Abstract
Purpose
To compare the diagnostic performance of photodynamic diagnosis (PDD) enhanced with oral 5-aminolaevulinic acid between the suspected upper tract urothelial carcinoma (UTUC) and bladder urothelial carcinoma (BUC) cases.
Methods
This retrospective study included 18 patients with suspected UTUC who underwent ureteroscopy (URS) with oral 5-ALA in the PDD-URS cohort between June 2018 and January 2019; and 110 patients with suspected BUC who underwent transurethral resection of bladder tumour (TURBT) in the PDD-TURBT cohort between January 2019 and March 2023. Sixty-three and 708 biopsy samples were collected during diagnostic URS and TURBT, respectively. The diagnostic accuracy of white light (WL) and PDD in the two cohorts was evaluated, and false PDD-positive samples were pathologically re-evaluated.
Results
The area under the receiver operating characteristic curve (AUC) of PDD was significantly superior to that of WL in both cohorts. The per biopsy sensitivity, specificity, and positive and negative predictive values of PDD in patients in the PDD-URS and PDD-TURBT cohorts were 91.2 vs. 71.4, 75.9 vs. 75.3, 81.6 vs. 66.3, and 88.0 vs. 79.4%, respectively. The PDD-URS cohort exhibited a higher AUC than did the PDD-TURBT cohort (0.84 vs. 0.73). Seven of four false PDD-positive samples (57.1%) in the PDD-URS cohort showed potential precancerous findings compared with eight of 101 (7.9%) in the PDD-TURBT cohort.
Conclusion
The diagnostic performance of PDD in the PDD-URS cohort was at least equivalent to that in the PDD-TURBT cohort.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Non-muscle-invasive bladder cancer is treated with endoscopic transurethral resection of bladder tumours (TURBT); however, it is highly recurrent and sometimes progresses into muscle-invasive bladder cancer (MIBC) [1, 2]. The advent of photodynamic diagnosis (PDD), such as oral 5-aminolevulinic acid hydrochloride (5-ALA) and intravesical hexaminolevulinate, has improved the detection of tiny tumours and flat lesions that would otherwise be missed by white light (WL) observation alone. Previous reports have shown that PDD with oral 5-ALA increases the tumour detection rate by approximately 20% compared with WL, and the diagnostic benefits of PDD have resulted in improved recurrence-free survival [3,4,5,6,7].
Although both bladder urothelial carcinoma (BUC) and upper urinary tract urothelial carcinoma (UTUC) are derived from the urothelium and thus share many characteristics [8], the diagnostic and therapeutic strategies for UTUC differ from those for BUC. Ureteroscopy (URS) is used to observe the upper urinary tract, which is somewhat inaccessible, and the acquisition of significant pathological specimens is often challenging. Although the feasibility of PDD for UTUC diagnosis has been investigated for decades [9,10,11,12,13,14,15,16], PDD has not been clinically approved for UTUC.
To the best of our knowledge, this is the first report to investigate the difference in diagnostic performance safety of oral 5-ALA for UTUC and BUC conducted in a single institution.
Materials and methods
Ethics statements
Administration of oral 5-ALA for URS was performed as a prospective single-arm clinical trial [9]. Data were collected retrospectively from a prospective trial database. TURBT data were retrospectively collected from electronic medical charts, and the use of the data was approved by the ethics board of Kansai Medical University (IRB No. 2018,036).
Patient selection and data collection
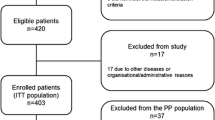
We reviewed 20 patients with suspected UTUC who were enrolled in a prospective clinical trial conducted between May 2018 and January 2019 and underwent URS diagnosis with pretreatment with oral 5-ALA (the PDD-URS cohort). Two patients were excluded because of a lack of sufficient information for analysis in the present study. We also reviewed the data of 114 consecutive patients with suspected BUC who underwent TURBT with oral 5-ALA between January 2019 and March 2023 (the PDD-TURBT cohort). Two patients who did not undergo tissue biopsy and two patients who had a history of radiation or systemic therapy for advanced BUC were excluded from the study. All procedures were performed at the Kansai Medical University Hospital. The recorded clinicopathological characteristics of the patients included age, sex, tumour appearance, history of URS for suspected UTUC, TURBT, intravesical UC treatment for BUC, preoperative urine cytology results, and laterality of the suspected upper urinary tract. Regarding tumour appearance, “irregular urothelium” referred to a thick, irregular, or reddened epithelium. Considering that a history of intravesical Bacillus Calmette-Guérin (BCG) treatment and/or TURBT has a negative effect on the diagnostic performance of PDD [17, 18], the PDD-TURBT cohort was further subdivided into PDD-TURBT-naïve (patients without previous TURBT), PDD-TURBT-recurrent (patients with a history of TURBT), and PDD-TURBT-BCG (patients with a history of intravesical BCG treatment) subgroups for assessment of diagnostic performance of WL and PDD.
