Abstract
Objective
This meta-analysis of published case–control and cohort studies sought to quantify the magnitude and direction of association between chronic UTI (defined as the infection of the urinary tract that either does not respond to treatment or keeps recurring) and risk of bladder carcinoma (BCa) (i.e., including mainly urothelial carcinoma, squamous cell carcinoma or adenocarcinoma).
Methods
A literature search was conducted using Medline, Embase, Ovid, Web of Science, Science Direct and Cochrane Library, which was supplemented with manual search of reference lists of the identified articles. Case–control and cohort studies examining UTI as a predictor of BCa risk published through June 2016 were eligible. Using random-effects models, odds ratios (OR) or relative risks (RR) from eligible studies were combined to synthesize summary effect estimates. The included studies were assessed for methodological quality and potential publication bias. Heterogeneity by study characteristics was examined by sub-group and meta-regression analyses.
Results
Eighteen case–control and three cohort studies published between 1963 and 2016 were eligible. Random-effects models showed that UTI was significantly associated with an increased BCa risk both in case–control studies (summary ORRE = 2.33; 95% CI 1.86, 2.92) and cohort studies (summary RRRE = 2.88; 95% CI 1.20, 6.89). The observed relationship of UTI with an increased BCa risk was independent of the study characteristics considered. No significant publication bias was detected.
Conclusions
Chronic UTI was significantly and independently associated with an increased BCa risk. However, due to the presence of high between-study heterogeneity and inconsistent patterns of adjusted confounding effects, more data are needed to clarify the role of chronic UTI in causation of BCa and if established, prompt and effective treatment of UTI may minimize a substantial proportion of BCa risk.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Urinary bladder carcinoma (BCa) ranks ninth among the most common cancers and comprises 3% of all malignancies worldwide [1]. BCa is uncommon before 50 years of age but its risk increases sharply thereafter, and males are affected more than females [2]. In addition to smoking, and occupational exposure to aromatic amines [3, 4], chronic inflammation is a recognized risk factor for BCa. Inflammation is self-limiting host defense mechanism against biological, physical and chemical agents. However, its chronicity may lead to cellular damage causing various diseases including cancer [5]. Urinary tract infection (UTI) refers to the presence of microbial pathogens in urethra or bladder (lower urinary tract) or ureter and pelvis of kidney (upper urinary tract). The most common causative agent for both uncomplicated and complicated UTI is uropathogenic Escherichia coli [6]. Both host and bacterial factors influence the probability whether asymptomatic colonization resolves spontaneously or progresses to symptomatic infection [7]. Human genetic variations particularly those affecting the immune response have been associated with recurrent UTI [7,8,9]. Furthermore, UTI has a propensity to recur and individuals with a history of one or more UTI episodes have 70% increased risk of recurrence [7, 10]. However, it is uncertain whether chronic and/or recurrent UTI plays any role in causation of urothelial carcinoma of bladder [11, 12].
Recently, epidemiological evidence for an association between chronic UTI and BCa was qualitatively summarized [11, 12], which showed that both men and women with chronic UTI had an increased BCa risk [13,14,15,16,17,18,19,20,21,22]. However, some other studies did not support this notion [23,24,25]. Studies on the number and duration of UTI episodes in relation to BCa diagnosis showed that positive association tended to weaken not only with an increasing time lag between UTI and BCa diagnosis [19, 21, 23], but also with a decreasing number of UTI episodes [17, 19]. In contrast, two other studies reported a decreased BCa risk with an increasing number of kidney and bladder infections with the lowest risk for more than three UTI episodes [16, 26]. However, these studies were conducted rather in small samples and/or as sub-group analyses [12], and not surprisingly, therefore, published evidence is largely inconsistent, with wide ranging effect size estimates. This study therefore, quantitatively assessed the magnitude and direction of an overall association between chronic UTI and BCa risk using the techniques of meta-analysis.
