Abstract
Purpose
To determine the efficacy and safety of dutasteride, alone or in combination, versus a placebo or control, used for the treatment of benign prostatic hyperplasia.
Methods
Pubmed® and the Cochrane Library were searched for randomized controlled trials longer than 6 months in duration. The subjects in the trials were men aged 40 or over, with moderate to severe symptoms of benign prostatic hyperplasia (BPH) as determined by International Prostate Symptom Score (IPSS). We pooled data from a total of nine different clinical trials.
Results
Dutasteride was superior to placebo in improving urinary symptoms measured by IPSS (∆ = −1.78, 95 % CI −3.01 to −0.55), peak urinary flow (Q max) (∆ = 1.27 mL/s, 95 % CI 0.97–1.57), and change in total prostate volume (TPV) (∆ = −17.40 cm3, 95 % CI −25.77 to −9.02) while it resulted in more frequent drug-related adverse events (RR 1.35, 95 % CI 1.19–1.54). Combination therapy with dutasteride and tamsulosin resulted in significantly greater improvements in IPSS and Q max than tamsulosin monotherapy (∆ = −1.80 mL/s, 95 % CI −1.81 to −1.79 and ∆ = 1.60 mL/s, 95 % CI 1.59–1.61, respectively). When comparing dutasteride with finasteride, no significant differences in symptom improvement or the rate of adverse events were observed.
Conclusions
Dutasteride can be used to improve urinary symptoms (IPSS and Q max) and reduce TPV but with awareness of its potential adverse events. Combination therapy with tamsulosin can be considered when further improvements in symptoms are desired.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Benign prostatic hyperplasia (BPH) almost inevitably afflicts men. About 40 % of men in their 50s and 90 % of men in their 80s have histological evidence of BPH [1]. Many individuals with BPH develop lower urinary tract symptoms, which are associated with prostatic enlargement and bladder outlet obstruction [2]. Men with BPH experience obstructive and irritative symptoms including decreased peak urinary flow rate, incomplete bladder emptying, and greater risks of acute urinary retention (AUR) and BPH-related surgery, all of which have an enormous negative impact on quality of life. Therefore, the goal of treatment of BPH focuses on relieving these bothersome and irritative symptoms.
Among diverse treatment options, pharmacological therapy is most commonly reserved for men with moderate to severe BPH because it helps to reduce BPH symptoms and relieve long-term risk of AUR and surgery. Of the prescription medications, alpha 1-adrenoreceptor antagonists (alpha-blockers) and five-alpha-reductase inhibitors (5-ARIs) are currently the most frequently considered to treat BPH. Alpha-blockers have been known to improve symptoms by reducing functional obstructions but do not decrease prostate volume. However, 5-ARIs such as dutasteride and finasteride decrease the risk of AUR and surgery by reducing prostate volume [3–5]. The 5-ARIs inhibit BPH-related disease progression by blocking 5-alpha reductase (5AR) enzyme that converts testosterone to dihydrotestosterone (DHT), the hormone responsible for prostate growth. Finasteride, a selective inhibitor of type II 5AR, reduces circulating DHT levels by approximately 70 % [4], whereas dutasteride is a dual inhibitor of both type I and type II 5AR, reducing DHT levels almost completely in both the serum and the prostate [6].
Although there has been a previous study that reviewed the clinical effectiveness and adverse events of finasteride [7], no study to date has systematically reviewed the comparative effectiveness and safety of dutasteride. This study summarizes the evidence of the efficacy and adverse events of dutasteride used for the treatment of symptomatic BPH.
Materials and methods
Search strategy and inclusion criteria
To identify previously published articles up to May 2013, the electronic database Pubmed® and the Cochrane Library were searched using the BPH synonyms (e.g., prostatic hyperplasia, BPH, benign prostatic hypertrophy, BPH) in combination with the term “dutasteride,” “avodart,” or “5AR inhibitor.” The search was limited to articles published in the English language.