Surgical procedures
Each patient was orally administered 20 mg/kg of 5-ALA (ALAGLIO®; SBI Pharmaceuticals Co., Tokyo, Japan) dissolved in 50 mL of water 2 to 4 h before URS or TURBT. In all cases, surgical procedures were performed under spinal anaesthesia, except for five cases of TURBT performed under general anaesthesia. The details of the URS system used in this study have been described in our previous study [9]. A D-Light C® (Karl Storz, Tuttlingen, Germany) with a removable protoporphyrin IX excitation eyepiece filter permitting blue-violet light (BL) (380–430 nm) was used for PDD.
Pathological assessment
All specimens were assessed by pathologists at the Department of Pathology, Kansai Medical University. One of the authors, C. O., reassessed the false-positive specimens of PDD regarding the existence of potential precancerous findings, such as suspicious lesions for malignancy (but not decisive for UC) and dysplasia.
Statistical analysis
Significant differences between the two groups were estimated using Student’s t-test for parametric data and the Mann–Whitney U test for nonparametric data. Fisher’s exact test or the chi-squared test was used to compare the contingency groups. All analyses were performed using GraphPad Prism version 9.4.1 (GraphPad Software, San Diego, CA, USA). Calculations of all diagnostic accuracy parameters, the area under a receiver operating characteristic curve (AUC), and statistical comparison of the AUC were performed using EZR. All reported P values were two-sided, and statistical significance was set at p < 0.05.
Results
Patients’ characteristics
The characteristics of the 18 and 110 patients who underwent PDD-URS and PDD-TURBT are shown in Table 1. Of the 18 patients with suspected UTUC, 11 (61.1%) showed visible tumour lesions on contrast-enhanced computed tomography (CT) scan or magnetic resonance imaging (MRI), whereas 48 (43.6%) showed papillary tumour lesions on cystoscopy in 110 patients with suspected BUC. In the PDD-URS cohort, seven patients were suspected to have UTUC in the right upper urinary tract and 11 patients in the left upper urinary tract. In the PDD-TURBT cohort, 30 patients (27.3%) received intravesical BCG treatment. Random biopsy was not performed in the PDD-URS cohort, whereas it was performed in most patients whose urine cytology results were suspicious or malignant in the PDD-TURBT cohort; 66 patients (60.0%) underwent random biopsy.
Overall pathological results per patient and sample
Eight of the 18 patients (44.4%) in the PDD-URS cohort did not show malignancy, another eight showed Ta tumours, and the other two each showed carcinoma in situ (CIS) and T1 tumours. Twenty-two of the 110 patients (20.0%) in the PDD-TURBT cohort did not show malignancy, 37 and 24 showed Ta tumours and CIS, respectively, and the remaining 27 patients showed invasive T1 or T2 tumours. Concomitant CIS was found in 18 patients.
In total, 63 biopsy samples were obtained in the PDD-URS cohort, 38 of which were positive for PDD (Table 2a). Twenty-two of 25 PDD-negative samples (88.0%) showed no malignancy, whereas only seven of 38 PDD-positive samples (18.4%) exhibited no malignancy. Out of 25 samples negative for PDD, only three samples were positive for malignancy. All 25 tumours visible with WL were PDD-positive, and only three samples (12.0%) did not show malignancy. When the urothelium appears irregular with the WL, PDD-negative samples showed no malignancy in six out of seven samples (85.7%), and PDD-positive samples showed malignancy in six out of eight samples (75.0%), suggesting the usefulness of PDD for irregular urothelium. Out of the five samples that appeared normal with WL but positive for PDD, three showed malignancy, whereas out of 18 PDD-negative samples from the normal urothelium, only two samples exhibited malignancy (Table 2a).
