Abstract
This review presents the fundamentals of atomic force microscopy (AFM) with microcantilever probes and their use as fluidic sensors for the measurement of micro/nanoscale transport properties. Over the last two decades, AFM has been widely used for, among other purposes, nanoscale topography, nanomechanical characterization, and intermolecular force spectroscopy. Furthermore, a microcantilever, an essential part of AFM, has been modified and exploited as a mechanical transducer for various sensing applications. Among many prospective uses, there appears to be great potential for micro/nanoscale sensing of fluid density and viscosity (Sect. 3.1), temperature (Sect. 3.2), pressure (Sect. 3.3), and flow velocity (Sect. 3.4). These micro/nanomechanical measurement techniques are expected to complement the advanced optical and electrical measurement techniques currently employed for micro/nanoscale fluidic sensors and also to fill the gap between microscale and nanoscale fluidic transport property measurements.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
An atomic force microscope (AFM), first developed by Binnig, Quate, and Gerber in 1986, is a scanning microscope that uses a sharp probe tip attached at the end of a microcantilever (Fig. 1). A microcantilever is a micrometer-sized single clamped suspended beam. The tip, which has a nanometric radius, scans across a specimen to map the surface nanotopography by monitoring interatomic forces between the probe tip and the specimen (Binnig et al. 1986). The interatomic forces between the probe tip and the specimen are detected via the deflection or the resonance frequency shift of the microcantilever (Gould et al. 1990; Prater et al. 1990; Rugar and Hansma 1990; Albrecht et al. 1991; Sarid 1994). These forces are then used as a form of feedback to control the z-position of a microcantilever and to record the surface nanotopography or to probe the surface nanomechanical characteristics of samples.
Compared with conventional optical microscopes, the nanoscale scanning tip of the AFM dramatically improves spatial resolutions in surface topographical imaging to sub-nanometer scale resolution for both conducting and non-conducting objects (Binnig and Rohrer 1999; Giessibl 2003; Giessibl and Quate 2006; Gross et al. 2009). In addition, AFM also allows local intermolecular force detection below the pico-Newton level with a high spatial resolution, thus opening new realms of force spectroscopy in biological substances (Heinz and Hoh 1999; Poggi et al. 2004).
A general AFM system consists of a microcantilever with a sharp probe tip; a piezoelectric scanner that controls the x–y–z movements of a sample with sub-nanometer accuracy; a laser and a position-sensitive photodetector that measures the light deflection and the frequency response of a microcantilever; feedback electronics to control the z-position of a sample; and a computer for electronic controls, data acquisition, display, and analysis. The deflection and the frequency response of the microcantilever are measured by reflecting a laser beam off the back of the cantilever and acquiring optical signals with a position-sensitive detector while the sharp probe tip is scanning the sample surface. The detected signals are sent to the feedback electronics circuit to maintain either a constant cantilever deflection for constant-force mode scanning, or a constant cantilever vibration amplitude for non-contact or tapping mode scanning (Albrecht et al. 1991; Zhong et al. 1993; Hansma et al. 1994; Anselmetti et al. 1994; Giessibl 1995). The corresponding x–y–z response of the piezoelectric scanner to the feedback signal generates ongoing surface topographic information (Meyer and Amer 1988; Alexander et al. 1989).
Beyond this conventional capability of AFM, various operation modes of AFM have been developed for their novel characterization purposes. Magnetization patterns in thin films have been revealed with a magnetized probe tip (Martin and Wickramasinghe 1987), and localized charges on an insulator surface have been imaged with a conducting probe tip (Stern et al. 1988). Surface elasticities of various samples have been measured (Burnham and Colton 1989; Maivald et al. 1991; Domke and Radmacher 1998; Chizhik et al. 1998; Dimitriadis et al. 2002) and the implications of this property for the response of biological entities have been extensively investigated (Pelham and Wang 1997; Wong et al. 2003; Discher et al. 2005; Engler et al. 2006; Kim et al. 2009b). The thermal conductivity variations of surfaces have been mapped (Nonnenmacher and Wickramasinghe 1992; Roh et al. 2006a, b), and the temperature variations of surfaces of electronic devices and interconnects have been imaged with a microfabricated thermocouple probe tip on a nanometer scale (Majumdar et al. 1993; Shi et al. 2000; Kim et al. 2008). Recently, nanoscale-resolution images of internal substructure of diverse materials have been acquired by combining an acoustic microscopy technique with AFM (Shekhawat and Dravid 2005; Tetard et al. 2008).
Meanwhile, a microcantilever, an essential part of AFM, has been modified and exploited as a mechanical transducer for various sensing applications (Butt 1996; Thundat et al. 1997; Berger et al. 1997; Pinnaduwage et al. 2005; Hansen and Thundat 2005). A microcantilever, as noted earlier, is a micrometer-sized single clamped suspended beam. Typical dimensions of microcantilevers are an order of magnitude smaller than the others, approximately 100-μm long, 10-μm wide and 1-μm thick. Advanced microfabrication techniques make it possible to fabricate very precise and reliable microcantilevers at low cost. In fact, the development of microcantilevers for AFM has been a major driving force for the fabrication of precise and reliable microcantilevers in various shapes and sizes. These microcantilevers have been used for various scanning probe microscopes (SPM) and diverse microelectromechanical systems (MEMS), including sensors and actuators, for over two decades. They have been exploited as mechanical transducers for biological, chemical, and physical sensing applications in various previous studies (Gimzewski et al. 1994; Boisen et al. 2000; Raiteri et al. 2001; Lang et al. 2002, 2005 Ziegler 2004; Lavrik et al. 2004).
The dynamic frequency responses and the static deflection of a microcantilever are two main transduction mechanisms for microcantilever sensors. The operation modes of microcantilever sensors are classified with these two transduction mechanisms. In the dynamic mode, the microcantilever’s peak resonance frequency, quality factor, amplitude, and phase are simultaneously measured. In the static mode, the deflections of the microcantilever are precisely measured for sensing applications. These frequency responses and deflections of a microcantilever can be measured by various readout techniques, including optical lever (Meyer and Amer 1988), piezoresistive (Tortonese et al. 1993), piezoelectric (Fujii et al. 1995; Lee and White 1996; Lee et al. 2002), embedded metal oxide semiconductor field-effect transistor (MOSFET) (Shekhawat et al. 2006), capacitance (Blanc et al. 1996; Britton et al. 2000), and electron tunneling (Binnig et al. 1986) methods with adequate precision.
Two of the most important features of microcantilever sensors are their sensitivity and specificity. Their high sensitivity is achieved by the miniaturization and optimization of the cantilever design, and the specificity is realized by the coatings of sensing materials on the microcantilever surface for particular types of physical, biological or chemical sensing applications (Carrascosa et al. 2006; Wang et al. 2007; Singamaneni et al. 2008; Senesac and Thundat 2008).
The field of micro- and nanoscale thermofluidics has recently attracted much attention with the marked progress of MEMS technology and its applications in the micro total analysis system (μTAS), Lab-on-a-Chip, and BioMEMS (Yosida 2005; Waggoner and Craighead 2007; Squires et al. 2008; Kim et al. 2009a). In order to understand micro/nanoscale fluidics and energy transport phenomena, it is essential to measure micro/nanoscale transport properties of fluids, especially their viscosity, density, temperature, pressure, and velocity, with high sensitivity. The main focus of this review, therefore, will be placed on the working principles of microcantilever sensors and their applications for micro/nanoscale transport property measurements in fluids.
2 Working principles of microcantilever sensors
Microcantilevers are basically physical sensors that interact with surrounding environments. The dynamic frequency response and the static deflection of a microcantilever are two main transduction mechanisms for microcantilever sensors. The dynamic mode of operation is very similar to that of other gravimetric sensors, such as quartz crystal microbalance (QCM) and surface acoustic wave (SAW) transducers (O’Sullivan and Guilbault 1999; Marx 2003; Ballantine et al. 1997). Figure 2 shows a schematic of a microcantilever mass sensor. In this mode of operation, the resonance frequency of a microcantilever f is measured before and after end tip mass loading and the added mass δm is calculated according to a simple approximate equation (Cleveland et al. 1993):
where k is the spring constant of a microcantilever, f 1 is the resonance frequency with the added mass, and f 0 is the initial resonance frequency of a microcantilever before mass addition. Recently, more rigorous analytical model for one-dimensionally distributed mass on cantilever was presented and experimentally validated (Ramos et al. 2006, 2007, 2008; Tamayo et al. 2006; Lee et al. 2009).