Studies were selected if they involved randomized controlled trials (RCTs) of greater than 6 months duration, comparing the efficacy and safety of dutasteride, alone or in combination, to placebo or control. To assess the long-term effects of treatments with dutasteride, we included randomized controlled studies, followed by open-label extension. However, we pooled these extended studies and the RCTs separately in our meta-analysis because patients in the extended studies were no longer randomized during the open-label phase. Subjects in the trials were limited to men aged 40 or over, with moderate to severe symptoms of BPH as determined by the International Prostate Symptom Score (IPSS). Studies were excluded if they were reviews or abstracts presented at meetings. If multiple studies were available from the same population but with different treatment durations, the longest study was selected for the same outcome measures.
The primary outcome of interest was improvement in urinary symptoms measured by IPSS, compared with placebo or control. Secondary outcomes included peak urinary flow (Q max) (mL/s), serum DHT (pg/mL), serum testosterone (pg/mL), total prostate volume (TPV) (cm3) or its change (%), and the incidence of adverse events and treatment withdrawal due to adverse events.
Data extraction and appraisal of study quality
Two reviewers (TP and JC) independently assessed whether the studies met the inclusion criteria and then extracted data from the selected studies. Any discrepancies were documented, discussed, and resolved by consensus. To assess the methodological quality of the included studies, we used a Jadad scale which considered the methods of randomization and blinding and the reason for patients’ withdrawals [8]. The risk of bias was considered as “high” or “low” when the Jadad score was in the range of 0–2 or 3–5, respectively.
Statistical analysis
Treatment effects were measured in accordance with the Cochrane handbook for systematic reviews of interventions [9]. Accordingly, risk ratio (RR) was calculated to indicate effective measures for dichotomous outcomes; mean difference was estimated for continuous outcomes, both with 95 % confidence intervals (CIs). To integrate the findings of multiple studies, a meta-analysis was conducted; continuous outcomes were investigated by comparing the weighted mean difference (WMD), and RR was examined for binary outcomes. If standard deviations (SDs) for pre- and post-treatment mean values were not reported in the original study, we imputed SDs using the arithmetic mean of available SDs rather than excluding the study to attenuate any loss in power and to avoid bias [10].
Heterogeneity was assessed using I 2 statistics. For outcomes that showed significant heterogeneity with an I 2 value exceeding 50 %, a random effects model was employed. Otherwise, a fixed effects model was used. Publication bias was assessed using the Begg-adjusted rank correlation test and visual inspection of funnel plots. We used Stata® version 11.0 for all analyses.
Results
Description of studies
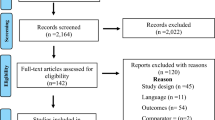
Figure 1 shows the number of articles identified, screened, and included. Our search strategy resulted in 11 articles being included in the final analysis. These 11 articles involving a total of 12,129 patients included seven different RCTs and two open-label extension studies after double-blind clinical trials. Different outcomes from the same clinical trials were sometimes reported in separate articles. Characteristics and methodological quality of included studies are presented in Table 1. Several studies compared the efficacy and safety of dutasteride to those of either placebo or finasteride. In the open-label extension studies, patients who initially received dutasteride in the previous double-blind phase were maintained on dutasteride during the open-label phase (D/D group), while those assigned to placebo in the prior phase were switched to open-label dutasteride (P/D group) [11–13]. To investigate whether combination therapy was more effective than monotherapy, some studies compared patients who received both dutasteride and tamsulosin to those with tamsulosin alone.
All included studies noted blinding and randomization in their trials except in two incidences: one study where blinding was not explicitly stated [14]; and open-label extensions of placebo-controlled trials [11–13]. However, the specific methods of blinding and random allocation were not clearly explained in these studies, placing their Jadad scores in the range of 3–5 (low risk of bias).
Efficacy of dutasteride interventions
Pooled data demonstrated a significantly greater reduction in symptom scores (IPSS) in the dutasteride group compared with placebo (WMD −1.78, 95 % CI −3.01 to −0.55) with evidence of heterogeneity (p = 0.04, I 2 = 69.0 %) (Fig. 2). In addition, dutasteride was significantly superior to placebo in increasing Q max (WMD 1.27, 95 % CI 0.97–1.57) and decreasing TPV (change in TPV (%), WMD −17.40, 95 % CI −25.77 to −9.02). The treatment effects of dutasteride were supported by a significant reduction in serum DHT (WMD −385.77, 95 % CI −394.60 to −376.95) and increase in serum testosterone (WMD 813.55, 95 % CI 717.32–909.77).