Methods
Search strategy
This study was undertaken based on a protocol developed in accordance with Preferred Reporting Items for Systematic Review and Meta-analysis (PRISMA) [27], and Meta-analysis of Observational Studies in Epidemiology (MOOSE) statements [28]. A literature search of electronic databases including Medline (PubMed), Embase, Ovid, Science Direct, Web of Science and Cochrane Library was conducted for case–control and cohort studies examining the association between chronic UTI and BCa risk and published through June 2016. Key terms combined in Boolean search included bladder or urinary bladder or transitional cell carcinoma (or urothelial carcinoma) or squamous cell carcinoma, adenocarcinoma, cancer or carcinoma or tumor, chronic or recurrent or persistent or continuous or long duration or frequent infection or disease or bacterial urinary tract infection or lower urinary tract or cystitis. Furthermore, the reference lists of retrieved articles were reviewed for undetected relevant studies. Only studies published in the English language were considered.
Inclusion criteria
The studies meeting the following criteria were included in the meta-analysis: (1) observational studies using case–control or cohort design; (2) exposure of interest chronic and/or recurrent bacterial infection of bladder (defined as the infection of the urinary tract that either does not respond to treatment or keeps recurring) in any gender; (3) outcome was histopathology-confirmed bladder carcinoma/cancer (i.e., including mainly urothelial carcinoma (transitional cell carcinoma), squamous cell carcinoma or adenocarcinoma); (4) provided measure of association as odds ratio (OR), relative risk (RR) or hazard ratio (HR) and their 95% confidence intervals (CI) obtained through stratified analysis or multivariable regression analysis. If the data were used in more than one study, the most recent complete article was included.
Data extraction
Data were extracted from each eligible study using standard data collection sheet which included the following variables; the first author’s name, publication year, country wherein study was conducted, participants’ gender (i.e., either one or both genders), study design, sample size, the effect estimates as OR, RR or HR, their 95% CIs and confounding variables accounted for in planning and/or in analysis.
Methodological quality assessment
To assess the methodological quality of selected case–control and cohort studies, the Cochrane Collaboration-recommended 9-point Newcastle–Ottawa Scale (NOS) for nonrandomized studies was used [29]. With NOS a maximum total of 9 points is possible and a study attaining a score of 6 or more is regarded as a high quality study [30]. The quality of case–control studies was assessed in the following aspects: adequate definition of bladder cancer cases, representativeness of cases, definition and selection of controls, control for the most important factor or the second most important factor, exposure assessment, same method of exposure ascertainment in cases and controls and their non-response rates (Table 1). The quality of selected cohort studies was assessed in following aspects: representativeness of exposed and non-exposed cohorts, ascertainment of exposure, documentation that the outcome was absent in members of both the cohorts at the beginning of follow-up, comparability of cohorts based on design or analysis and unbiased assessment of outcome (Table 1).
Statistical analysis
Meta-analysis was carried out using random-effects models that took into account within and between studies’ heterogeneity [31]. Heterogeneity across the included studies was evaluated using Cochran Q statistic and was considered statistically significant if the associated p value was < 0.05. I2 metric was used to estimate the proportion of total variation across the included studies due to heterogeneity rather than by chance and was interpreted as absent (I2: 0–25%), low (I2: 25.1–50%), moderate (I2: 50.1–75%) or high (I2: 75.1–100%) [32]. A pre-defined set of sub-group analyses were conducted to examine between-group differences in random-effects summary estimates of BCa risks by chronic UTI across the studies. Sub-groups were based on study location (USA, Europe and others), study period (before and/or during 1990 vs. after 1990) and exposure definition (cystitis, chronic UBI or chronic/recurrent UTI). A random-effects meta-regression analysis was conducted for quantitative factors including sample size, proportion of male cases and NOS score to examine their potential modulating effects on the relationship between Chronic UTI and BCa risk. Potential publication bias was assessed using funnel plot, Egger’s regression asymmetry test and Begg’s rank correlation test [32, 33].
Sensitivity analysis
An influence analysis was conducted quantitatively by examining the sensitivity of heterogeneity metrics to exclusion of studies one at a time and in combination of two or more thereof. Accordingly, a sequential algorithm was used to identify which of the studies singly or in combination minimizes I2, and \(\tau^{2}\), and Higgins’ H statistics less than their threshold values for low, moderate, or high heterogeneity [32, 34]. The objective of this uncertainty analysis was to assess the robustness of conclusions concerning the size and the direction of adjusted effect estimates yielded by the meta-analysis [35].
