Abstract
Purpose
To assess the effect of our new classification on surgical outcomes after flexible ureteroscopy (fURS) for kidney stones.
Methods
We retrospectively examined 128 patients after single renal fURS procedures performed using ureteral access sheaths (UASs) with the fragmentation technique. Based on the gap (calculated by subtracting the ureteroscope diameter from the UAS diameter), enrolled patients were divided into three groups: small (< 0.6 mm), medium (0.6 to < 1.2 mm), and large space groups (≥ 1.2 mm). Stone-free (SF) status was defined as either complete absence of stones (SF) or the presence of stones < 4 mm in diameter on non-contrast computed tomography (NCCT).
Results
The SF rate was significantly lower in the small space group (50% in small, 97.9% in medium, 89.2% in large; p = 0.001). Perioperative complications over Clavien–Dindo Grade I were observed in 16.7%, 4.2%, and 8.1% of patients, respectively (p = 0.452). The ratio of stone volume and operative time (efficiency of stone removal) was significantly higher in the large space group compared to the small and medium space groups (0.009 ± 0.003 ml/min, 0.013 ± 0.005 ml/min, 0.027 ± 0.012 ml/min, respectively; p < 0.001).
Conclusion
Our findings that gaps > 0.6 mm (1.8 Fr), including the combination of a 9.5-Fr UAS and a small caliber ureteroscope, improve SF rates, and larger gaps facilitate stone removal efficiency providing the basis for future development of clinical protocols aimed at improving outcomes.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The technological advancements in flexible ureteroscopy (fURS) have improved postoperative stone-free (SF) rates and overall safety [1,2,3,4,5,6,7]. Thus, fURS is gradually becoming a more common procedure for the treatment of renal stones [8, 9]. To identify patients who may receive more benefit from fURS than shock wave lithotripsy or percutaneous nephrolithotomy, many researchers have analyzed the usefulness of preoperative and perioperative parameters to predict the SF rate and perioperative complications following fURS.
On the other hand, surgical instruments such as the ureteral access sheath (UAS) have kept improving, thereby improving the safety and efficiency of fURS. A UAS provides the advantage of fast, repeatable, and safe access to the upper urinary tract with improved vision [10, 11]. It also provides the benefit of reduction of intrarenal pressure [12, 13]. One prospective study that enrolled 2239 patients showed that fURS with UAS reduced postoperative infectious complications without any increase in ureteral damage or bleeding [14]. The size of the UAS has also been evaluated, because it is thought to influence both SF rates and complications because larger UASs facilitate passive fragment elimination, allowing for extraction of larger stone fragments and preventing an increase in intrarenal pressure [15]. It has been reported that a larger UAS can improve the SF rate without an increase in perioperative complications [16].
In this study, we focused on the inside space of the UAS where backflow of the irrigation fluids can pass, meaning the gap between the UAS and the ureteroscope. Because this directly reflects the capability of passive elimination of fragments and irrigation of the fluidics, it is thought to be a good parameter to evaluate the influence of the UAS on fURS.
This study evaluated a new classification based on the gap between the UAS and the ureteroscope to determine the optimal UAS and ureteroscope combination to improve SF rates after fURS for renal stones.
Materials and methods
Patient data
We evaluated the data of 245 patients who underwent fURS for renal stones between December 2018 and February 2020. Various surgeons performed these procedures, and all procedures were conducted at Ohguchi East General Hospital in Japan. Patients who underwent multiple fURS procedures for the same stone (nine patients), those with coexisting ureteral stones (78 patients), and those receiving bilateral fURS (30 patients) were excluded. Finally, a total of 128 patients were retrospectively analyzed. All patients received fURS with UAS.
The gap was calculated by subtracting the diameter of the ureteroscope from the diameter of the UAS. The outer diameter of the insertion tube was adapted to the diameter of the ureteroscope. Due to the lack of inner diameter description of the outer cylinder of the UAS, the diameter of the UAS was determined as that of the inner tube. These values were obtained from the manufacturer’s catalogs. Based on this calculation, the major diameter differences varied from 0.52 mm to 2.02 mm (Table 1). In this study, patients were divided into three groups based on gap size: small (< 0.6 mm), medium (0.6 to < 1.2 mm), and large space groups (≥ 1.2 mm).