A schematic drawing of a microcantilever mass sensor. (Berger et al. 1997)
Mass detection using the cantilever resonance frequency, i.e. Eq. 1, is fairly well suited for measuring mass in a vacuum or in air. When operated in liquid, however, its mass resolution is very poor and the simple approximate Eq. 1 breaks down with large discrepancies. Thus, various attempts to derive more comprehensive analytical models for the cantilever resonance in liquids have been proposed by several research groups (Inaba et al. 1993; Chen et al. 1994; Hosaka et al. 1995; Oden et al. 1996; Salapaka et al. 1997; Elmer and Dreier 1997). Recently, Sader successfully presented a comprehensive theoretical model to predict the frequency response of cantilever beams immersed in viscous fluids (Sader 1998). In his model, the Euler–Bernoulli beam equation with external loading function was solved simultaneously with the Navier–Stokes equation for the velocity field of the surrounding fluid. The model rigorously accounts for both dissipative and inertial effects in the fluid, and is therefore capable of making detailed predictions of the thermal frequency spectra of microcantilevers in viscous fluids.
The peak resonance response frequency and the quality factor of a microcantilever are two main dynamic characteristics that are very sensitive to the density and viscosity of the surrounding fluid (Bergaud et al. 1999; Chon et al. 2000; Bergaud and Nicu 2000; Patois et al. 2000; Shih et al. 2001; Ahmed et al. 2001). Therefore, the microscale viscosity and density of a fluid can be determined by analyzing the frequency response of a cantilever in the fluid, as shown in Fig. 3 (Boskovic et al. 2002). Green and Sader recently modified their previous theoretical models to predict the thermally driven frequency response of cantilever beams immersed in viscous fluids near a solid surface (Green and Sader 2005). Their model includes both inertial and dissipative effects in fluid to account for the variations of frequency responses observed when a cantilever is brought close to a solid surface. This study elucidated the basic mechanisms of the broadening of the resonance peaks and the lowering of the peak resonance frequency as the microcantilever beam/surface separation distance decreased. The study also laid a theoretical foundation for the analysis of frequency responses of cantilever beams in atomic force microscopy.
Schematic illustrating the technique of using a microcantilever in rheological measurements of fluids. (Boskovic et al. 2002)
In order to examine the comprehensive effect of temperature on the frequency response of a microcantilever immersed in a fluid, Sader’s model was extended to individually account for the fluid viscosity η(T), density ρ(T), and the Young’s modulus of the microcantilever E(T), and the extended model was experimentally validated (Kim and Kihm 2006, 2007b). Based on this extension, microscale temperature measurement in aqueous medium was achieved by analyzing the frequency response of a microcantilever (Kim et al. 2007).
Although successful measurements of microscale viscosity, density, and temperature of a simple fluid have been achieved by analyzing the frequency response of a cantilever, the interpretation of the measured frequency response becomes more complicated when we are dealing with complex chemical and biological fluids. Both flexural rigidity changes due to the adsorbate and adsorption-induced surface stress and added mass due to the adsorbate and surface condition change should be additionally considered in order to correctly analyze the measured frequency response of a microcantilever in viscous fluids (Thundat et al. 1994; Chen et al. 1995; Cherian and Thundat 2002; Lu et al. 2005; Tamayo et al. 2006; Kwon et al. 2007; Lachut and Sader 2007; Kim and Kihm 2008).
Assuming evenly distributed adsorbate on the microcantilever surface, the governing Euler–Bernoulli beam equation for the dynamic deflection function w(x,t) of the cantilever is modified to the following:
where E is Young’s modulus, I is the moment of inertia of the cantilever, δEI is the flexural rigidity change of the cantilever due to adsorbate and adsorption-induced surface stress, μ is the mass per unit length of the cantilever, δμ is the adsorbate mass per unit length of the cantilever, F is the external applied force per unit length, x is the spatial coordinate along the length of the cantilever, and t is time. However, the variation of the hydrodynamic loading due to the adsorption of biological molecules on the cantilever surface in biological fluid needs to be properly modeled and combined in the externally applied force function F(x,t) for accurate determination of adsorbed molecular mass (Kwon et al. 2007; Rijal and Mutharasan 2007).
In addition to the aforementioned microscale transport property measurements in fluids, the measurement of absolute gas pressure over a range of six decades, from the atmospheric pressure of 105 Pa down to a mere 10−1 Pa, was accomplished using a microcantilever sensor by monitoring the shift of both the resonance frequency and the quality factor according to the variations in ambient damping (Blom et al. 1992; Hane et al. 1992; Kumazaki et al. 1996; Yasumura et al. 2000; Mertens et al. 2003; Sandberg et al. 2005; Bianco et al. 2006; Keskar et al. 2008). In the molecular region where absolute pressure is between 10−1 Pa and 102 Pa, the ambient damping is caused by independent collisions of non-interacting gas molecules with the moving surface of the vibrating microcantilever beam. In this case, the drag force can be determined with the kinetic theory of gases and the pressure dependence of the quality factor can be evaluated using Christian’s model (Christian 1966) for a rectangular cantilever beam in free space.
For the first resonance mode, the pressure dependent quality factor Q is expressed as
where ρ c is the mass density of the cantilever, h is the thickness of the cantilever, ω 0 is the undamped fundamental angular resonance frequency of the cantilever, R is the universal gas constant, T is the absolute temperature, M is the molar mass of the gas, and p is the pressure. Here, the undamped fundamental angular resonance frequency ω 0 can be evaluated as
where L is the length of the cantilever.
In the viscous region where absolute pressure ranges between 102 Pa and 105 Pa, the gas acts as a viscous fluid and the ambient damping can be calculated using fluid mechanics. In this case, both the resonance frequency and the quality factor of a microcantilever can be expressed as functions of pressure (Hosaka et al. 1995; Bianco et al. 2006). The normalized fundamental angular resonance frequency shift is given as
where b is the width of a cantilever and η is the dynamic viscosity of gas. The pressure-dependent quality factor Q is expressed as
Thus, the combination of information from the resonance frequency and quality factor of a microcantilever, from Eqs. 3, 4, 5 and 6, enables us to obtain absolute pressure measurements over a wide range of pressures.
The micro/nanoscale fluid velocity can be measured with the static deflection of a microcantilever by means of the fluid drag force (Gass et al. 1993a, b; Nishimoto et al. 1994; Su et al. 1996, 2002; Barth et al. 2005; Quist et al. 2006; Piorek et al. 2006). Assuming a rectangular microcantilever with dimensions L × b × h (lengthxwidthxthickness) and that the fluid flow is normal to the cantilever surface with a high speed (i.e. high Reynolds number, Re > 1,000), the drag force F d is given as
where ρ is the mass density of the fluid, C d is the drag coefficient, and v is the mean fluid velocity normal to the cantilever surface. Then the deflection of the microcantilever d is given by
where the drag coefficient is taken to be independent of the Reynolds number due to the small dimensions and the very sharp edges of the cantilever (Barth et al. 2005).
When the fluid velocity is very low or the characteristic length is very small (i.e. low Reynolds number, Re ≪ 1), the drag force is approximately proportional to the velocity. For the special case of small spherical objects (Stokes flow), the drag force F d is given as
where r is the radius of the sphere and V denotes the flow speed. This linear relationship between the drag force and velocity is the general basis for micro/nanoscale fluid velocity measurements. Although there is no simple analytical equation for the drag force of a microcantilever, the fluid velocity still can be correlated with the deflection of a microcantilever.
3 Micro/nanoscale transport property measurements
3.1 Fluid density and viscosity measurements
Conventional viscometers and densimeters have been successfully developed over the past few decades to measure the viscosity and density of fluids over a wide range of thermodynamic states with high precision (Tropea et al. 2007). In contrast to traditional rheometers, which demand large sample volumes and probing areas, the microcantilever sensors are capable of rapid and in situ measurements requiring only minute amounts of fluids (<1 nL) and small probing areas. The working principle is analogous to the vibrating-wire viscometer (Tough et al. 1964; Assael et al. 1991; Wilhelm and Vogel 2001; da Mata et al. 2001) or the vibrating-wire or -tube densitometer (Dix et al. 1991; Padua et al. 1994; Blencoe et al. 1996; Hynek et al. 1997; Padua et al. 1998; Audonnet and Padua 2001). However, the rapid and in situ micro/nanoscale measurement capabilities, unlike the traditional rheometers, are realized by the innovative microcantilever sensors, contributing to the state-of-the-art micro/nanofabrication technology and the enhanced understanding of the complicated frequency response of a microcantilever in fluids (Sader 1998; Kirstein et al. 1998; Chon et al. 2000; Boskovic et al. 2002; Green and Sader 2005; Van Eysden and Sader 2006; Van Eysden and Sader 2007; Van Eysden and Sader 2009).