Results
Selection process
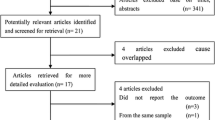
A total of 2740 potentially relevant articles identified by the search of PubMed (522), Embase (372), web of Science (122), Ovid (468), Cochrane Library (258) and Science Direct (998). Duplicate articles (294) articles were excluded. Additionally, 2419 studies were excluded by screening the titles and/or abstracts. Remaining 27 articles were retrieved for full text review. From the reference lists of the retrieved articles, four relevant articles were identified for inclusion in the meta-analysis. Subsequently, after detailed evaluation,10 articles were excluded for various reasons (Fig. 1). Finally, remaining 21 studies (18 case–control and 3 cohort studies) were included in the meta-analysis.
Study characteristics
Eighteen case–control [12,13,14,15,16,17,18,19,20, 22,23,24,25,26, 36,37,38,39], and three cohort studies [21, 40, 41] met the inclusion criteria. Of 18 case–control studies, 9 were published from USA [14,15,16,17, 20, 22, 25, 26, 37], 2 each from Germany [13, 18] and Italy [19, 23], and 1 each from UK [42], Denmark [24], Iran [38], Turkey [36], and Netherlands [12]. Seven of 18 case–control studies were pair-matched. The included 18 case–control studies widely varied in their sample sizes (cases/controls) from the smallest (173/173) to the largest (4915/21718). Only one study exclusively included female cases and controls, whereas, remaining 17 studies included both males and females among cases and controls. The proportion (%) of male cases ranged from zero to 90% in 18 case–control studies. Eight of 18 case–control studies presented the OR and its 95% CI, while remaining 10 studies presented RR and its 95% CI as a measure of association between chronic UTI and BCa. The number and type of adjusted confounding effects varied across the 18 case–control studies (Table 1a).
Three cohort studies included in this meta-analysis were reported from Sweden [40], Taiwan [21], and USA [41]. These three cohort studies reported a total of 2,606,179 person-years at risk and 1105 BCa cases developed during the follow-up period [21, 40, 41]. One of the cohort studies, retrospectively tracked the BCa patients (12,195; men = 9326; women = 2869), who reportedly either had hematuria (81.4% men; 60.5% women or UTI (18.6% men; 39.5% women) as a presenting claim at the index visit within one year (follow up of 1 year) preceding the diagnosis of BCa [41]. One cohort study presented the standardized incidence ratio [40]. while remaining two cohort studies presented adjusted hazard ratio (HR) along with 95% CIs as a measure of association between chronic UTI and BCa [21, 41]. Follow-up period in three cohort studies ranged from 1 to 25 years (Table 1b).
Association between chronic UTI and bladder carcinoma risk
Case–control and cohort studies
The random-effects model based summary estimate from 18 case–control studies showed that chronic UTI was significantly and independently associated with an increased BCa risk (ORRE = 2.33; 95%CI 1.86, 2.92) (Fig. 2). This meta-analysis of case–control studies detected a significant (p < 0.001) heterogeneity (I2 = 91.7%; 95% UI 88.3, 94.4; Higgins’ H = 3.6; 95% UI 3.0, 4.2). The random-effects model based pooled effect-estimate from three cohort studies showed that patients with chronic UTI had significantly increased BCa risk compared to individuals without chronic UTI (RRRE = 2.88; 95% CI 1.20, 6.89). The summary RRRE from three cohort studies had nearly the same magnitude as that of summary estimate ORRE sought from 18 case–control studies (Fig. 2). Furthermore, a statistically significant (p < 0.001) heterogeneity across the effect-estimates from three cohort studies was also detected (I2 = 96.2%, 95% UI 92.0, 98.2; Higgins’ H statistic = 7.3; 95% CI 4.8, 11.1).
Sub-group and random-effects meta-regression analyses
To evaluate the possible sources of between-study heterogeneity, 18 case–control studies on chronic UTI and BCa risk were further examined either by sub-group analyses (study location, study period, exposure definition) or by random-effects meta-regression analysis (sample size, proportion of male cases, NOS score). Sub-group analyses showed that the increased BCa risk associated with chronic UTI was independent of studies’ geographic location (p = 0.061), study period (p = 0.440), and exposure definition (p = 0.843) (Table 2). Additionally, no considerable evidence of modulating effect was found by study sample size (p = 0.421), proportion of male cases (p = 0.395) and NOS score (p = 0.583) on the association between chronic UTI and BCa risk.