Surgical techniques
Surgical procedures were performed as described in our previous studies [6, 17]. Briefly, each fURS began with an observation of the upper urinary tract using a 6/7.5-Fr semi-rigid ureteroscope (Wolf™; Richard Wolf GmBH, Knittlingen, Germany) to determine the adequate diameter of the UAS. After the placement of the UAS [mainly 9.5- or 12/14-Fr (Cook Medical, Bloomington, IN, USA), or 11/13 or 13/15-Fr (Boston Scientific, Natick, MA, USA), or 10/12-Fr or 14/16-Fr (Coloplast A/A, Humlebæk, Denmark)], lithotripsy was performed based on the fragmentation technique using a 6-Fr flexible ureteroscope (Olympus P-5™, P-6™, P-7™, or V2™; Olympus, Tokyo, Japan or Flex X2™; Karl Storz, Germany) with a 200 μm holmium:yttrium aluminum–garnet laser. A UAS was used in all cases. The renal pelvis was the target insertion point of the access sheath, and when the surgeon felt resistance during insertion, the access sheath was inserted as close as possible to the renal pelvis. Such a situation was defined as a narrow ureter. In all cases, 1.5-Fr tipless nitinol baskets were used for stone removal and clearance of residual fragments. After fURS, injury in the upper urinary tract was evaluated by retrograde pyelography and observation of the lumen through endoscopy.
After fURS, overnight ureteral catheterization or conventional postoperative stenting was performed. In patients at high risk of perioperative complications, such as those with a severely narrow ureter that posed difficulty in accessing the renal pelvis using a ureteroscope and inserting a 9.5-Fr UAS, those with a large stone over 2 cm3, or those with a prolonged procedure time over 2 h, conventional postoperative stenting tended to be selected. In patients who required overnight ureteral catheterization [5-Fr Tigertail ureteral catheter (Bard Medical Division, Covington, GA, USA)], the ureteral catheter was removed on postoperative day 1. A conventional ureteral stent was usually removed 3–4 weeks after the surgery. Preventive infusion of antibiotics was performed at the beginning of the surgery.
Preoperative and postoperative evaluation
Preoperative parameters included sex, age, height, body weight, pyuria, side of intervention (right or left), Hounsfield units of the stone and stone volume (cm3), preoperative hydronephrosis, presence of lower calyx stones, pre-stenting, and a narrow ureter. The side of involvement, the presence of lower calix stones, and hydronephrosis were confirmed by preoperative NCCT. The volume and Hounsfield units of the stones were obtained from 5 mm axial and 3.5 mm reconstructed coronal NCCT images by the software, as previously reported [18]. Postoperative evaluation consisted of SF rates and perioperative complications related to surgery. Stone status and hydronephrosis was evaluated by NCCT within 2 months of surgery. In cases with postoperative stenting, a computed tomography (CT) scan was performed about 2 weeks after the removal of the ureteral stent. In patients without postoperative stenting, a CT scan was performed 2–4 weeks after fURS. SF was defined as either complete absence or presence of stones < 4 mm in diameter on NCCT. To evaluate perioperative complications, precise examinations such as blood examination, urinalysis, ultrasound, kidney–ureter–bladder X-ray imaging, and NCCT images were performed when patients showed symptoms or abnormal vital signs.
Statistical analysis
Continuous variables were compared using Student’s t tests and expressed as mean [standard deviation (SD)]. Proportions of categorical variables were compared using a Fisher’s exact test. Statistical significance was set at p < 0.05, and all reported p values were two-sided. Treatment efficiency was calculated as the ratio of stone volume and operative time (cm3/min).
Univariate logistic regression models were used, measuring the effect of all parameters on SF rate and perioperative complications. The odds ratio (OR) estimates, 95% OR confidence intervals (CIs), and p values were calculated. Multivariate logistic regression models were used to assess SF rates and perioperative complications. The selection criterion of either p < 0.1, or the smallest p value and second smallest p value was used for the elimination of variables that were statistically different.
All statistical analyses were performed with EZR (Saitama Medical Center, Jichi Medical University, Saitama, Japan), which is a graphical user interface for R (The R Foundation for Statistical Computing, Vienna, Austria) and R software version 3.5.3 (R Foundation for Statistical Computing). More precisely, EZR is a modified version of the R Commander designed to add statistical functions that are frequently used in biostatistics [19].
Results
Classification based on the gap between the UAS and ureteroscope
The combination of a 9.5-Fr UAS and a Flex X2 ureteroscope diameter was classified as a medium space. Other combinations of a 9.5-Fr UAS and a P6/P7 or V2 ureteroscope diameter were classified as small spaces. The combination of a 10-Fr UAS and a P5 or V2 ureteroscope diameter was classified as a small space. Other combinations with 10-Fr UASs were classified as medium spaces. 11-Fr UASs were classified as medium spaces and 12-Fr UASs or above were classified as large spaces. Classifications of the mismatch between the gap and sheath size are summarized in Table 1.