In the double-blind studies followed by the open-label phases, the D/D group improved the symptom scores (IPSS) greater than the P/D group (WMD −0.98, 95 % CI −1.55 to −0.41). Although an increase in Q max and a reduction in TPV were slightly greater in the D/D group, the differences between the two groups were not significant (p = 0.60 and p = 0.50, respectively).
Combination therapy with dutasteride and tamsulosin resulted in significantly greater improvement in the symptom scores (IPSS) and Q max compared with tamsulosin monotherapy (WMD −1.80, 95 % CI −1.81 to −1.79 and WMD 1.60, 95 % CI 1.59–1.61, respectively).
Safety of dutasteride interventions
Pooled data indicated adverse events and drug-related adverse events were more significantly common in patients treated with dutasteride compared with placebo (RR 1.04, 95 % CI 1.00–1.07 and RR 1.35, 95 % CI 1.19–1.54, respectively) (Fig. 3). They also more frequently reported sexual adverse events, including erectile dysfunction, decreased libido, and gynecomastia (RR 1.83, 95 % CI 1.42–2.36, RR 2.00, 95 % CI 1.42–2.83, and RR 3.11, 95 % CI 1.79–5.40, respectively). However, the occurrence rates of withdrawal due to adverse events, serious events, and stomach discomfort were not significantly different between the two groups (p’s = 0.92, 0.25, and 0.98 respectively).
Forest plot and meta-analysis of dutasteride safety. aPatients who had been taking tamsulosin before participating in the trial were excluded in our analysis. a Dutasteride versus placebo. b Dutasteride/dutasteride versus placebo/dutasteride during open-label period. c Combination therapy versus monotherapy. d Dutasteride versus finasteride
Between the D/D and P/D groups, no significant differences were observed in adverse events, withdrawal due to adverse events, drug-related adverse events, and serious events during the open-label phase period (p’s = 0.713, 0.396, 0.956, and 0.351, respectively).
Patients who received combination therapy experienced more sexual adverse events such as erectile dysfunction and decreased libido compared with the tamsulosin monotherapy group (RR 1.82, 95 % CI 1.41–2.36 and RR 2.02, 95 % CI 1.36–3.00, respectively). Although retrograde ejaculation occurred more frequently in the combination group, it was not statistically significant (p = 0.23). There was no significant difference in the incidence of dizziness between the groups (p = 0.91).
When comparing patients treated with dutasteride and finasteride, no significant differences in adverse events and drug-related adverse events were observed (p = 0.75 and p = 0.23, respectively).
Begg-adjusted rank correlation tests and funnel plots indicated no obvious publication bias in our analyses (data were not shown).
Discussion
To our knowledge, this is the first systematic review and meta-analysis to quantify the efficacy and safety of dutasteride for the treatment of BPH. Our results demonstrated that dutasteride, compared with placebo, significantly improved the symptom score (IPSS), peak urinary flow, and TPV. Treatment effects of dutasteride were observed with evidence of significantly reduced DHT and increased testosterone in the serum. However, adverse events including drug-related adverse events and sexual adverse events were more commonly reported in the treatment group than in the placebo group. We also found a greater reduction in IPSS for the D/D group compared with the P/D group although there were no significant differences in Q max, TPV, and the frequency of adverse events between the two groups. Compared with tamsulosin monotherapy, combination therapy showed improvement in symptoms measured by IPSS and Q max along with more frequently reported sexual adverse events such as erectile dysfunction and decreased libido. Direct comparisons between dutasteride and finasteride were limited to only two studies, each with different efficacy outcome measures [6, 15]. These studies showed that dutasteride and finasteride led to similar outcomes in TPV reduction, improvement in IPSS and Q max, and the rate of adverse events.