Publication bias
Visual examination of funnel plot from meta-analysis of 18 case–control studies showed little asymmetry. Quantitatively, no evidence of significant publication bias was observed using Egger’s regression asymmetry test (Egger’s p = 0.053) and Begg’s rank correlation test (Begg’s p = 0.192).
Sensitivity analysis
Sensitivity analysis by excluding the studies with the highest or the lowest effect estimates singly or in combination did not show a substantial reduction in value of I2 (approximate proportion of heterogeneity in adjusted summary ORRE for BCa). Furthermore, the direction and magnitude of adjusted summary ORRE did not change meaningfully (Table 3). Additionally, cumulative analyses of 18 case–control and three cohort studies showed no meaningful change in trend of reporting BCa risk over the studies’ period. Thus, all the studies considered in sensitivity analysis were retained in the final meta-analysis.
Discussion
Infection is one of the main contributors to cancer incidence. During 2008, 12.7 million new cancer cases occurred worldwide and of these about 2 million (16%) were attributable to infectious agents. This fraction was higher in less developed (22.9%) than developed (7.4%) regions of the world [43]. BCa is the 13th most common cause of cancer-related deaths worldwide [44]. Epidemiologic studies have inconsistent results regarding UTI as a risk factor for urothelial carcinoma (previously known as transitional cell carcinoma) of the bladder, which accounts for about 90% of the bladder cancers [45, 46]. Therefore, this study quantitatively assessed the magnitude of an overall association between the chronic UTI and BCa risk using the techniques of meta-analysis.
Main findings
The meta-analysis of eligible 18 case–control and 3 cohort studies provided robust evidence for an increased BCa risk among those with chronic/recurrent UTI compared to those without it. This seems to be the first study that has meta-analyzed the association between chronic/recurrent UTI and BCa risk. Therefore, the results from any related previous meta-analysis of case–control and/or cohort studies exclusively addressing this question were unavailable for relative appraisal. Chronic UTI and resultant cystitis have been implicated in the occurrence and progression of BCa [44, 47]. Globally bladder cancer cases attributable to infection have declined to 1.6% over the years [48]. Despite this decline, still a very high population risk of BCa is attributed to chronic UTI (41%; 95% CI 36, 48%) [44].
Several mechanistic pathways have been proposed by which infectious agents contribute in causation of BCa. Of infectious agents, E. coli is a predominant (70–80%) uropathogen. Additionally, evidence from an experimental study suggested that UTI with E. coli play a key additive and synergistic role during bladder carcinogenesis [49]. Because of recurrent bacterial UTI, chronic inflammation may lead to carcinogenesis and one of the key molecules that link chronic inflammation and cancer is represented by the NF-κB family of transcription factors [50, 51]. Furthermore, it has been argued that once the cystitis is initiated by infectious agents, it can mediate pathogenesis of BCa by stimulating cancer cell growth, invasion and metastasis through the recruitment of inflammatory cells and signaling molecules [52]. Cystitis may also increase absorption of the carcinogens. Furthermore, bacterial flora in the urine may contribute to the production of nitrites that are converted to carcinogenic nitrosamines [53,54,55]. Paradoxically, however, it has been suggested that the patients with recurrent UTI have reduced BCa risk, probably due to anti-cancer effect of antibiotic treatment for bladder infection, higher exposure to non-steroidal anti-inflammatory drugs of patients with history of bladder infection and/or immune response triggered by UTI [26]. Furthermore, it may well be that cystitis of infectious origin may merely represent a complication of cancer during its early growth before clinical diagnosis [17].
Adjustment for confounding
Although sub-group-specific risk estimates showed statistically non-significant differences for study location, study period and exposure definition, yet these differences reflected the existence of some residual confounding. For example, regarding the study location, sub-group-specific summary ORRE turned out to be relatively stronger for seven studies from Europe, whereas, this relationship was attenuated for nine studies conducted in USA than the overall summary ORRE for 18 studies together. Similarly, sub-group-specific summary ORRE was relatively stronger for studies published after 1990 than of those published during or before 1990. Additionally, differences among sub-group-specific estimates by exposure definitions were statistically non-significant, though studies wherein exposure was defined as chronic UTI suggested slightly stronger relationship with BCa. Random-effects meta-regression analyses showed statistically non-significant effect of proportion of male cases, sample size and NOS score on the adjusted summary ORRE from the main analysis.
