resolution. In either case, following sounds well at rates up to 1,250/s with a temporal resolution shorter than 1 ms may be sufficient for echolocating porpoises because their inter-click-intervals are not less than several ms, varying typically between 10 and 80 ms (Akamatsu et al. 2007).
Because more sensitive hearing abilities were found using longer duration, high-frequency pulses, it seems that both signal duration and perhaps the center frequency component are vital to detection of short sounds in the present subjects. The finless porpoise hearing may better detect their longer, narrower pulses. It is uncertain whether this is also the case for delphinids which produce shorter duration, broader band clicks. It has been shown that T. truncatus detects short, broadband echoes at slightly lower energy levels relative to longer tones (Au et al. 2002). However, this behavioral study used repeating, complex echoes of 500 μs duration. We suggest detection of single pulses be further explored in delphinids and other porpoises to compare how differing odontocete species detect and process temporally varied sounds.
Abbreviations
- AEP:
-
Auditory evoked potential
- FFT:
-
Fast Fourier transform
- MRTF:
-
Modulation rate transfer function
- pe rms:
-
Peak-equivalent root-mean square
- p-p:
-
Peak-to-peak
References
Akamatsu T, Teilmann J, Miller LA, Tougaard J, Dietz R, Wang D, Wang K, Siebert U, Naito Y (2007) Comparison of echolocation behaviour between coastal and riverine porpoises. Deep Sea Res II 54:290–297. doi:10.1016/j.dsr2.2006.11.006
Au WWL (1993) The sonar of dolphins. Springer, New York
Au WWL, Lemonds DW, Vlachos S, Nachtigall PE, Roitblat HL (2002) Atlantic bottlenose dolphin (Tursiops truncatus) hearing thresholds for brief broadband signals. J Comp Psychol 116:151–157
Au WWL, Moore PWB, Pawloski DA (1988) Detection of complex echoes in noise by an echolocating dolphin. J Acoust Soc Am 83:662–668. doi:10.1121/1.396161
Bullock TH, Grinnell AD, Ikezono F, Kameda K, Katsuki Y, Nomoto M, Sato O, Suga N, Yanagisava K (1968) Electrophysiological studies of the central auditory mechanisms in cetaceans. Z Vergl Physiol 59:117–156
Cook MLH, Verela RA, Goldstein JD, McCulloch SD, Bossart GD, Finneran JJ, Houser DS, Mann DA (2006) Beaked whale auditory evoked potential hearing measurements. J Comp Physiol A 192:489–495. doi:10.1007/s00359-005-0086-1
Finneran JJ, Houser DS (2006) Comparison of in-air evoked potential and underwater behavioral hearing thresholds in four bottlenose dolphins (Tursiops truncatus). J Acoust Soc Am 119:3181–3192. doi:10.1121/1.2180208
Finneran JJ, London HR, Houser DS (2007) Modulation rate transfer functions in bottlenose dolphins (Tursiops truncatus) with normal hearing and high-frequency hearing loss. J Comp Physiol A 193:835–843. doi:10.1007/s00359-007-0238-6
Johnson CS (1966) Auditory thresholds of the bottlenosed porpoise (Tursiops truncatus, Montagu). U.S. Naval Ordnance Test Station, China Lake
Johnson CS (1968) Relation between absolute threshold and duration-of-tone pulses in the bottlenosed porpoise. J Acoust Soc Am 43:757–763. doi:10.1121/1.1910893
Kastelein RA, Bunskoek P, Hagedoorn M, Au WWL, de Haan D (2002) Audiogram of a harbor porpoise (Phocoena phocoena) measured with narrow-band frequency-modulated signals. J Acoust Soc Am 112:334–344. doi:10.1121/1.1480835
Klishin VO, Popov VV, Supin AY (2000) Hearing capabilities of a beluga whale, Delphinapterus leucas. Aquat Mamm 26:212–228
Li S, Wang D, Wang K, Akamatsu T (2006) Sonar gain control in echolocating finless porpoises (Neophocaena phocaenoides) in an open water (L). J Acoust Soc Am 120:1803–1806. doi:10.1121/1.2335674
Li S, Wang K, Wang D, Akamatsu T (2005) Echolocation signals of the free-ranging Yangtze finless porpoise (Neophocaena phocaenoides asiaeorientalis). J Acoust Soc Am 117:3288–3296. doi:10.1121/1.1882945
Madsen PT (2005) Marine mammals and noise: Problems with root mean square sound pressure levels for transients. J Acoust Soc Am 117:3957–3962. doi:10.1121/1.1921508
Madsen PT, Wahlberg M (2007) Recording and quantification of ultrasonic echolocation clicks from free-ranging toothed whales. Deep Sea Res I 54:1421–1444. doi:10.1016/j.dsr.2007.04.020
Mooney TA, Nachtigall PE, Taylor KA, Miller LA, Rasmussen M (2009) Comparative auditory temporal resolution of the white-beaked dolphin (Lagenorhynchus albirostris). J Comp Physiol A 195:375–384. doi:10.1007/s00359-009-0415-x