In total, 708 biopsy samples were obtained from the PDD-TURBT cohort, of which 309 were positive for PDD (Table 2b). Of the 399 PDD-negative samples, 317 (79.4%) showed no malignancy, whereas 104 of the 309 PDD-positive samples (33.7%) demonstrated no malignancy. Ninety-four of the 101 visible tumours with WL (93.1%) were PDD-positive, and among them, 85 samples (90.4%) showed malignancy. PDD was negative in seven visible tumours (6.9%), and five samples (71.4%) were malignant. In the irregular urothelium with WL observation, as many as 47 of 113 PDD-positive samples (41.6%) were negative for malignancy, and 10 of 34 PDD-negative samples (29.4%) were malignant. These findings suggested inferior diagnostic performance of PDD for irregular urothelium in the PDD-TURBT cohort compared to that in the PDD-URS cohort. Sixty-five of the 358 lesions that appeared normal and PDD negative (18.2%) were positive for malignancy, suggesting the importance of random biopsy. Moreover, of the 102 samples from the normal urothelium with PDD positivity, 48 (47.1%) showed no malignancy, indicating a high incidence of false PDD positivity in the normal urothelium, as was also observed in the irregular urothelium (Table 2b).
Diagnostic performance of PDD in suspected BUC and UTUC
The diagnostic accuracies of WL and PDD in 63 biopsies from 18 patients in the PDD-URS cohort estimated with AUC were 0.74 and 0.84, respectively (p = 0.011). The sample sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) for WL and PDD were 85.3% and 91.2%; 62.1 and 75.9%; 72.5 and 81.6%; and 78.3 and 88.0%, respectively (Table 3).
The diagnostic accuracies of WL and PDD in 708 biopsies from 110 patients in the PDD-TURBT cohort estimated with AUC were 0.69 and 0.73, respectively (p = 0.00028). The sample sensitivity, specificity, PPV, and NPV values for WL and PDD were 57.8%, 71.4, 80.5%, 75.3, 66.9%, 66.3, 73.7%, and 79.4%, respectively. As expected, PDD biopsy for patients in the PDD-TURBT-naïve subgroup showed the highest AUC, followed by that for patients in the PDD-TURBT-recurrent subgroup and then for patients in the PDD-TURBT-BCG subgroup. Among the estimated parameters, the specificity and PPV showed the same trend as the AUC values (Table 3).
All estimated diagnostic parameters of PDD were better in the total PDD-URS cohort than in the total PDD-TURBT cohort, and the AUC value of PDD biopsy in the total PDD-URS cohort was higher than that in the total PDD-TURBT cohort. In particular, the sensitivity (91.2 vs. 71.4%) and PPV (81.6 vs. 66.3%) were much better in the total PDD-URS cohort than in the total PDD-TURBT cohort. Next, we compared the AUC values of PDD between the total PDD-URS cohort and the PDD-TURBT-naïve subgroup, which showed the best diagnostic performance among the PDD-TURBT subgroups, and found that the AUC value in the total PDD-URS cohort was slightly higher than that in the total PDD-TURBT-naïve subgroup (0.84 vs. 0.76) (Table 3). Thus, the diagnostic performance of PDD in the PDD-URS cohort can be considered comparable to that in the PDD-TURBT-naïve subgroup.
Pathological findings of false-positive samples
In the PDD-URS cohort, four out of seven false-positive samples of PDD (57.1%) had features of potential precancerous lesions (one suspicious for malignancy and three for dysplasia). In contrast, only eight of 101 false-positive samples of PDD (7.9%) in the PDD-TURBT cohort showed precancerous features (four suspicious for malignancy and four for dysplasia). Thus, false PDD-positive samples in the PDD-URS cohort showed a significantly higher incidence of precancerous findings than did those in the PDD-TURBT cohort (p = 0.0027).
Discussion
Although several studies have demonstrated the potential clinical benefits of PDD for UTUC [9,10,11, 13,14,15,16, 19, 20], there are no previous reports comparing the diagnostic performance of PDD between URS and TURBT. This study provides valuable basic information regarding differences in the diagnostic performance of PDD depending on the location within the urinary tract.
The diagnosis of UTUC is often challenging because of the limited accessibility to the upper urinary tract and potential modification of the mucosa caused by the manipulation of guidewires and ureteroscopes. Consequently, diagnostic accuracy is influenced by the skill and experience of operators. When malignant lesions in the upper urinary tract are not visible, the diagnosis becomes extremely challenging because a random biopsy of the upper urinary tract, as performed in the bladder, is not routinely feasible. PDD for UTUC appears to offer a promising solution for addressing these issues.