Heterogeneity analysis
In this meta-analysis, a high between-study heterogeneity across the size of effect estimates was noted both for 18 case–control and 3 cohort studies on a previously described scale [56]. However, this heterogeneity could not be explained in sub-group analyses by study location, study period, exposure definition, sample size, proportion of male cases and/or NOS score. Nonetheless, this observed high between-study heterogeneity might be due to one or more of the life styles, occupational and nutritional factors that might be moderating the associations between chronic UTI and BCa risk across studied populations. Previously, disproportionate prevalence of risk factors such as cigarette smoking, environmental exposure to tobacco smoke, occupational exposures, quality of drinking water, dietary factors, including animal fats, processed meet, insufficient fruit and vegetables consumption, genetic polymorphism and use of drugs (e.g., phenacetin cyclophosphamide) with fluctuating strengths of their associations with BCa have been reported [57]. These known BCa risk factors have not been substantively considered in the studies included in this meta-analysis. Therefore, varying prevalence of these known factors might have interplayed with chronic UTI in increasing the BCa risk in studied populations and contributed to high between-study heterogeneity in this meta-analysis. Evaluation of such factors may be an impetus to future research.
Strengths and limitations of this study
This study seems to be the first one, which meta-analyzed the relationship between chronic UTI and BCa based on 18 case–control and three cohort studies. Furthermore, no significant publication bias could be detected. Additionally, the results were substantially robust on sub-group analyses, meta-regression and sensitivity analysis. Moreover, all the included case–control and cohort studies employed histopathologic evidence as a standard diagnostic tool for BCa.
This study has few limitations. First, there were only three cohort studies, therefore, temporal relationship between chronic UTI and BCa was assumed mainly based on analysis of case–control studies. However, the direction and strength of association between chronic UTI and BCa sought in meta-analyses of two groups of studies under two different study designs, conducted at various times and on different populations were consistent. Second; though the usage of varying exposure definition (i.e., chronic UTI) across the studies did not turn out to be a significant source of between-study heterogeneity, yet a consistent exposure definition and its assessment would have been reassuring about the observed association. Third, all the case–control studies did not report duration of chronic UTI before BCa diagnosis, therefore, median induction period for this exposure could not be ascertained. Therefore, future studies, may examine this variable in relation to BCa risk. Finally, the association between chronic UTI and BCa in majority of the case–control studies included in this meta-analysis came from sub-group analyses. Therefore, the statistical power of the studies to estimate the effect measure of interest could have been an issue. Future research on this question may consider this aspect.
In conclusion, the finding of this meta-analysis of 18 case–control and 3 cohort studies suggested a significantly increased BCa risk among patients with chronic infection of lower urinary tract/cystitis. Nonetheless, due to the presence of inevitable between-study heterogeneity and inconsistent patterns of adjusted confounding effects by the included studies, more data are needed to clarify the role of chronic UTI in causation of BCa and if established, prompt and effective treatment of UTI may minimize a substantial proportion of BCa risk.
Abbreviations
- UTI:
-
Urinary tract infection
- BCa:
-
Bladder carcinoma
- OR:
-
Odds ratio
- RR:
-
Relative risk
- Hazard ratio:
-
Hazard ratio
- PRISMA:
-
Preferred Reporting Items for Systematic Review and Meta-analysis
- MOOSE:
-
Meta-analysis of Observational Studies in Epidemiology Statements
- NOS:
-
Newcastle–Ottawa Scale
References
Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F (2015) Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 136(5):E359–386. https://doi.org/10.1002/ijc.29210
Bladder Cancer Statistics (2016) http://www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/bladder-cancer. Accessed 18 Mar 2017
WHO I (1982) Monographs on the evaluation of the carcinogenic risk of chemicals to humans. Chemicals, industrial processes and industries associated with cancer in humans. In: Group IW (ed) Some Industrial Chemicals and Dyestuffs
Marrett LD, Hartge P, Meigs JW (1986) Bladder cancer and occupational exposure to leather. Br J Ind Med 43(2):96–100
Leibovici D, Grossman HB, Dinney CP, Millikan RE, Lerner S, Wang Y, Gu J, Dong Q, Wu X (2005) Polymorphisms in inflammation genes and bladder cancer: from initiation to recurrence, progression, and survival. J Clin Oncol 23(24):5746–5756. https://doi.org/10.1200/jco.2005.01.598
Flores-Mireles AL, Walker JN, Caparon M, Hultgren SJ (2015) Urinary tract infections: epidemiology, mechanisms of infection and treatment options. Nat Rev Microbiol 13(5):269–284. https://doi.org/10.1038/nrmicro3432
Foxman B (2010) The epidemiology of urinary tract infection. Nat Rev Urol 7(12):653–660
Hawn TR, Scholes D, Wang H, Li SS, Stapleton AE, Janer M, Aderem A, Stamm WE, Zhao LP, Hooton TM (2009) Genetic variation of the human urinary tract innate immune response and asymptomatic bacteriuria in women. PLoS One 4(12):e8300. https://doi.org/10.1371/journal.pone.0008300
Lundstedt AC, Leijonhufvud I, Ragnarsdottir B, Karpman D, Andersson B, Svanborg C (2007) Inherited susceptibility to acute pyelonephritis: a family study of urinary tract infection. J Infect Dis 195(8):1227–1234. https://doi.org/10.1086/512620
Hooton TM, Scholes D, Hughes JP, Winter C, Roberts PL, Stapleton AE, Stergachis A, Stamm WE (1996) A prospective study of risk factors for symptomatic urinary tract infection in young women. N Engl J Med 335(7):468–474. https://doi.org/10.1056/nejm199608153350703
Anderson-Otunu O, Akhtar S (2016) Chronic infections of the urinary tract and bladder cancer risk: a systematic review. Asian Pac J Cancer Prev 17(8):3805–3807
Vermeulen SH, Hanum N, Grotenhuis AJ, Castano-Vinyals G, van der Heijden AG, Aben KK, Mysorekar IU, Kiemeney LA (2015) Recurrent urinary tract infection and risk of bladder cancer in the Nijmegen bladder cancer study. Br J Cancer 112(3):594–600. https://doi.org/10.1038/bjc.2014.601
Claude J, Kunze E, Frentzel-Beyme R, Paczkowski K, Schneider J, Schubert H (1986) Life-style and occupational risk factors in cancer of the lower urinary tract. Am J Epidemiol 124(4):578–589
Dunham LJ, Rabson AS, Stewart HL, Frank AS, Young JL (1968) Rates, interview, and pathology study of cancer of the urinary bladder in New Orleans, Louisiana. J Natl Cancer Inst 41(3):683–709
Howe GR, Burch JD, Miller AB, Cook GM, Esteve J, Morrison B, Gordon P, Chambers LW, Fodor G, Winsor GM (1980) Tobacco use, occupation, coffee, various nutrients, and bladder cancer. J Natl Cancer Inst 64(4):701–713
Jhamb M, Lin J, Ballow R, Kamat AM, Grossman HB, Wu X (2007) Urinary tract diseases and bladder cancer risk: a case-control study. Cancer Causes Control 18(8):839–845. https://doi.org/10.1007/s10552-007-9028-2
Kantor AF, Hartge P, Hoover RN, Narayana AS, Sullivan JW, Fraumeni JF (1984) Urinary tract infection and risk of bladder cancer. Am J Epidemiol 119(4):6
Kunze E, Chang-Claude J, Frentzel-Beyme R (1992) Life style and occupational risk factors for bladder cancer in Germany. A case-control study. Cancer 69(7):1776–1790
La Vecchia C, Negri E, D’Avanzo B, Savoldelli R, Franceschi S (1991) Genital and urinary tract diseases and bladder cancer. Can Res 51(2):629–631
Sturgeon SR, Hartge P, Silverman DT, Kantor AF, Linehan WM, Lynch C, Hoover RN (1994) Associations between bladder cancer risk factors and tumor stage and grade at diagnosis. Epidemiology (Cambridge, Mass) 5(2):218–225