Patient characteristics and surgical outcomes
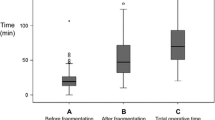
Patient characteristics are summarized in Table 2. The small, medium, and large gap group included 6, 48, and 74 patients, respectively. There was no difference between the three groups in terms of sex, age, height, body weight, laterality, preoperative hydronephrosis, and lower calix stones. However, pyuria and CT density were significantly different in the groups (p = 0.006 and p < 0.001, respectively). Stone volume in the large space group was significantly higher than in the small and medium space groups (1.03 ± 0.39 cm3, 1.06 ± 0.66 cm3, and 2.14 ± 1.11 cm3, respectively; p < 0.001). The rate of preoperative ureteral stent increased significantly with increases in the gap space (33.3%, 56.2%, and 93.2%, respectively; p < 0.001). Conversely, the rate of narrow ureter was higher in the small space group, followed by the medium and large space groups (66.7%, 14.6%, and 8.1%, respectively; p < 0.001). SF rates and perioperative complications are summarized in Table 2. The SF rate was significantly different in the three groups (50% [small], 97.9% [medium], and 89.2% [large]; p = 0.001). Operation time was also prolonged in the small space group (111.17 ± 20.59 min vs. 76.73 ± 35.42 min and 81.45 ± 28.93 min in the medium and large space groups, respectively; p = 0.042). Although the perioperative complication rate in the small space group was high, there was no significant difference between the three groups (33.3%, 6.2%, and 16.2%, respectively; p = 0.095). Clinically important complications over Clavien–Dindo Grade I were observed in 16.7%, 4.2%, and 8.1% of patients in the small, medium, and large space groups, respectively (p = 0.452). In the small space group, there was one febrile patient who did not require antibiotics and one febrile patient who did. In the medium space group, there was one febrile patient who did not require antibiotics and two febrile patients who did. In the large space group, there were seven febrile patients who did not required antibiotics and two febrile patients who did, and one septic patient who required intensive therapy. Injury to the upper urinary tract after fURS was not observed in this study. The rate of postoperative ureteral stenting and the period of hospital stay did not differ significantly. Postoperative hydronephrosis was observed on NCCT in five patients (3.9%) within 2 months of surgery. Because there were no signs of renal dysfunction, urinary tract inflammation, or other symptoms, they were monitored conservatively (Table 3).
To compare efficiency more directly, the ratio of stone volume and operative time was calculated. There was significantly better efficiency in the large space group compared to the small and medium space groups (0.009 ± 0.003 cm3/min, 0.013 ± 0.005 cm3/min, and 0.027 ± 0.012 cm3/min, respectively; p < 0.001). In the univariate analysis of the predictive parameters for SF rate, a significant difference was only shown for gap classification (small vs. medium, OR = 0.02, p = 0.017). The second lowest value was observed for the presence of lower calyx stones (p = 0.22). Multivariate analysis of these two parameters showed significant differences according to gap classification (small vs. medium OR = 0.02, p = 0.0026; small vs. large OR = 0.09, p = 0.014). The presence or absence of lower calyx stones was not significantly different in the three groups (p = 0.18). In terms of perioperative complications, the gap did not show a significant influence. Preoperative ureteral stent and Hounsfield units of the stone did have a significant influence (p = 0.027 and p = 0.0065, respectively) (Table 4).
Discussion
Several reports have demonstrated the benefit of a UAS on SF rates and a decrease in perioperative complications during fURS for renal stones [10, 11, 14]. Our evaluation of the size of the UAS showed that larger UAS sizes improved SF rates [14, 16]. However, the size of UAS only indicated the irrigation space without the ureteroscope and could not reflect the irrigation space during observation, reposition, and fragmentation with the ureteroscope. Because the ureteroscope is almost always inserted into the UAS during fURS, the gap between the UAS and the ureteroscope should be used to assess the effect of the UAS on fURS for renal stones.