In the present study, all estimated diagnostic parameters of PDD were better in the PDD-URS cohort than in the PDD-TURBT cohort (Table 3). The AUC values in the PDD-URS cohort appeared to be equivalent, even when compared to the PDD-TURBT-naïve subgroup, which showed the best diagnostic performance among the PDD-TURBT subgroups. Moreover, the incidence of precancerous findings in false PDD-positive samples in the PDD-URS cohort was much higher than that in the PDD-TURBT cohort (57.1% vs. 7.9%, p = 0.00028). Although a direct statistical comparison of the diagnostic performance of PDD between the PDD-URS and PDD-TURBT cohorts is not possible practically, our findings support the efficacy of PDD in the diagnosis of UTUC.
The sensitivity of PDD for BUC was lower than that reported in previous studies (Table 3) [21, 22]. This is probably because as many as 60% of the patients in the present study underwent random biopsy, which often detected malignancy negative for PDD. A high false-positive rate has been recognised as one of the issues with PDD for BUC [18, 23]. The false-positive rate of PDD for BUC was also high in the present study. However, when the PDD-TURBT cohort was subclassified into the PDD-TURBT-naïve, PDD-TURBT-recurrent, and PDD-TURBT-BCG subgroups, the false-positive rate of the PDD-TURBT-naïve subgroup was only 18.9%, whereas those of PDD-TURBT-recurrent and PDD-TURBT-BCG subgroups were approximately 50% (Table 3), suggesting that false positivity is caused by reactive changes in previous TURBT and intravesical BCG treatment.
Our study had some limitations. First, we could not conclude the advantages of applying PDD for UTUC to clinical standards because of the retrospective design and small number of patients, especially in the PDD-URS cohort, in the present study. Second, the procedures were conducted at different time periods in the PDD-URS and PDD-TURBT cohorts, which may have affected the diagnostic performance, even though most surgeons overlapped between the time periods.
Conclusion
PDD with oral administration of 5-ALA improved the diagnostic performance of BUC and UTUC. Among the subgroups in the PDD-TURBT cohort, the PDD-TURBT-naïve subgroup showed the best diagnostic performance, and a history of TURBT and intravesical BCG considerably decreased diagnostic accuracy. The diagnostic performance of PDD in the PDD-URS cohort was equivalent to that in the PDD-TURBT-naïve subgroup, supporting the efficacy of PDD for UTUC.
References
Babjuk M, Oosterlinck W, Sylvester R et al (2008) EAU guidelines on non-muscle-invasive urothelial carcinoma of the bladder. Eur Urol 54:303–314
Sylvester RJ, Rodríguez O, Hernández V et al (2021) European Association of Urology (EAU) prognostic factor risk groups for non-muscle-invasive bladder Cancer (NMIBC) incorporating the WHO 2004/2016 and WHO 1973 classification systems for Grade: an update from the EAU NMIBC guidelines Panel. Eur Urol 79:480–488
Kausch I, Sommerauer M, Montorsi F et al (2010) Photodynamic diagnosis in non-muscle-invasive bladder cancer: a systematic review and cumulative analysis of prospective studies. Eur Urol 57:595–606
Burger M, Grossman HB, Droller M et al (2013) Photodynamic diagnosis of non-muscle-invasive bladder cancer with hexaminolevulinate cystoscopy: a meta-analysis of detection and recurrence based on raw data. Eur Urol 64:846–854
Chou R, Selph S, Buckley DI et al (2017) Comparative effectiveness of fluorescent Versus White Light Cystoscopy for initial diagnosis or surveillance of bladder Cancer on clinical outcomes: systematic review and Meta-analysis. J Urol 197:548–558
Bach T, Bastian PJ, Blana A et al (2017) Optimised photodynamic diagnosis for transurethral resection of the bladder (TURB) in German clinical practice: results of the noninterventional study OPTIC III. World J Urol 35:737–744
Gakis G, Ngamsri T, Rausch S et al (2015) Fluorescence-guided bladder tumour resection: impact on survival after radical cystectomy. World J Urol 33:1429–1437
Green DA, Rink M, Xylinas E et al (2013) Urothelial carcinoma of the bladder and the upper tract: disparate twins. J Urol 189:1214–1221
Yoshida T, Setsuda S, Ishizuka M et al (2020) Photodynamic diagnosis with oral 5-Aminolevulinic acid for Upper urinary tract carcinoma: a prospective clinical trial. J Endourol 34:509–515