Inaba et al. rigorously investigated the frequency response of cantilevers in millimeter sizes and suggested liquid density sensing by using a photothermal vibration (Inaba and Hane 1992; Inaba et al. 1992). They concluded that the resonance frequency shift was caused mainly not by viscosity drag but by the sound radiation, which depends on the liquid density. This conclusion is in line with the Chu’s report and his inviscid fluid model for a macroscopic rectangular cantilever beam (Chu 1963; Lindholm et al. 1965). With Chu’s model, the fluid density ρ is determined from
where f vac and f fluid are the fundamental resonance frequencies of the cantilever in vacuum and fluid, respectively. Fairly good accuracy was demonstrated in comparison with experimental measurements.
In addition to the liquid density sensing, the possibility of liquid viscosity sensing was also shown by relating the half-power width of a peak resonance frequency spectrum to the liquid viscosity (Inaba et al. 1993). In parallel to these efforts, effects of the viscosity and density of a fluid on the dynamic behavior of microcantilevers have been also investigated by other groups. They developed a forced one-dimensional simple harmonic oscillator (SHO) model to delineate the effects of fluid properties on the resonance frequency (Butt et al. 1993; Chen et al. 1994; Roters and Johannsmann 1996).
Figure 4 shows the resonance responses of a microcantilever in various mixtures of glycerol/water (mass percentage of glycerol to water) as well as in air. A wide range of the viscosity and density of fluids could be determined from the resonance response of microcantilevers by using the simple harmonic oscillator model and its modifications (Oden et al. 1996; Thundat et al. 1997; Patois et al. 2000; Shih et al. 2001; Ahmed et al. 2001). In these cases, however, unknown geometrical factors had to be determined by fitting the cantilever response data. Therefore, the determination of the viscosity and density of a fluid is not straightforward and these models are not readily applicable to highly viscous fluids.
Resonance response of a microcantilever in various mixtures of glycerol/water (mass percentage of glycerol to water) as well as in air. (Oden et al. 1996)
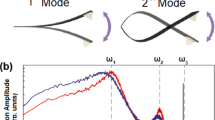
Sader successfully presented more comprehensive models that rigorously accounted for true geometry of the cantilever and laid the foundation for straightforward evaluation of the viscosity and density of arbitrary fluids by analyzing the frequency response of a microcantilever without any fitting parameters (Sader 1998). Figure 5a shows that the Viscous Model (Sader 1998) predicts well and shows fairly good agreement with the experimental data for all tested Reynolds number ranges, while the Inviscid Model (Chu 1963) shows excessive deviations that grow with decreasing Reynolds numbers. The Simple Harmonic Oscillator (SHO) model (Chon et al. 2000) derived in the limit of small dissipative effects, i.e., when the quality factor greatly exceeds unity, on the other hand as shown in Fig. 5b, agrees well with the Viscous Model and experimental data with the exception occurring at low Reynolds number due to the violation of small dissipative effects assumption.
Plot of f theory/f exp (f is the resonant frequency) where theory and exp refer to results obtained theoretically and experimentally, for modes 1 and 2 of all the practical cantilevers immersed in the four liquids. Results plotted as a function of the Reynolds number Re evaluated at the experimental resonant frequencies in liquid, for a the viscous and inviscid models, b the SHO and inviscid models. The straight line indicates exact correspondence between theory and experiment. (Chon et al. 2000)
The basic assumptions and limitations of Chu’s inviscid model, SHO model, and Sader’s viscous model have been thoroughly discussed and verified by various research groups (Sader 1998; Chon et al. 2000; Bergaud and Nicu 2000; Kim and Kihm 2006), and rheological measurements using microcantilevers have been successfully demonstrated with several different gases (air, CO2, Ar, He, N2) and liquids (acetone, CCl4, water, 1-butanol) (Boskovic et al. 2002). Recently, an extended Sader’s model for higher vibrational modes was presented (Van Eysden and Sader 2007) and was applied to find comprehensive characterization of Newtonian fluids (Ghatkesar et al. 2008). Microscale viscosity and density variation near a solid surface also could be estimated by the progress of theoretical models that describe the dynamics of AFM microcantilever vibration in viscous fluids near a solid surface (Naik et al. 2003; Green and Sader 2005; Clarke et al. 2006).
Local concentration measurements of a binary mixture solution are one of the simple applications of microscale viscosity and density determination with a microcantilever. The resonance frequency of a microcantilever can be correlated with the concentration of glycerol, sucrose, ethanol, sodium chloride, polyethylene glycol, or bovine plasma albumin in aqueous solution (Ahmed et al. 2001). However, as mentioned earlier, both flexural rigidity change due to the adsorbate and adsorption-induced surface stress and added mass due to the adsorbate and surface condition change should be comprehensively considered in order to correctly analyze the measured frequency response of a microcantilever to determine local concentrations (Kim and Kihm 2008). Figure 6 shows the resonance frequency shifts from the reference frequency as a function of NaCl concentration in de-ionized water. Each symbol represents the averaged frequency shift of 10 individual measurements, with the error bar corresponding to a 95% confidence interval. The solid curve shows the calculated frequency shift based on Sader’s viscous model (Sader 1998) that assumes both flexural rigidity and added mass changes to be zero. Thus, the discrepancies between the experimental data and the theoretical predictions imply the need for a careful calibration or more comprehensive model in order to correctly determine the mixture concentration.
In contrast to the previous approaches that used an immersed cantilever in fluids, a vibrating-tube densitometer has been devised using a hollow microcantilever, also called a suspended microchannel resonator (SMR), for very precise and accurate liquid density measurements in a sub-nanoliter volume (Burg and Manalis 2003; Burg et al. 2006; Godin et al. 2007). Figure 7 shows an SMR that is essentially a microcantilever with a microfluidic channel fabricated inside the cantilever, and the dimensions of a microchannel define the sample volume. Since the liquid is inside the cantilever, which is vibrating in a vacuum chamber, very high quality factor can be achieved, and this enables very precise and accurate liquid density measurements (Burg et al. 2009). Using a similar device, weight measurements for biomolecular masses, such as a single cell and a single nanoparticle in liquid, have been demonstrated (Burg et al. 2007). Table 1 provides a summary of viscosity or density measurements with different cantilever configurations.
3.2 Fluid temperature measurements
Various approaches to micro/nanoscale thermometry techniques have been tried for over a decade, and a number of researchers have attempted to explore the limits of spatial and temporal measurement resolutions. The ratiometric laser-induced fluorescence (LIF) technique using fluorescent dye molecules in liquid has achieved microscale spatial measurement resolution, but the measurement uncertainties were excessive particularly in the near-wall or near-meniscus region because of the surface interference of the fluorescent emission light (Sakakibara and Adrian 1999; Kim et al. 2003). Similarly, the optical serial sectioning microscopy (OSSM) technique showed good potential as a microscale thermometry system for nanoparticle suspension fluids where the nanoparticle Brownian motion was related with the local temperature (Park et al. 2005). This technique, however, becomes less accurate as the solid surface is approached because of the near-wall hindered and biased Brownian motion (Park et al. 2005).
In addition to the excessive measurement uncertainties of these optical thermometry techniques, the presence of dye molecules or dye-coated nanospheres may alter the thermal characteristics of the tested fluid. To alleviate this shortcoming, which is associated with the foreign trace particles, a label-free, real-time, full-field surface plasmon resonance (SPR) reflectance sensing technique has been exploited to map microscale temperature distribution in the aqueous medium (Kim and Kihm 2007a). However, the detection range of this technique is confined within the extremely small penetration depth of the evanescent wave field, approximately an order of 100 nm from the solid surface.
Temperature dependence of the resonance frequency of microcantilevers in vacuum or in gaseous environments has been extensively studied theoretically as well as experimentally (Shen et al. 2001; Han et al. 2002; Mertens et al. 2003; Chua et al. 2004; Sandberg et al. 2005; Lee et al. 2008). Resonance frequency shifts of partially or fully gold-coated silicon microcantilevers, due to the mismatching of thermal expansion coefficients and temperature-dependent material properties, have been numerically investigated, and the temperature-dependent material properties were found to be the dominating factor for the resonance frequency shifts (Shen et al. 2001). A theoretical model for the temperature dependence of the fundamental resonance frequency of a bi-layer cantilever (silicon dioxide covered silicon) in air has been presented and compared with experimental results for the temperature range of 20–95°C (Han et al. 2002). The effect of temperature on the resonance frequency shifts of silicon nitride or silicon dioxide microcantilevers with or without gold coating in high vacuum has also been demonstrated (Mertens et al. 2003; Sandberg et al. 2005), and the resonance frequency shift of a diamond-like amorphous carbon cantilever during the annealing process has been shown as well (Chua et al. 2004).
Generally, the resonant frequency of silicon or silicon nitride cantilevers in air under atmospheric pressure decreases with increasing temperature. However, cantilevers made of silicon oxide or diamond-like amorphous carbon show that the resonant frequency increases with increasing temperature. In addition to the consideration of temperature-dependent material properties, variations of viscosity and density of surrounding fluid due to temperature change should be considered in order to correctly establish the relationship between the resonant frequency of a microcantilever and the surrounding fluid temperature. In addition to the effects of the cantilever materials and the fluidic properties, including viscosity and density, the resonance frequency of a microcantilever changes with the measurement location when approaching the solid surface (Kim and Kihm 2006, 2007b).