Sun LM, Lin CL, Liang JA, Liu SH, Sung FC, Chang YJ, Kao CH (2013) Urinary tract infection increases subsequent urinary tract cancer risk: a population-based cohort study. Cancer Sci 104(5):619–623. https://doi.org/10.1111/cas.12127
Wynder EL, Onderdonk J, Mantel N (1963) An epidemiological investigation of cancer of the bladder. Cancer 16:1388–1407
Gonzalez CA, Errezola M, Izarzugaza I, Lopez-Abente G, Escolar A, Nebot M, Riboli E (1991) Urinary infection, renal lithiasis and bladder cancer in Spain. Eur J Cancer (Oxford, England: 1990) 27(4):498–500
Kjaer SK, Knudsen JB, Sorensen BL, Moller Jensen O (1989) The Copenhagen case-control study of bladder cancer. V. Review of the role of urinary-tract infection. Acta Oncolog (Stockholm, Sweden) 28(5):631–636
Piper JM, Matanoski GM, Tonascia J (1986) Bladder cancer in young women. Am J Epidemiol 123(6):1033–1042
Jiang X, Castelao JE, Groshen S, Cortessis VK, Shibata D, Conti DV, Yuan JM, Pike MC, Gago-Dominguez M (2009) Urinary tract infections and reduced risk of bladder cancer in Los Angeles. Br J Cancer 100(5):834–839. https://doi.org/10.1038/sj.bjc.6604889
Moher D, Liberati A, Tetzlaff J, Altman DG (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ (Clinical research ed) 339:b2535. https://doi.org/10.1136/bmj.b2535
Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, Moher D, Becker BJ, Sipe TA, Thacker SB (2000) Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA 283(15):2008–2012
Stang A (2010) Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol 25(9):603–605. https://doi.org/10.1007/s10654-010-9491-z
Wells GA SB, O’Connell D, Peterson J, Welch V, Losos M, GA Wells, Tugwell P (2012) The Newcastle-Ottawa Scale (NOS) for assessing the quality if nonrandomized studies in meta-analyses. Ottawa Hospital Research Institute, Canada
DerSimonian R (1996) Meta-analysis in the design and monitoring of clinical trials. Stat Med 15(12):1237–1248. (discussion 1249-1252). https://doi.org/10.1002/(SICI)1097-0258(19960630)15:12<1237::AID-SIM301>3.0.CO;2-N
Higgins JP, Thompson SG, Deeks JJ, Altman DG (2003) Measuring inconsistency in meta-analyses. BMJ (Clinical research ed) 327(7414):557–560. https://doi.org/10.1136/bmj.327.7414.557
Egger MDSG, Schneider M, Minder C (1997) Bias in meta-analysis detected by a simple, graphical test. BMJ (Clinical research ed) 315:6
Patsopoulos NA, Evangelou E, Ioannidis JP (2008) Sensitivity of between-study heterogeneity in meta-analysis: proposed metrics and empirical evaluation. Int J Epidemiol 37(5):1148–1157. https://doi.org/10.1093/ije/dyn065
Higgins JP (2008) Commentary: heterogeneity in meta-analysis should be expected and appropriately quantified. Int J Epidemiol 37(5):1158–1160. https://doi.org/10.1093/ije/dyn204
Erdurak K, Dundar PE, Ozyurt BC, Negri E, La Vecchia C, Tay Z (2014) Smoking, occupation, history of selected diseases and bladder cancer risk in Manisa, Turkey. Eur J Cancer Prev 23(1):58–61. https://doi.org/10.1097/CEJ.0b013e3283631dde
Hartge P, Harvey EB, Linehan WM, Silverman DT, Sullivan JW, Hoover RN, Fraumeni JF Jr (1990) Unexplained excess risk of bladder cancer in men. J Natl Cancer Inst 82(20):1636–1640
Shakhssalim N, Hosseini SY, Basiri A, Eshrati B, Mazaheri M, Soleimanirahbar A (2010) Prominent bladder cancer risk factors in Iran. Asian Pac J Cancer Prev 11(3):601–606
Shephard EA, Stapley S, Neal RD, Rose P, Walter FM, Hamilton WT (2012) Clinical features of bladder cancer in primary care. The British journal of general practice: the journal of the Royal College of General Practitioners 62(602):e598–604. https://doi.org/10.3399/bjgp12X654560
Chow WH, Lindblad P, Gridley G, Nyren O, McLaughlin JK, Linet MS, Pennello GA, Adami HO, Fraumeni JF Jr (1997) Risk of urinary tract cancers following kidney or ureter stones. J Natl Cancer Inst 89(19):1453–1457
Richards KA, Ham S, Cohn JA, Steinberg GD (2016) Urinary tract infection-like symptom is associated with worse bladder cancer outcomes in the Medicare population: implications for sex disparities. Int J Urol 23(1):42–47. https://doi.org/10.1111/iju.12959