To the best of our knowledge, this is the first report to focus on the gap to evaluate surgical outcomes after fURS for renal stones. Our classification based on the gap could predict the SF rate. The larger gap space was thought to facilitate passive fragment elimination to improve SF rate and irrigation of the fluidics to prevent the increase of intrarenal pressure. In addition, the gap classification simplifies the UAS parameter regarding the irrigation space because it unifies the size of the UAS and ureteroscope. It might be useful to compare the UAS parameters between published studies using a meta-analysis. Given that there were various sizes of ureteroscope, of the four patients with 9.5-Fr UAS generally thought to be a small sheath, two were classified into the small space group and two into the medium space group (Table 1). This study showed that a 9.5-Fr UAS with a small caliber ureteroscope could maintain the gaps over 0.6 mm and improve SF rates. However, the gap may show a low accurate value due to the lack of an official description of the UAS diameter and ureteroscope, as well as manufacturing errors associated with these products. Integer values using Fr units may also reduce the accuracy. Detailed data regarding the size of UAS and ureteroscope should be made available by the manufacturers.
Preoperative ureteral stent, stone volume, Hounsfield units of the stone, and lower calyx stones are generally thought to be the parameters affecting SF rate. In this study, the high proportion of preoperative stenting (76.6%) may have masked the positive effect on SF rates. In terms of the stone volume and Hounsfield units of the stone, the fragmentation technique and the removal of fragments over 2 mm in diameter are recommended in our institute. Lower calyx stones are repositioned as soon as possible if they can be grasped by the stone-retrieval baskets. These surgical procedures would cancel the effect of the parameters. The assistance of the expert urologist, who had performed over 200 surgeries, may have also prevented a low SF rate due to these parameters. In terms of the perioperative complications, preoperative ureteral stent and Hounsfield units of the stone were shown to be the predictive parameters in the univariate analysis. However, higher Hounsfield units of the stone only increased the risk of perioperative complications in the multivariable analysis. The influence of the gap on complications was not observed in this study. Repeated manual washing of the intrarenal space through the tunnel of the ureteroscope using a syringe after the insertion of the UAS is routinely performed in our institute until debris and muddiness disappear. Because the wash decreased the amounts of bacteria and endotoxic substrates that may induce urinary tract infection and sepsis, this was thought to have strongly masked the effect of the gap. In general, the rates of complications over grade 1 and septic shock (grade 4) were 6.3% and 0.78%, respectively.
This study had several limitations. Because the enrolled patients received fURS for renal stones using fragmentation techniques, our results do not apply to patients with both renal and ureteral stones or patients receiving fURS using dusting techniques. Also, as the small gap groups only included six patients, a further assessment with a larger number of cases should be performed. Although the difference in the SF rate could be detected by the gap classification, the gap classification could not evaluate the risk of ureteral injury because it did not consider the size of the UAS. To assess ureteral injury, the size of the sheath should be considered. Although there were no cases in this study, a large UAS does carry the possibility of increasing the rate of urinary injury. This risk should be considered when fURS is performed with a UAS. This study only evaluated early complications within 2 months after surgery, and late complications were not assessed. Further, long-term observations for ureteric stenosis after fURS with UAS seem necessary. As the presence of preoperative urinary tract infection and urine culture were not included due to a high rate of missing medical records, only the presence of pyuria was included. Further research including these factors should be organized to assess the risk of urinary tract infection. Due to the retrospective nature of the analysis, these results should be confirmed by randomized controlled trials.
Conclusions
The gap classification could evaluate the effect of UAS on fURS for renal stones with the fragmentation technique accurately. To calculate the objective value of the gap, the new classification has removed the complexity of the various combinations between UAS and ureteroscope, thereby simplifying the UAS parameter. To improve UAS and ureteroscope outcomes, our results show that a gap over 0.6 mm (1.8 Fr), including the combination of a 9.5-Fr UAS and a small caliber ureteroscope, increases the SF rate, and that larger gaps facilitate the efficiency of stone removal, which should become protocol.