Figure 8 shows a measured resonance frequency shift for the case of an NSC12-C microcantilever (Mikromasch Inc.) immersed in de-ionized water with the error bars showing 95% confidence intervals. The curves represent the theoretical predictions by the extended Sader’s model. The net frequency shift, Δf = Δf η + Δf ρ + Δf E , comprises the individual contributions due to the liquid viscosity Δf η , to the liquid density Δf ρ , and to the Young’s modulus of cantilever Δf E . The dissipative effect due to the liquid viscosity, Δf η , is the dominating factor for the net frequency shifts of the entire range of tested temperatures. The inertial effect due to the liquid density Δf ρ gradually increases with temperature, but its maximum is approximately 15% of the net shift. The frequency shifts due to the Young’s modulus changes, Δf E , are negative because the stiffness of a gold-coated silicon cantilever decreases with increasing temperature. However, the effect is minimal compared to the previous two effects.
Figure 9 shows the resonant frequency data of a TL-NCH microcantilever (Nanosensors Inc.) immersed in de-ionized water as a function of specified temperatures at four different z-measurement locations. The inset schematic illustrates the experimental setup, which provides a well-controlled and steady temperature field for the fluid surrounding the sensor. Using the calibration data in Fig. 9, we measured the near-wall microscale temperature profiles for the probing site less than 40 μm from the solid surface, and the measurements were compared fairly well with the two-dimensional CFD calculation results as shown in Fig. 10 (Kim et al. 2007).
Experimental near-wall correlation of the microcantilever peak resonance frequency with aqueous medium temperature at four different separation distances (z = 5, 10, 20, and 40 μm) between the lower end of the cantilever and the glass substrate surface. (Kim et al. 2007)
Measured and calculated steady-state temperature profiles of an aqueous medium in the vicinity of the microheater surface at four different z-locations (5, 10, 20, and 40 μm) for each of three different heater temperatures (50, 70, or 90°C). Each symbol represents the average of ten data realization measurements, and the three curves show the calculated temperature profiles based on the two-dimensional CFD calculations. (Kim et al. 2007)
In addition, the static deflection of a microcantilever due to the bimetallic effect was also exploited to monitor liquid temperature in a small reaction chamber (50 μL volume). Marie et al. used a piezoresistive cantilever array sensor to monitor the temperature of a polymerase chain reaction (PCR) buffer solution during multiple temperature cycles ranging from 20 to 95°C and obtained the stable temperature signal as shown in Fig. 11 (Marie et al. 2003). A highly persistent correlation was observed between the output voltage variation (the plain line) and the surrounding temperature variation (the dashed line).
Temperature (dashed) and output voltage (plain) of the sensor during five typical PCR temperature cycles. The temperature is initially maintained at 95°C for 5 min and then for 1 min at 55, 72, and 95°C successively. The heating/cooling rate is constant at 0.2°C/s. (Marie et al. 2003)
3.3 Pressure measurements
Various pressure sensors have been developed, from a simple liquid manometer to a complex ionization pressure gauge, and each pressure sensor has its own merits as well as limitations (Dushman and Lafferty 1962). The vibration of an object has been widely utilized in the development of a miniaturized pressure gauge such as the quartz oscillator gauge (Christen 1983; Kokubun et al. 1984, 1985; Hirata et al. 1985; Ono et al. 1985, 1986). The effects of air or gas pressure on the vibration of miniature quartz tuning forks have been investigated, and the relative resonant frequency shift has been linearly correlated with the air pressure for the range from 0.1 bar up to a few bars (Christen 1983). Also, a pressure sensor using a small, solid, and stable quartz tuning fork oscillator has been studied for the pressure range from 10−4 to 103 Torr (Kokubun et al. 1984, 1985; Hirata et al. 1985; Ono et al. 1985, 1986). The frequency dependence of this oscillator on pressure was found to be negligible for the molecular region (below ~1 Torr), but the resonance impedance of this quartz oscillator was successfully exploited to measure gas pressure in the molecular region as well as in the viscous region (between 10−3 and 103 Torr).
Similarly, Inaba and Hane investigated the resonance frequency shifts of a photothermal vibration of millimeter-sized cantilevers in the pressure range of 10−4–760 Torr for air or rare gases (Inaba and Hane 1991a, 1991b). They showed that the resonance frequency of a cantilever decreased in the higher pressure region above ~100 Torr due to the viscous drag of the gas, but increased in the medium vacuum region between 10−3 and 1 Torr due to the static thermal stress of the cantilever. Meanwhile, Blom and coworkers studied the dependence of the quality factor (the ratio of the peak energy stored in the resonator to that of the energy dissipated per cycle) of millimeter-sized cantilevers on pressure and geometry and showed the potential of exploiting the quality factor to measure a wide range of gas pressure between 1 and 105 Pa (Blom et al. 1992).
Microscale pressure measurement was envisioned by the fabrication of microcantilevers and utilization of optical or electrical readout system for acquiring the frequency response of a microcantilever (Hane et al. 1992; Zook et al. 1992). The pressure dependence of the mechanical quality factor Q of various microcantilevers was rigorously investigated in the molecular region (Yasumura et al. 2000; Mertens et al. 2003). Attempts have been made to improve the theoretical model and to develop a molecular dynamics simulation code to provide more accurate quality factor predictions that will account for the additional damping effects caused by the nearby wall in the molecular region (Bao et al. 2002; Hutcherson and Ye 2004).
The pressure dependence of the resonance frequency of various microcantilevers has also been rigorously studied and found to be significant in the viscous region, but not in the molecular region (Mertens et al. 2003; Sandberg et al. 2005; Bianco et al. 2006). Figure 12 shows the variation of quality factors and resonance frequency shifts of four different microcantilevers as a function of N2 gas pressure (Mertens et al. 2003). These experimental data clearly suggest that the combined data from the resonance frequency and quality factor of a microcantilever enable us to obtain absolute pressure measurement over a wide range of gas pressures. Theoretical predictions from Eqs. 3, 4, 5 and 6 were also compared with experimental measurements and shown to be in fair agreement with experimental data (Bianco et al. 2006). More recently, a unified model of gas damping of flexible microcantilevers at a low ambient pressure region was presented and experimentally validated (Bidkar et al. 2009).
Pressure dependence of the quality factor and resonance frequency of four different microcantilevers as a function of N2 gas pressure. (Mertens et al. 2003)
In addition to the measurement of gas pressure, a method of gas composition analysis was proposed by measuring the resonance frequency shift of a microcantilever in the viscous region (Xu et al. 2006). As seen in Eq. 5, the resonance frequency shift is a function of the molar mass, dynamic viscosity, temperature as well as the pressure of a gas. By measuring the resonance frequency at specified temperature and pressure, it is possible to obtain the molar mass of an unknown gas as long as the effect of dynamic viscosity can be held down to be negligibly small. Figure 13 shows the resonance frequency shifts as functions of pressure for four different gases; the results clearly indicate the molar mass dependence on the resonance frequency shift (Xu et al. 2006).
The relative resonance frequency as a function of pressure from 10 to 760 Torr in helium (diamond), methane (open circle), nitrogen (triangle), and argon (square) environments. Inset: the relative resonance frequency shift of a resonator for the four gases. (Xu et al. 2006)
3.4 Flow velocity measurements
Micro/nanoscale fluid velocity measurements are a subject of great interest in building an understanding of microflows and the development of analytical microfluidic chips; a number of microscale flow visualization techniques have been developed (Sinton 2004; Lee and Kim 2009). Among them, the application of microscopic particle image velocimetry (PIV) is becoming a preeminent technique in determining the velocity distribution in liquid flows within microfluidic devices (Santiago et al. 1998; Meinhart et al. 1999; Cummings 2000; Zettner and Yoda 2003; Park et al. 2004; Liu et al. 2005). Although the micro PIV is the most powerful technique to date, the implementation of this method demands expensive optical components and software for data processing, and more importantly, the optical diffraction limits the spatial resolution to 0.5–1.0 μm even under ideal optical conditions.
On the other hand, the need for a simple flow sensor integrated on a chip has emerged with the applications of μTAS, Lab-on-a-Chip, and BioMEMS. A simple flow-rate sensor based on the drag force measurement of a millimeter-sized piezoresistive cantilever has been developed to regulate the liquid flow of a silicon micropump (Gass et al. 1993a, b, 1994; Nishimoto et al. 1994). The airflow velocity profile in a small steel pipe with an inner diameter of 7.0 mm has been directly measured with a piezoresistive microcantilever paddle based on normal pressure drag, and the minimum detectable airflow velocity was shown to be 7.0 cm/s (Su et al. 1996, 2002).