Although improvement in the symptom score (IPSS) with dutasteride was statistically significant, its clinical significance has yet to be determined. A clinically important change in IPSS was established as a decrease of 2 or more points for men with a score of <20 points and 6 or more for men with a score of ≥20 points [16]. Because we included the studies where the subjects’ scores were 8 or greater, men with either moderate symptoms (a score of 8–19) or severe symptoms (a score of 20–35) were included in this analysis. Our pooled data suggested that the WMD in the symptom scores (IPSS) for dutasteride was −1.78 points (95 % CI −3.01 to −0.55) compared with placebo. This magnitude of improvement seems to be marginally insufficient to achieve clinically meaningful improvement in symptoms for men with moderate symptoms. Moreover, the required decrease of 6 or more points in IPSS for men with severe symptoms is much higher than the observed mean decrease in the symptom scores. Since the original studies included in this analysis did not separately report results for men with different severity of symptoms, it is difficult to determine whether patients treated with dutasteride resulted in clinically meaningful symptom improvement in general without considering their symptom severity. It is not clear whether patients with severe symptoms improved to a greater extent than those with moderate symptoms (or vice versa) after administering dutasteride. For the same reason, it is hard to determine whether the combination therapy would bring clinically meaningful symptom improvement for patients with moderate to severe symptoms compared with monotherapy. In addition, the peak urinary flow rate was recommended to be above 15 mL/s, making the probability of bladder outlet obstruction very low [17]. Our analysis comprising men with Q max ≤ 15 mL/s showed that the WMD was 1.27 mL/s (95 % CI 0.97–1.57) compared with placebo. This suggests that men whose Q max was below 16.27 mL/s (95 % CI 15.97–16.57) at baseline could likely prevent potential functional obstructions using dutasteride. Similarly, given the WMD of 1.60 mL/s (95 % CI 1.59–1.61) for the combination therapy, men whose Q max was below 16.60 mL/s (95 % CI 16.59–16.61) could experience clinically meaningful benefit from the combination therapy. Moreover, the evidence showed that the relative risk of AUR in patients with prostate volume >30 cm3 was three times greater than that in those with a prostate volume <30 cm3 [18]. Assuming that a prostate volume of 30 cm3 is a critical point, the change in TPV (%) of −17.40 (95 % CI −25.77 to −9.02) indicates clinically meaningful improvement for those whose TPV at baseline was below 35.22 cm3 (95 % CI 32.71–37.73 cm3).
This study is subject to several limitations. Mainly, the number of studies available for meta-analysis was limited. The final outcomes were obtained based on a single study or pooled from two to four studies. In addition, the weights for meta-analysis were skewed in their assignment to the studies. For example, the overall RR of dutasteride for withdrawal due to adverse events compared with a placebo was obtained by pooling two studies [12, 13]. The pooled RR of 0.99 (95 % CI 0.82–1.20) was obtained largely based on Schulman et al.’s [12] study in which RR was reported as 0.98 (95 % CI 0.80–1.18), but little weight was assigned to Na et al.’s [13] study that found a RR of 7.06 (95 % CI 0.37–135.20). Before publishing the results, Schulman et al. [5, 11] and the authors of two other studies had already pooled data from three large clinical trials (ARIA 3,001, ARIA 3,002, and ARIB 3,003), resulting in large sample sizes in their studies. Accordingly, our analyses assigned greater weight to these studies compared with other studies with smaller sample sizes where data had been collected from only a single clinical trial. Thus, although this pooled RR was largely dependent on only Schulman et al.’s [19] study, this estimate actually reflected the pooled data from 2,340 patients in three different trials across 19 countries. Nevertheless, the results relying largely or entirely upon a single study still need to be interpreted with caution. Since data from different clinical trials were already pooled in the study, the weights for different trials could not be distinguished, and heterogeneity among these trials could not be assessed. Therefore, more evidence from independent studies is necessary to confirm the robustness of these findings. Furthermore, we included two open-label extension studies after double-blind clinical trials although the subjects were no longer randomized in the open-label phase to assess the long-term effects of dutasteride use. Our findings suggested a favorable influence of long-term dutasteride use on symptom improvement. We included only studies published in English in journals. We relied on published information about methods and did not contact the authors for clarification of poorly reported information. Finally, all included studies in our analysis had been sponsored by the manufacturers of dutasteride except one study. As Als-Nielsen pointed out, industry-sponsored trials are likely to draw pro-industry conclusions [20]. That is, conclusions in trials funded by for-profit companies may be more positive due to biased interpretation of trial results. This caveat may also apply to our study.