Mooney TA, Nachtigall PE, Yuen MML (2006) Temporal resolution of the Risso’s dolphin, Grampus griseus, auditory system. J Comp Physiol A 192:373–380. doi:10.1007/s00359-005-0075-4
Moore PWB, Hall RW, Friedl WA, Nachtigall PE (1984) The critical interval in dolphin echlocation: what is it? J Acoust Soc Am 76:314–317
Mulsow J, Reichmuth CJ (2007) Electrophysiological assessment of temporal resolution in pinnipeds. Aquat Mamm 33:122–131
Nachtigall PE, Mooney TA, Taylor KA, Miller LA, Rasmussen M, Akamatsu T, Teilmann J, Linnenschidt M, Vikingsson GA (2008) Shipboard measurements of the hearing of the white-beaked dolphin, Lagenorynchus albirostris. J Exp Biol 211:642–647
Nachtigall PE, Mooney TA, Taylor KA, Yuen MML (2007) Hearing and auditory evoked potential methods applied to odontocete cetaceans. Aquat Mamm 33:6–13. doi:10.1578/AM.33.1.2007.6
Ostroff J, McDonald K, Schneider B, Alain C (2003) Aging and the processing of sound duration in human auditory cortex. Hear Res 181:1–7. doi:10.1016/S0378-5955(03)00113-8
Popov VV, Supin AY (2001) Contribution of various frequency bands to ABR in dolphins. Hear Res 151:250–260. doi:10.1016/S0378-5955(00)00234-3
Popov VV, Supin AY, Klishin VO (1996) Frequency tuning curves of the dolphin’s hearing: envelope-following response study. J Comp Physiol A 178:571–578. doi:10.1007/BF00190187
Popov VV, Supin AY, Wang D, Wang K (2006) Nonconstant quality of auditory filters in the porpoises, Phocoena phocoena, and Neophocaena phocaenoides (Cetacea, Phocoenidae). J Acoust Soc Am 119:3173–3180. doi:10.1121/1.2184290
Popov VV, Supin AY, Wang D, Wang K, Xiao J, Li S (2005) Evoked-potential audiogram of the Yangtze finless porpoise Neophocaena phocaenoides asiaeorientalis (L). J Acoust Soc Am 117:2728–2731. doi:10.1121/1.1880712
Skinner PH, Jones HC (1968) Effects of signal duration and rise time on the auditory evoked potential. J Speech Hear Res 11:301–306
Supin AY, Popov VV (1995) Envelope-following response and modulation rate transfer function in the dolphin’s auditory system. Hear Res 92:38–45. doi:10.1016/0378-5955(95)00194-8
Supin AY, Popov VV, Mass AM (2001) The sensory physiology of aquatic mammals. Kluwer, Boston
Szymanski MD, Supin AY, Bain DE, Henry KR (1998) Killer whale auditory evoked potentials to rhythmic clicks. Mar Mamm Sci 14:676–691. doi:10.1111/j.1748-7692.1998.tb00756.x
Urick RJ (1983) Principles of underwater sound. Mc-Graw-Hill, New York
Vermeister NF (1979) Temporal modulation transfer functions based on modulation thresholds. J Acoust Soc Am 66:1364–1380. doi:10.1121/1.383531
Viemeister NF, Plack CJ (1993) Time analysis. In: Yost WA, Popper AN, Fay RR (eds) Human Psychophysics. Springer, New York, pp 116–154
Yuen MML, Nachtigall PE, Breese M, Supin AY (2005) Behavioral and auditory evoked potential audiograms of a false killer whale (Pseudorca crassidens). J Acoust Soc Am 118:2688–2695. doi:10.1121/1.2010350
Acknowledgments
The authors are grateful to the staff and students at the Baiji Aquarium, Institute of Hydrobiology, for their assistance in data collection and travels in China. We appreciate the advice, encouragement, and support from Alexander Supin, Vladimir Popov, Scott Cramer, Julie Arruda, Maya Yamato and Paul Nachtigall. The comments of two excellent reviewers greatly improved this manuscript. The work was supported by the Office of Naval Research, a WHOI Mellon Joint Initiatives Award, the Chinese National Natural Science Foundation (grant No: 30730018) and the Institute of Hydrobiology of the Chinese Academy of Sciences, and we thank them all for their contribution. The care and use of the animals was performed under China’s Wildlife Protection Act, 1989, Implementation Bylaw on Aquatic Wildlife Conservation, and the Woods Hole Oceanographic Institution’s Animal Care and Utilization Committee (protocol number DRK #3).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mooney, T.A., Li, S., Ketten, D.R. et al. Auditory temporal resolution and evoked responses to pulsed sounds for the Yangtze finless porpoises (Neophocaena phocaenoides asiaeorientalis). J Comp Physiol A 197, 1149–1158 (2011). https://doi.org/10.1007/s00359-011-0677-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00359-011-0677-y