Figure 14 shows the measured air velocity distributions with the corresponding theoretical laminar flow velocity profiles (Su et al. 2002). This application showed the potential of direct fluid velocity measurement with the use of a microcantilever. Quist et al. demonstrated a highly sensitive piezoresistive microcantilever to measure flow properties in microfluidic channels and showed the dependence of cantilever deflection on the fluid viscosity and velocity (Quist et al. 2006). Mechler et al. exploited the nanoprobe “whisker” tip of a microcantilever to investigate the nanoscale velocity-drag force relationship in thin liquid layers (Mechler et al. 2004), and Piorek et al. used a torsional AFM velocimeter to successfully measure the fluid velocity with a submicron resolution (Piorek et al. 2006). Figure 15 shows the measured liquid velocity profile with 0.102 μm spatial resolution in a microchannel.
The experimental airflow velocity distributions in a small steel pipe with an inner diameter of 7.0 mm when the mean airflow velocity varies from 6.5 to 10.83 m/s and the corresponding Reynolds number Re ranges from 2,886 to 4,808. (Su et al. 2002)
Temporally averaged liquid flow velocity profile in a microchannel of dimensions of 1.5 mm long, 1.5 μm deep, and 15 μm wide. Open circles represent measured data with a best-fit parabola solid line. (Piorek et al. 2006)
A laser-cantilever anemometer based on the AFM optical lever technique was also developed for high-resolution velocimetry and specifically for turbulent flow measurements that require investigation of small-scale correlations (Barth et al. 2005). That study used microcantilevers with a typical length of 160 μm, a width of 30 μm, and a thickness of 1–3 μm as sensing elements, and the researchers presented their results as favorable, compared with hot-wire anemometer measurement results for both cases of air and water flows.
4 Conclusions and perspectives
The marked progress in MEMS/NEMS technology and its applications to micro total analysis system (μTAS), Lab-on-a-Chip, and BioMEMS/NEMS has demanded the development of a fundamental understanding of micro/nanoscale fluidics and energy transport phenomena. To reveal the basic physics of micro/nanoscale fluidics and energy transport phenomena, it is essential to measure micro/nanoscale transport properties of fluids such as viscosity, density, temperature, pressure, and velocity. It is anticipated that microcantilever sensors will provide real-time, in situ measurements of these physical properties of fluids in sufficiently fine spatial resolution. We have presented a comprehensive review of the working principles of AFM and its microcantilever sensors and their applications for micro/nanoscale transport property measurements in fluid flows. This micro/nanomechanical measurement technique is expected to complement the advanced optical and electrical measurement techniques currently employed for microscale thermofluidic sensors and also to fill the gap between microscale and nanoscale fluidic transport property measurements.
References
Ahmed N, Nino DF, Moy VT (2001) Measurement of solution viscosity by atomic force microscopy. Rev Sci Instrum 72:2731–2734
Albrecht TR, Grütter P, Horne D, Rugar D (1991) Frequency modulation detection using high-Q cantilevers for enhanced force microscope sensitivity. J Appl Phys 69:668–673
Alexander S, Hellemans L, Marti O, Schneir J, Elings V, Hansma PK, Longmire M, Gurley J (1989) An atomic-resolution atomic-force microscope implemented using an optical lever. J Appl Phys 65:164–167
Anselmetti D, Lüthi R, Meyer E, Richmond T, Dreier M, Frommer JE, Güntherodt HJ (1994) Attractive-mode imaging of biological materials with dynamic force microscopy. Nanotechnology 5:87–94
Assael MJ, Papadaki M, Dix M, Richardson SM, Wakeham WA (1991) An absolute vibrating-wire viscometer for liquids at high pressures. Int J Thermophys 12:231–244
Audonnet F, Padua AAH (2001) Simultaneous measurements of density and viscosity of n-pentane from 298 to 383 K and up to 100 MPa pressure using a vibrating-wire instrument. Fluid Phase Equilibr 181:147–161
Ballantine DS, White RM, Martin SJ, Ricco AJ, Zellers ET, Frye GC, Wohltjen H (1997) Acoustic wave sensors. Academic Press, San Diego
Bao M, Yang H, Yin H, Sun Y (2002) Energy transfer model for squeeze-film air damping in low vacuum. J Micromech Microeng 12:341–346
Barth S, Koch H, Kittel A, Peinke J, Burgold J, Wurmus H (2005) Laser-cantilever anemometer: a new high-resolution sensor for air and liquid flows. Rev Sci Instrum 76:075110
Bergaud C, Nicu L (2000) Viscosity measurements based on experimental investigations of composite cantilever beam eigenfrequencies in viscous media. Rev Sci Instrum 71:2487–2491
Bergaud C, Nicu L, Martinez A (1999) Multi-mode air damping analysis of composite cantilever beams. Jpn J Appl Phys 38:6521–6525
Berger R, Ch Gerber, Lang HP, Gimzewski JK (1997) Micromechanics: a toolbox for femtoscale science: “towards a laboratory on a tip”. Microelect Eng 35:373–379
Bianco S, Cocuzza M, Ferrero S, Giuri E, Piacenza G, Pirri CF, Ricci A, Scaltrito L, Bich D, Merialdo A, Schina P, Correale R (2006) Silicon resonant microcantilevers for absolute pressure measurement. J Vac Sci Technol B 24:1803–1809
Bidkar RA, Tung RC, Alexeenko AA, Sumali H, Raman A (2009) Unified theory of gas damping of flexible microcantilevers at low ambient pressures. Appl Phys Lett 94:163117
Binnig G, Rohrer H (1999) In touch with atom. Rev Mod Phys 71:S324–S330
Binnig G, Quate CF, Ch Gerber (1986) Atomic force microscope. Phys Rev Lett 56:930–933
Blanc M, Brugger J, de Rooji NF, Dürig U (1996) Scanning force microscopy in the dynamic mode using microfabricated capacitive sensors. J Vac Sci Technol B 14:901–905
Blencoe JG, Drummond SE, Seitz JC, Nesbitt BE (1996) A vibrating-tube densimeter for fluids at high pressures and temperatures. Int J Thermophys 17:179–190
Blom FR, Bouwstra S, Elwenspoek M, Fluitman JHJ (1992) Dependence of the quality factor of micromachined silicon beam resonators on pressure and geometry. J Vac Sci Technol B 10:19–26
Boisen A, Thaysen J, Jensenius H, Hansen O (2000) Environmental sensors based on micromachined cantilevers with integrated read-out. Ultramicroscopy 82:11–16
Boskovic S, Chon JWM, Mulvaney P, Sader JE (2002) Rheological measurements using microcantilevers. J Rheol 46:891–899
Britton CL Jr, Jones RL, Oden PI, Hu Z, Warmack RJ, Smith SF, Bryan WL, Rochelle JM (2000) Multiple-input microcantilever sensors. Ultramicroscopy 82:17–21
Burg TP, Manalis SR (2003) Suspended microchannel resonators for biomolecular detection. Appl Phys Lett 83:2698–2700
Burg TP, Mirza AR, Milovic N, Tsau CH, Popescu GA, Foster J, Manalis SR (2006) Vacuum-packaged suspended microchannel resonant mass sensor for biomolecular detection. J Microelectromech Syst 15:1466–1476
Burg TP, Godin M, Knudsen SM, Shen W, Carlson G, Foster J, Bobcock K, Manalis SR (2007) Weighing of biomolecules, single cells and single nanoparticles in fluid. Nature 446:1066–1069
Burg TP, Sader JE, Manalis SR (2009) Nonmonotonic energy dissipation in microfluidic resonators. Phys Rev Lett 102:228103
Burnham NA, Colton RJ (1989) Measuring the nanomechanical properties and surface forces of materials using an atomic force microscope. J Vac Sci Technol A 7:2906–2913
Butt HJ (1996) A sensitive method to measure changes in the surface stress of solids. J Colloid Interf Sci 180:251–260
Butt HJ, Siedle P, Seifert K, Seeger T, Fendler K, Bamberg E, Goldie K, Engel A (1993) Scan speed limit in atomic force microscopy. J Microsc 169:75–84
Carrascosa LG, Moreno M, Alvarez M, Lechuga LM (2006) Nanomechanical biosensors: a new sensing tool. Trends Anal Chem 25:196–206