Aboumarzouk OM, Ahmad S, Moseley H et al (2012) Accuracy of photodynamic diagnosis in the detection and follow-up of patients with upper urinary tract lesions: initial 3-year experience. Arab J Urol 10:138–142
Fukuhara H, Kurabayashi A, Furihata M et al (2020) 5-aminolevulinic acid-mediated photodynamic diagnosis using fluorescence ureterorenoscopy for urinary upper tract urothelial carcinoma ∼Preliminary prospective single centre trial∼. Photodiagnosis Photodyn Ther 29:101617
Kata SG, Zreik A, Ahmad S et al (2016) Concurrent bladder cancer in patients undergoing photodynamic diagnostic ureterorenoscopy: how many lesions do we miss under white light cystoscopy? Cent Eur J Urol 69:334–340
Audenet F, Traxer O, Yates DR et al (2012) Potential role of photodynamic techniques combined with new generation flexible ureterorenoscopes and molecular markers for the management of urothelial carcinoma of the upper urinary tract. BJU Int 109:608–613 discussion 613 – 604
Osman E, Alnaib Z, Kumar N (2017) Photodynamic diagnosis in upper urinary tract urothelial carcinoma: a systematic review. Arab J Urol 15:100–109
Ahmad S, Aboumarzouk O, Somani B et al (2012) Oral 5-aminolevulinic acid in simultaneous photodynamic diagnosis of upper and lower urinary tract transitional cell carcinoma - a prospective audit. BJU Int 110:E596–600
Wada K, Araki M, Tanimoto R et al (2021) Photodynamic diagnostic ureteroscopy using the VISERA ELITE video system for diagnosis of upper-urinary tract urothelial carcinoma: a prospective cohort pilot study. BMC Urol 21:45
Ray ER, Chatterton K, Khan MS et al (2010) Hexylaminolaevulinate fluorescence cystoscopy in patients previously treated with intravesical bacille Calmette-Guérin. BJU Int 105:789–794
Draga RO, Grimbergen MC, Kok ET et al (2010) Photodynamic diagnosis (5-aminolevulinic acid) of transitional cell carcinoma after bacillus Calmette-Guérin immunotherapy and mitomycin C intravesical therapy. Eur Urol 57:655–660
Waidelich R, Hofstetter A, Stepp H et al (1998) Early clinical experience with 5-aminolevulinic acid for the photodynamic therapy of upper tract urothelial tumors. J Urol 159:401–404
Kata SG, Nabi G, Eljamel S et al (2014) Photodynamic diagnostic ureterorenoscopy with orally administered 5-aminolaevulinic acid as photosensitiser: how I do it. Urol Int 93:384–388
Inoue K, Anai S, Fujimoto K et al (2015) Oral 5-aminolevulinic acid mediated photodynamic diagnosis using fluorescence cystoscopy for non-muscle-invasive bladder cancer: a randomized, double-blind, multicentre phase II/III study. Photodiagnosis Photodyn Ther 12:193–200
Nakai Y, Inoue K, Tsuzuki T et al (2018) Oral 5-aminolevulinic acid-mediated photodynamic diagnosis using fluorescence cystoscopy for non-muscle-invasive bladder cancer: a multicenter phase III study. Int J Urol 25:723–729
Draga RO, Grimbergen MC, Kok ET et al (2009) Predictors of false positives in 5-aminolevulinic acid-induced photodynamic diagnosis of bladder carcinoma: identification of patient groups that may benefit most from highly specific optical diagnostics. Urology 74:851–856
Acknowledgements
We express our gratitude to SBI Pharmaceuticals Co., Ltd. for supplying 5-aminolaevulinic acid for our previous prospective study, whose data was partly shown also in this study. We would like to thank Editage (www.editage.com) for English language editing.
Author information
Authors and Affiliations
Contributions
T. S.: project development, data collection, data analysis, manuscript writing/editing; T.Y.: project development, data collection, and manuscript revision; T. N.: data collection and manuscript revision; O.C.: data analysis and manuscript revision. H.T.: manuscript revision, M.Y.: manuscript revision, H.K.: study supervision and manuscript revision.
Corresponding author
Ethics declarations
Ethical approval
This retrospective study was approved by the Institutional Review Board of Kansai Medical University (IRB No. 2018036).
Conflict of interest
The authors have no competing interests to declare that are relevant to the content of this article.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sano, T., Yoshida, T., Nakamoto, T. et al. Diagnostic performance of photodynamic diagnosis with oral 5-aminolevulinic acid for upper tract- and bladder urothelial carcinoma: a single-centre, retrospective analysis. World J Urol 42, 389 (2024). https://doi.org/10.1007/s00345-024-05083-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00345-024-05083-1