Shephard E, Neal R, Rose P, Walter F, Hamilton WT (2013) Clinical features of kidney cancer in primary care: a case-control study using primary care records. Br J Gen Pract 63(609):e250–255. https://doi.org/10.3399/bjgp13X665215
de Martel C, Ferlay J, Franceschi S, Vignat J, Bray F, Forman D, Plummer M (2012) Global burden of cancers attributable to infections in 2008: a review and synthetic analysis. Lancet Oncol 13(6):607–615. https://doi.org/10.1016/s1470-2045(12)70137-7
Parkin DM, Pisani P, Fau-Lopez AD, Lopez AD, Fau-Masuyer E, Masuyer E At least one in seven cases of cancer is caused by smoking. Global estimates for 1985. (0020-7136 (Print))
Abol-Enein H (2008) Infection: is it a cause of bladder cancer? Scand J Urol Nephrol Suppl 218:79–84. https://doi.org/10.1080/03008880802325309
Pelucchi C, Bosetti C, Negri E, Malvezzi M, La Vecchia C (2006) Mechanisms of disease: the epidemiology of bladder cancer. Nat Clin Pract Urol 3(6):327–340. https://doi.org/10.1038/ncpuro0510
Pasin E, Josephson DY, Mitra AP, Cote RJ, Stein JP (2008) Superficial bladder cancer: an update on etiology, molecular development, classification, and natural history. Rev Urol 10(1):31–43
Parkin DM (2008) The global burden of urinary bladder cancer. Scand J Urol Nephrol 218:12–20
El-Mosalamy H, Salman TM, Ashmawey AM, Osama N (2012) Role of chronic E. coli infection in the process of bladder cancer- an experimental study. Inf Agents Cancer 7(1):19. https://doi.org/10.1186/1750-9378-7-19
Karin M, Greten FR (2005) NF-kappaB: linking inflammation and immunity to cancer development and progression. Nat Rev Immunol 5(10):749–759. https://doi.org/10.1038/nri1703
Lax AJ, Thomas W (2002) How bacteria could cause cancer: one step at a time. Trends Microbiol 10(6):293–299
Nesi G, Nobili S, Cai T, Caini S, Santi R (2015) Chronic inflammation in urothelial bladder cancer. Virchows Archiv 467(6):623–633. https://doi.org/10.1007/s00428-015-1820-x
Hicks R, Walters C, Elsebai I, Aasser A, Merzabani M, Gough T (1977) Demonstration of nitrosamines in human urine: preliminary observations on a possible etiology for bladder cancer in association with chronic urinary tract infections. Proc R Soc Med 70(6):1
Johansson S, Wahlqvist L (1977) Tumours of urinary bladder and ureter associated with abuse of phenacetin-containing analgesics. Acta Pathol Microbiol Scand Sect A Pathol 85A(6):768–774. https://doi.org/10.1111/j.1699-0463.1977.tb03892.x
Radomski JL, Greenwald D, Hearn WL, Block NL, Woods FM (1978) Nitrosamine formation in bladder infections and its role in the etiology of bladder cancer. J Urol 120(1):48–50
Higgins JP, Thompson SG (2002) Quantifying heterogeneity in a meta-analysis. Stat Med 21(11):1539–1558. https://doi.org/10.1002/sim.1186
Jankovic S, Radosavljevic V (2007) Risk factors for bladder cancer. Tumori 93(1):4–12
Acknowledgements
The senior author works for Faculty of Medicine, Kuwait University as a Professor of Epidemiology and gratefully acknowledges their support.
Funding
This study did not receive any specific funding.
Author information
Authors and Affiliations
Contributions
SA conceived the idea, did literature search. SA, AA, JA assessed the studies and extracted the relevant data. SA analyzed the data and wrote the first draft. All authors contributed to further drafts and final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflicts of interest.
Ethical approval
Ethical approval for this study was neither required nor was it solicited.
Research involving Human Participants and/or Animals
This study did require direct contact with human participants.
Informed consent
The requirement of seeking informed consent was not applicable in this study.
Rights and permissions
About this article
Cite this article
Akhtar, S., Al-Shammari, A. & Al-Abkal, J. Chronic urinary tract infection and bladder carcinoma risk: a meta-analysis of case–control and cohort studies. World J Urol 36, 839–848 (2018). https://doi.org/10.1007/s00345-018-2206-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00345-018-2206-x