Chen GY, Warmack RJ, Thundat T, Allison DP, Huang A (1994) Resonance response of scanning force microscopy cantilevers. Rev Sci Instrum 65:2532–2537
Chen GY, Thundat T, Wachter EA, Warmack RJ (1995) Adsorption-induced surface stress and its effects on resonance frequency of microcantilevers. J Appl Phys 77:3618–3622
Cherian S, Thundat T (2002) Determination of adsorption-induced variation in the spring constant of a microcantilever. Appl Phys Lett 80:2219–2221
Chizhik SA, Huang Z, Gorbunov VV, Myshkin NK, Tsukruk VV (1998) Micromechanical properties of elastic polymeric materials as probed by scanning force microscopy. Langmuir 14:2606–2609
Chon JWM, Mulvaney P, Sader JE (2000) Experimental validation of theoretical models for the frequency response of atomic force microscope cantilever beams immersed in fluids. J Appl Phys 87:3978–3988
Christen M (1983) Air and gas damping of quartz tuning forks. Sens Actuators 4:555–564
Christian RG (1966) The theory of oscillating-vane vacuum gauges. Vacuum 16:175–178
Chu WH (1963) Vibration of fully submerged cantilever plates in water. Technical report no. 2. DTMB, South-west Research Institute, San Antonio, Texas
Chua DHC, Tay BK, Zhang P, Teo EHT, Lim LTW, O’Shea S, Miao J, Milne WI (2004) Vibratory response of diamond-like amorphous carbon cantilevers under different temperatures. Diam Relat Mater 13:1980–1983
Clarke RJ, Jensen OE, Billingham J, Pearson AP, Williams PM (2006) Stochastic elastohydrodynamics of a microcantilever oscillating near a wall. Phys Rev Lett 96:050801
Cleveland JP, Manne S, Bocek D, Hansma PK (1993) A nondestructive method for determining the spring constant of cantilevers for scanning force microscopy. Rev Sci Instrum 64:403–405
Cummings EB (2000) An image processing and optimal nonlinear filtering technique for particle image velocimetry of microflows. Exp Fluids Suppl S42-S50
da Mata JLCG, Fareleira JMNA, Oliveira CMBP, Caetano FJP, Wakeham WA (2001) A new instrument to perform simultaneous measurements of density and viscosity of fluids by a dual vibrating-wire technique. High Temp High Press 33:669–676
Dimitriadis EK, Horkay F, Maresca J, Kachar B, Chadwick RS (2002) Determination of elastic moduli of thin layers of soft material using the atomic force microscope. Biophys J 82:2798–2810
Discher DE, Janmey P, Wang Y-L (2005) Tissue cells feel and respond to the stiffness of their substrate. Science 310:1139–1143
Dix M, Fareleira JMNA, Takaishi Y, Wakeham WA (1991) A vibrating wire densimeter for measurements in fluids at high pressures. Int J Thermophys 12:357–370
Domke J, Radmacher M (1998) Measuring the elastic properties of thin polymer films with the atomic force microscope. Langmuir 14:3320–3325
Dushman S, Lafferty JN (1962) Scientific foundations of vacuum technique, 2nd edn. Wiley, New York
Elmer F-J, Dreier M (1997) Eigenfrequencies of a rectangular atomic force microscope cantilever in a medium. J Appl Phys 81:7709–7714
Engler AJ, Sen S, Sweeney HL, Discher DE (2006) Matrix elasticity directs stem cell lineage specification. Cell 126:677–689
Fujii T, Watanabe S, Suzuki M, Fujiu T (1995) Application of lead zirconate titanate thin film displacement sensors for the atomic force microscope. J Vac Sci Technol B 13:1119–1122
Gass V, van der Schoot BH, de Rooij NF (1993) Nanofluid handling by micro-flow-sensor based on drag force measurements. In: Proceedings of MEMS’93 IEEE, pp 167–172
Gass V, van der Schoot BH, Jeanneret S, de Rooij NF (1993b) Micro liquid handling using a flow-regulated silicon micropump. J Micromech Microeng 3:214–215
Gass V, van der Schoot BH, Jeanneret S, de Rooij NF (1994) Integrated flow-regulated silicon micropump. Sens Actuators A 43:335–338
Ghatkesar MK, Rakhmatullina E, Lang HP, Gerber C, Hegner M, Braun T (2008) Multi-parameter microcantilever sensor for comprehensive characterization of Newtonian fluids. Sens Actuators B 135:133–138
Giessibl FJ (1995) Atomic resolution of the silicon (111)-(7×7) surface by atomic force microscopy. Science 267:68–71
Giessibl FJ (2003) Advances in atomic force microscopy. Rev Mod Phys 75:949–983
Giessibl FJ, Quate CF (2006) Exploring the nanoworld with atomic force microscopy. Phys Today 59:44–50
Gimzewski JK, Ch Gerber, Meyer E, Schlittler RR (1994) Observation of a chemical reaction using a micromechanical sensor. Chem Phys Lett 217:589–594
Godin M, Bryan AK, Burg TP, Babcock K, Manalis SR (2007) Measuring the mass, density, and size of particles and cells using a suspended microchannel resonator. Appl Phys Lett 91:123121
Gould SAC, Drake B, Prater CB, Weisenhorn AL, Manne S, Kelderman GL, Butt HJ, Hansma H, Hansma PK (1990) The atomic force microscope: a tool for science and industry. Ultramicroscopy 33:93–98
Green CP, Sader JE (2005) Frequency response of cantilever beams immersed in viscous fluids near a solid surface with applications to the atomic force microscope. J Appl Phys 98:114913
Gross L, Mohn F, Moll N, Liljeroth P, Meyer G (2009) The chemical structure of a molecule resolved by atomic force microscopy. Science 325:1110–1114
Han J, Zhu C, Liu J, He Y (2002) Dependence of the resonance frequency of thermally excited microcantilever resonators on temperature. Sens Actuators A 101:37–41
Hane K, Iwatuki T, Inaba S, Okuma S (1992) Frequency shift on a micromachined resonator excited photothermally in vacuum. Rev Sci Instrum 63:3781–3782
Hansen KM, Thundat T (2005) Microcantilever biosensors. Methods 37:57–64
Hansma PK, Cleveland JP, Radmacher M, Walters DA, Hilner PE, Bezanilla M, Fritz M, Vie D, Hansma HG, Prater CB, Massie J, Fukunaga L, Gurley J, Elings V (1994) Tapping mode atomic force microscopy in liquids. Appl Phys Lett 64:1738–1740
Heinz WF, Hoh JH (1999) Spatially resolved force spectroscopy of biological surfaces using the atomic force microscope. Trends Biotechnol 17:143–150
Hirata M, Kokubun K, Ono M, Nakayama K (1985) Size effect of a quartz oscillator on its characteristics as a friction vacuum gauge. J Vac Sci Technol A 3:1742–1745
Hosaka H, Itao K, Kuroda S (1995) Damping characteristics of beam-shaped micro-oscillators. Sens Actuators A 49:87–95
Hutcherson S, Ye W (2004) On the squeeze-film damping of micro-resonators in the free-molecular regime. J Micromech Microeng 14:1726–1733
Hynek V, Obsil M, Majer V, Quint J, Grolier JPE (1997) A vibrating-tube flow densitometer for measurements with corrosive solutions at temperatures up to 723 K and pressures up to 40 MPa. Int J Thermophys 18:719–732
Inaba S, Hane K (1991a) Resonance frequency shifts of a photothermal vibration in vacuum. J Vac Sci Technol A 9:2138–2139
Inaba S, Hane K (1991b) Resonance frequency of a photothermal vibration for a polyethylene thin plate in medium vacuum region. J Vac Sci Technol A 9:3173–3174
Inaba S, Hane K (1992) Photothermal vibration for a membrane in water. J Appl Phys 71:3631–3632
Inaba S, Okuhara Y, Hane K (1992) Liquid density sensing by using photothermal vibration. Sens Actuators A 33:163–166
Inaba S, Akaishi K, Mori T, Hane K (1993) Analysis of the resonance characteristics of a cantilever vibrated photothermally in a liquid. J Appl Phys 73:2654–2658
Keskar G, Elliot B, Gaillard J, Skove MJ, Rao AM (2008) Using electric actuation and detection of oscillations in microcantilevers for pressure measurements. Sens Actuators A 147:203–209
Kim S, Kihm KD (2006) Experimental verification of the temperature effects on Sader’s model for multilayered cantilevers immersed in an aqueous medium. Appl Phys Lett 89:061918
Kim IT, Kihm KD (2007a) Full-field and real-time SPR imaging thermometry. Opt Lett 32:3456–3458
Kim S, Kihm KD (2007b) Temperature dependence of the near-wall oscillation of microcantilevers submerged in liquid environment. Appl Phys Lett 90:081908