References
Breda A, Ogunyemi O, Leppert JT, Schulam PG (2009) Flexible ureteroscopy and laser lithotripsy for multiple unilateral intrarenal stones. Eur Urol 55:1190–1197. https://doi.org/10.1016/j.eururo.2008.06.019
El-Nahas AR, Ibrahim HM, Youssef RF, Sheir KZ (2012) Flexible ureterorenoscopy versus extracorporeal shock wave lithotripsy for treatment of lower pole stones of 10–20 mm. BJU Int 110:898–902. https://doi.org/10.1111/j.1464-410X.2012.10961.x
Cohen J, Cohen S, Grasso M (2013) Ureteropyeloscopic treatment of large, complex intrarenal and proximal ureteral calculi. BJU Int. https://doi.org/10.1111/j.1464-410X.2012.11352.x
Breda A, Ogunyemi O, Leppert JT et al (2008) Flexible ureteroscopy and laser lithotripsy for single intrarenal stones 2 cm or greater-is this the new frontier? J Urol 179:981–984. https://doi.org/10.1016/j.juro.2007.10.083
Pearle MS, Lingeman JE, Leveillee R et al (2008) Prospective randomized trial comparing shock wave lithotripsy and ureteroscopy for lower pole caliceal calculi 1 cm or less. J Urol 179:2005–2009. https://doi.org/10.1016/j.juro.2008.03.140
Komeya M, Usui K, Asai T et al (2018) Outcome of flexible ureteroscopy for renal stone with overnight ureteral catheterization: a propensity score-matching analysis. World J Urol. https://doi.org/10.1007/s00345-018-2328-1
Komeya M, Odaka H, Asano J et al (2019) Development and internal validation of a nomogram to predict perioperative complications after flexible ureteroscopy for renal stones in overnight ureteral catheterization cases. World J Urol. https://doi.org/10.1007/s00345-019-03023-y
Raheem OA, Khandwala YS, Sur RL et al (2017) Burden of urolithiasis: trends in prevalence, treatments, and costs. Eur Urol Focus 3:18–26. https://doi.org/10.1016/j.euf.2017.04.001
Giusti G, Proietti S, Villa L et al (2016) Current standard technique for modern flexible ureteroscopy: tips and tricks. Eur Urol 70:188–194. https://doi.org/10.1016/j.eururo.2016.03.035
Vanlangendonck R, Landman J (2004) Ureteral access strategies: Pro-access sheath. Urol Clin North Am 31:71–81
Breda A, Territo A, López-Martínez JM (2016) Benefits and risks of ureteral access sheaths for retrograde renal access. Curr Opin Urol 26:70–75. https://doi.org/10.1097/MOU.0000000000000233
Rehman J, Monga M, Landman J et al (2003) Characterization of intrapelvic pressure during ureteropyeloscopy with ureteral access sheaths. Urology 61:713–718
Aboumarzouk OM, Monga M, Kata SG et al (2012) Flexible ureteroscopy and laser lithotripsy for stones >2cm: a systematic review and meta-analysis. J Endourol 26:1257–1263
Traxer O, Wendt-Nordahl G, Sodha H et al (2015) Differences in renal stone treatment and outcomes for patients treated either with or without the support of a ureteral access sheath: the clinical research office of the endourological society ureteroscopy global study. World J Urol 33:2137–2144. https://doi.org/10.1007/s00345-015-1582-8
Noureldin YA, Kallidonis P, Ntasiotis P et al (2019) The effect of irrigation power and ureteral access sheath diameter on the maximal intra-pelvic pressure during ureteroscopy: in vivo experimental study in a live anesthetized pig. J Endourol 33:725–729
Tracy CR, Ghareeb GM, Paul CJ, Brooks NA (2018) Increasing the size of ureteral access sheath during retrograde intrarenal surgery improves surgical efficiency without increasing complications. World J Urol 36:971–978. https://doi.org/10.1007/s00345-018-2204-z
Ito H, Kawahara T, Terao H et al (2012) The most reliable preoperative assessment of renal stone burden as a predictor of stone-free status after flexible ureteroscopy with holmium laser lithotripsy: a single-center experience. Urology 80:524–528. https://doi.org/10.1016/j.urology.2012.04.001
Yallappa S, Metcalfe J, Subramonian K (2018) The natural history of asymptomatic calyceal stones. BJU Int 122:263–269. https://doi.org/10.1111/bju.14354
Kanda Y (2013) Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant 48:452–458. https://doi.org/10.1038/bmt.2012.244
Funding
This study was funded by a Grant-in-Aid for Scientific Research (C) 19K09718 (to M.J. and K.M.)
Author information
Authors and Affiliations
Contributions
MK: project development, data collection, and manuscript writing. KO: data collection. TW: data collection. HK: data collection. TO: project development and manuscript editing. MY: project development. JM: project development.
Corresponding author
Ethics declarations
Conflict of interest statement
There are no conflicts of interest to report.
Ethics approval
This study was approved by the Institutional Ethics Committee of Ohguchi East General Hospital.
Informed consent statement
All study participants, or their legal guardian, provided informed written consent prior to study enrollment.
Data sharing statement
No additional data are available.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Komeya, M., Odaka, H., Watanabe, T. et al. Gap between UAS and ureteroscope predicts renal stone-free rate after flexible ureteroscopy with the fragmentation technique. World J Urol 39, 2733–2739 (2021). https://doi.org/10.1007/s00345-020-03459-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00345-020-03459-7