Kim S, Kihm KD (2008) Effect of adsorption-induced surface stress change on the stiffness of a microcantilever used as a salinity detection sensor. Appl Phys Lett 93:081911
Kim HJ, Kihm KD, Allen JS (2003) Examination of ratiometric laser induced fluorescence thermometry for microscale spatial measurement resolution. Int J Heat Mass Transfer 46:3967–3974
Kim S, Kim KC, Kihm KD (2007) Near-field thermometry sensor based on the thermal resonance of a microcantilever in aqueous medium. Sensors 7:3156–3165
Kim K, Chung J, Won J, Kwon O, Lee JS, Park S, Choi Y (2008) Quantitative scanning thermal microscopy using double scan technique. Appl Phys Lett 93:203115
Kim JK, Junkin M, Kim D-H, Kwon S, Shin YS, Wong K, Gale BK (2009a) Applications, techniques, and microfluidic interfacing for nanoscale biosensing. Microfluid Nanofluid 7:149–167
Kim S, English AE, Kihm KD (2009b) Surface elasticity and charge concentration-dependent endothelial cell attachment to copolymer polyelectrolyte hydrogel. Acta Biomater 5:144–151
Kirstein S, Mertesdorf M, Schönhoff M (1998) The influence of a viscous fluid on the vibration dynamics of scanning near-field optical microscopy fiber probes and atomic force microscopy cantilevers. J Appl Phys 84:1782–1790
Kokubun K, Hirata M, Murakami H, Toda Y, Ono M (1984) A bending and stretching mode crystal oscillator as a friction vacuum gauge. Vacuum 34:731–735
Kokubun K, Hirata M, Ono M, Murakami H, Toda Y (1985) Frequency dependence of a quartz oscillator on gas pressure. J Vac Sci Technol A 3:2184–2187
Kumazaki H, Inaba S, Hane K (1996) Pressure dependence of resonance characteristics of the microcantilever fabricated from optical fiber. Vacuum 47:475–477
Kwon TY, Eom K, Park JH, Yoon DS, Kim TS, Lee HL (2007) In situ real-time monitoring of biomolecular interactions based on resonating microcantilevers immersed in a viscous fluid. Appl Phys Lett 90:223903
Lachut MJ, Sader JE (2007) Effect of surface stress on the stiffness of cantilever plates. Phys Rev Lett 99:206102
Lang HP, Hegner M, Meyer E, Ch Gerber (2002) Nanomechanics from atomic resolution to molecular recognition based on atomic force microscopy technology. Nanotechnology 13:R29–R36
Lang HP, Hegner M, Ch Gerber (2005) Cantilever array sensors. Mater Today 8:30–36
Lavrik NV, Sepaniak MJ, Datskos PG (2004) Cantilever transducers as a platform for chemical and biological sensors. Rev Sci Instrum 75:2229–2253
Lee SJ, Kim S (2009) Advanced particle-based velocimetry techniques for microscale flows. Microfluid Nanofluid 6:577–588
Lee SS, White RM (1996) Self-excited piezoelectric cantilever oscillators. Sens Actuators A 52:41–45
Lee JH, Yoon KH, Kim TS (2002) Characterization of resonant behavior and sensitivity using micromachined PZT cantilever. Integr Ferroelectron 50:43–52
Lee J, Goericke F, King WP (2008) Temperature-dependent thermomechanical noise spectra of doped silicon microcantilevers. Sens Actuators A 145–146:37–43
Lee D, Kim S, Jung N, Thundat T, Jeon S (2009) Effects of gold patterning on the bending profile and frequency response of a microcantilever. J Appl Phys 106:024310
Lindholm US, Kana DD, Chu WH, Abramson HN (1965) Elastic vibration characteristics of cantilever plates in water. J Ship Res 9:11–36
Liu D, Garimella SV, Wereley ST (2005) Infrared micro-particle image velocimetry in silicon-based microdevices. Exp Fluids 38:385–392
Lu P, Lee HP, Lu C, O’Shea SJ (2005) Surface stress effects on the resonance properties of cantilever sensors. Phys Rev B 72:085405
Maivald P, Butt HJ, Gould SAC, Prater CB, Drake B, Gurley JA, Elings VB, Hansma PK (1991) Using force modulation to image surface elasticities with the atomic force microscope. Nanotechnology 2:103–106
Majumdar A, Carrejo JP, Lai J (1993) Thermal imaging using the atomic force microscope. Appl Phy Lett 62:2501–2503
Marie R, Thaysen J, Christensen CBV, Boisen A (2003) A cantilever-based sensor for thermal cycling in buffer solution. Microelect Eng 67–68:893–898
Martin Y, Wickramasinghe HK (1987) Magnetic imaging by “force microscopy” with 1000 Å resolution. Appl Phys Lett 50:1455–1457
Marx KA (2003) Quartz crystal microbalance: a useful tool for studying thin polymer films and complex biomolecular systems at the solution-surface interface. Biomacromolecules 4:1099–1120
Mechler A, Piorek B, Lal R, Banerjee S (2004) Nanoscale velocity-drag force relationship in thin liquid layers measured by atomic force microscopy. Appl Phys Lett 85:3881–3883
Meinhart CD, Wereley ST, Santiago JG (1999) PIV measurements of a microchannel flow. Exp Fluids 27:414–419
Mertens J, Finot E, Thundat T, Fabre A, Nadal M-H, Eyraud V, Bourillot E (2003) Effects of temperature and pressure on microcantilever resonance response. Ultramicroscopy 97:119–126
Meyer G, Amer NM (1988) Novel optical approach to atomic force microscopy. Appl Phys Lett 53:1045–1047
Naik T, Longmire EK, Mantell SC (2003) Dynamic response of a cantilever in liquid near a solid wall. Sens Actuators A 102:240–254
Nishimoto T, Shoji S, Esashi M (1994) Buried piezoresistive sensors by means of MeV ion implantation. Sens Actuators A 43:249–253
Nonnenmacher M, Wickramasinghe HK (1992) Scanning probe microscopy of thermal conductivity and subsurface properties. Appl Phys Lett 61:168–170
O’Sullivan CK, Guilbault GG (1999) Commercial quartz crystal microbalances-theory and applications. Biosens Bioelectron 14:663–670
Oden PI, Chen GY, Steele RA, Warmack RJ, Thundat T (1996) Viscous drag measurements utilizing microfabricated cantilevers. Appl Phys Lett 68:3814–3816
Ono M, Hirata M, Kokubun K, Murakami H, Tamura F, Hojo H, Kawashima H, Kyogoku H (1985) Design and performance of a quartz oscillator vacuum gauge with a controller. J Vac Sci Technol A 3:1746–1749
Ono M, Hirata M, Kokubun K, Murakami H, Hojo H, Kawashima H, Kyogoku H (1986) Quartz friction vacuum gauge for pressure range from 0.001 to 1000 Torr. J Vac Sci Technol A 4:1728–1731
Padua AAH, Fareleira JMNA, Calado JCG, Wakeham WA (1994) A vibrating-wire densimeter for liquids at high pressures: the density of 2, 2, 4-trimethylpentane from 298.15 to 348.15 K and up to 100 MPa. Int J Thermophys 15:229–243
Padua AAH, Fareleira JMNA, Calado JCG, Wakeham WA (1998) Electromechanical model for vibrating-wire instruments. Rev Sci Instrum 69:2392–2399
Park JS, Choi CK, Kihm KD (2004) Optically sliced micro-PIV using confocal laser scanning microscopy (CLSM). Exp Fluids 37:105–119
Park JS, Choi CK, Kihm KD (2005) Temperature measurement for a nanoparticle suspension by detecting the Brownian motion using optical serial sectioning microscopy (OSSM). Meas Sci Technol 16:1418–1429
Patois R, Vairac P, Cretin B (2000) Near-field acoustic densimeter and viscosimeter. Rev Sci Instrum 71:3860–3863
Pelham RJ, Wang Y-L (1997) Cell locomotion and focal adhesions are regulated by substrate flexibility. Proc Natl Acad Sci USA 94:13661–13665
Pinnaduwage LA, Ji HF, Thundat T (2005) Moore’s law in homeland defense: an integrated sensor platform based on silicon microcantilevers. IEEE Sensors J 5:774–785
Piorek B, Mechler A, Lal R, Freudenthal P, Meinhart C, Banerjee S (2006) Nanoscale resolution microchannel flow velocimetry by atomic force microscopy. Appl Phys Lett 89:153123
Poggi MA, Gadsby ED, Bottomley LA, King WP, Oroudjev E, Hansma H (2004) Scanning probe microscopy. Anal Chem 76:3429–3443
Prater CB, Butt HJ, Hansma PK (1990) Atomic force microscopy. Nature 345:839–840
Quist A, Chand A, Ramachandran S, Cohen D, Lal R (2006) Piezoresistive cantilever based nanoflow and viscosity sensor for microchannels. Lab Chip 6:1450–1454
Raiteri R, Grattarola M, Butt HJ, Skladal P (2001) Micromechanical cantilever-based biosensors. Sens Actuators B 79:115–126
Ramos D, Tamayo J, Mertens J, Calleja M, Zaballos A (2006) Origin of the response of nanomechanical resonators to bacteria adsorption. J Appl Phys 100:106105
Ramos D, Calleja M, Mertens J, Zaballos A, Tamayo J (2007) Measurement of the mass and rigidity of adsorbates on a microcantilever sensor. Sensors 7:1834–1845
Ramos D, Tamayo J, Mertens J, Calleja M, Villanueva LG, Zaballos A (2008) Detection of bacteria based on the thermomechanical noise of a nanomechanical resonator: origin of the response and detection limits. Nanotechnology 19:035503
Rijal K, Mutharasan R (2007) PEMC-based method of measuring DNA hybridization at femtomolar concentration directly in human serum and in the presence of copious noncomplementary strands. Anal Chem 79:7392–7400
Roh HH, Lee JS, Kim DL, Park JS, Kim KT, Kwon O, Park SH, Choi YK, Majumdar A (2006a) Novel nanoscale thermal property imaging technique: the 2ω method. I. Principle and the 2ω signal measurement. J Vac Sci Technol B 24:2398–2404
Roh HH, Lee JS, Kim DL, Park JS, Kim KT, Kwon O, Park SH, Choi YK, Majumdar A (2006b) Novel nanoscale thermal property imaging technique: the 2ω method. II. Demonstration and comparison. J Vac Sci Technol B 24:2405–2411
Roters A, Johannsmann D (1996) Distance-dependent noise measurements in scanning force microscopy. J Phys Condens Matter 8:7561–7577
Rugar D, Hansma P (1990) Atomic force microscopy. Phys Today 43:23–30
Sader JE (1998) Frequency response of cantilever beams immersed in viscous fluids with applications to the atomic force microscope. J Appl Phys 84:64–76
Sakakibara J, Adrian RJ (1999) Whole field measurement of temperature in water using two-color laser induced fluorescence. Exp Fluids 26:7–15
Salapaka MV, Bergh HS, Lai J, Majumdar A, McFarland E (1997) Multi-mode noise analysis of cantilevers for scanning probe microscopy. J Appl Phys 81:2480–2487
Sandberg R, Svendsen W, Mølhave K, Boisen A (2005) Temperature and pressure dependence of resonance in multi-layer microcantilevers. J Micromech Microeng 15:1454–1458
Santiago JG, Wereley ST, Meinhart CD, Beebe DJ, Adrian RJ (1998) A particle image velocimetry system for microfluidics. Exp Fluids 25:316–319
Sarid D (1994) Scanning force microscopy. Revised edition. Oxford University Press, NewYork
Senesac L, Thundat T (2008) Nanosensors for trace explosive detection. Mater Today 11:28–36
Shekhawat GS, Dravid VP (2005) Nanoscale imaging of buried structures via scanning near-field ultrasound holography. Science 310:89–92
Shekhawat GS, Tark S-H, Dravid VP (2006) MOSFET-embedded microcantilevers for measuring deflection in biomolecular sensors. Science 311:1592–1595
Shen F, Lu P, O’Shea SJ, Lee KH, Ng TY (2001) Thermal effects on coated resonant microcantilevers. Sens Actuators A 95:17–23
Shi L, Plyasunov S, Bachtold A, McEuen PL, Majumdar A (2000) Scanning thermal microscopy of carbon nanotubes using batch-fabricated probes. Appl Phys Lett 77:4295–4297
Shih WY, Li X, Gu H, Shih W-H, Aksay IA (2001) Simultaneous liquid viscosity and density determination with piezoelectric unimorph cantilevers. J Appl Phys 89:1497–1505
Singamaneni S, LeMieux MC, Lang HP, Ch Gerber, Lam Y, Zauscher S, Datskos PG, Lavrik NV, Jiang H, Naik RR, Bunning TJ, Tsukruk VV (2008) Bimaterial microcantilevers as a hybrid sensing platform. Adv Mater 20:653–680
Sinton D (2004) Microscale flow visualization. Microfluid Nanofluid 1:2–21
Squires TM, Messinger RJ, Manalis SR (2008) Making it stick: convection, reaction and diffusion in surface-based biosensors. Nature Biotechnol 26:417–426
Stern JE, Terris BD, Mamin HJ, Rugar D (1988) Deposition and imaging of localized charge on insulator surfaces using a force microscope. Appl Phys Lett 53:2717–2719
Su Y, Evans AGR, Brunnschweiler A (1996) Micromachined silicon cantilever paddles with piezoresistive readout for flow sensing. J Micromech Microeng 6:69–72
Su Y, Evans AGR, Brunnschweiler A, Ensell G (2002) Characterization of a highly sensitive ultra-thin piezoresistive silicon cantilever probe and its application in gas flow velocity sensing. J Micromech Microeng 12:780–785
Tamayo J, Ramos D, Mertens J, Calleja M (2006) Effect of the adsorbate stiffness on the resonance response of microcantilever sensors. Appl Phys Lett 89:224104
Tetard L, Passian A, Venmar KT, Lynch RM, Voy BH, Shekhawat GS, Dravid VP, Thundat T (2008) Imaging nanoparticles in cells by nanomechanical holography. Nat Nanotech 3:501–505
Thundat T, Warmack RJ, Chen GY, Allison DP (1994) Thermal and ambient-induced deflections of scanning force microscope cantilevers. Appl Phys Lett 64:2894–2896
Thundat T, Oden PI, Warmack RJ (1997) Microcantilever sensors. Microscale Thermophys Eng 1:185–199
Tortonese M, Barrett RC, Quate CF (1993) Atomic resolution with an atomic force microscope using piezoresistive detection. Appl Phys Lett 62:834–836
Tough JT, McCormick WD, Dash JG (1964) Vibrating wire viscometer. Rev Sci Instrum 35:1345–1348
Tropea C, Yarin AL, Foss JF (2007) Springer handbook of experimental fluid mechanics. Springer, Berlin
Van Eysden CA, Sader JE (2006) Resonant frequencies of a rectangular cantilever beam immersed in a fluid. J Appl Phys 100:114916
Van Eysden CA, Sader JE (2007) Frequency response of cantilever beams immersed in viscous fluids with applications to the atomic force microscope: arbitrary mode order. J Appl Phys 101:044908
Van Eysden CA, Sader JE (2009) Frequency response of cantilever beams immersed in compressible fluids with applications to the atomic force microscope. J Appl Phys 106:094904
Waggoner PS, Craighead HG (2007) Micro- and nanomechanical sensors for environmental, chemical, and biological detection. Lab Chip 7:1238–1255
Wang C, Wang D, Mao Y, Hu X (2007) Ultrasensitive biochemical sensors based on microcantilevers of atomic force microscope. Anal Biochem 363:1–11
Wilhelm J, Vogel E (2001) Viscosity measurements on gaseous propane. J Chem Eng Data 46:1467–1471
Wong JY, Velasco A, Rajagopalan P, Pham Q (2003) Directed movement of vascular smooth muscle cells on gradient-compliant hydrogels. Langmuir 19:1908–1913
Xu Y, Lin JT, Alphenaar BW, Keynton RS (2006) Viscous damping of microresonators for gas composition analysis. Appl Phys Lett 88:143513
Yasumura KY, Stowe TD, Chow EM, Pfafman T, Kenny TW, Stipe BC, Rugar D (2000) Quality factors in micron- and submicron-thick cantilevers. J Microelectromech Syst 9:117–125
Yosida H (2005) The wide variety of possible applications of micro-thermofluid control. Microfluid Nanofluid 1:289–300
Zettner CM, Yoda M (2003) Particle velocity field measurements in a near-wall flow using evanescent wave illumination. Exp Fluids 34:115–121
Zhong Q, Innis D, Kjoller K, Elings VB (1993) Fractured polymer/silica fiber surface studied by tapping mode atomic force microscopy. Surf Sci Lett 290:L688–L692
Ziegler C (2004) Cantilever-based biosensors. Anal Bioanal Chem 379:946–959
Zook JD, Burns DW, Guckel H, Sniegowski J, Engelstad RL, Feng Z (1992) Characteristics of polysilicon resonant microbeams. Sens Actuators A 35:51–59
Acknowledgments
We would like to thank our colleagues and collaborators cited in this review for their contributions. S. Kim and T. Thundat would like to thank DOE BER for its support. Oak Ridge National Laboratory is managed by UT-Battelle under contract No. DE-AC05-00OR22725. The present study was supported partially by the WCU (World Class University) Program through the National Research Foundation of Korea funded by the Ministry of Education, Science and Technology (R31-2008-000-10083-0).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kim, S., Kihm, K.D. & Thundat, T. Fluidic applications for atomic force microscopy (AFM) with microcantilever sensors. Exp Fluids 48, 721–736 (2010). https://doi.org/10.1007/s00348-010-0830-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00348-010-0830-3