Conclusions
Dutasteride can be used as one of the treatment options for the purpose of improving BPH symptoms measured by IPSS and Q max and reducing TPV with cautions of its adverse events. Adding dutasteride to tamsulosin can be considered if further improvements in symptoms (IPSS and Q max) are required beyond that achieved by tamsulosin monotherapy. However, the incidence of sexual adverse events may be higher in the combination therapy. Because the efficacy and safety profiles were similar for dutasteride and finasteride, other factors such as drug cost and individual preference should be considered in selection.
References
Berry SJ, Coffey DS, Walsh PC et al (1984) The development of human benign prostatic hyperplasia with age. J Urol 132(3):474–479
O’Leary MP, Roehrborn CG, Black L (2008) Dutasteride significantly improves quality of life measures in patients with enlarged prostate. Prostate Cancer Prostatic Dis 11(2):129–133
Djavan B, Marberger M (1999) A meta-analysis on the efficacy and tolerability of alpha 1-adrenoceptor antagonists in patients with lower urinary tract symptoms suggestive of benign prostatic obstruction. Eur Urol 36(1):1–13
McConnell JD, Bruskewitz R, Walsh P et al (1998) The effect of finasteride on the risk of acute urinary retention and the need for surgical treatment among men with benign prostatic hyperplasia. N Engl J Med 338(9):557–563
Roehrborn CG, Boyle P, Nickel JC et al (2002) Efficacy and safety of a dual inhibitor of 5-alpha-reductase types 1 and 2 (dutasteride) in men with benign prostatic hyperplasia. Urology 60(3):434–441
Clark RV, Hermann DJ, Cunningham GR et al (2004) Marked suppression of dihydrotestosterone in men with benign prostatic hyperplasia by dutasteride, a dual 5 alpha-reductase inhibitor. J Clin Endocrinol Metab 89(5):2179–2184
Tacklind J, Fink HA, Macdonald R et al (2010) Finasteride for benign prostatic hyperplasia. Cochrane Database Syst Rev 10
Halpern SH, Douglas MJ (2007) Appendix: Jadad scale for reporting randomized controlled trials, in evidence-based obstetric anesthesia. Blackwell, London
Higgins JPT, Green S (2011) Cochrane handbook for systematic reviews of interventions version 5.1.0. [updated March 2011]. The Cochrane Collaboration
Thiessen Philbrook H, Barrowman N, Garg AX (2007) Imputing variance estimates do not alter the conclusions of a meta-analysis with continuous outcomes: a case study of changes in renal function after living kidney donation. J Clin Epidemiol 60(3):228–240
Debruyne F, Barkin J, van Erps P et al (2004) Efficacy and safety of long-term treatment with the dual 5 alpha-reductase inhibitor dutasteride in men with symptomatic benign prostatic hyperplasia. Eur Urol 46(4):488–494
Schulman C, Pommerville P, Hofner K et al (2006) Long-term therapy with the dual 5 alpha-reductase inhibitor dutasteride is well tolerated in men with symptomatic benign prostatic hyperplasia. BJU Int 97(1):73–79
Na Y, Ye Z, Zhang S et al (2012) Efficacy and safety of dutasteride in Chinese adults with symptomatic benign prostatic hyperplasia: a randomized, double-blind, parallel-group, placebo-controlled study with an open-label extension. Clin Drug Investig 32(1):29–39
Hong SK, Min GE, Ha SB et al (2010) Effect of the dual 5 alpha-reductase inhibitor, dutasteride, on serum testosterone and body mass index in men with benign prostatic hyperplasia. BJU Int 105(7):970–974
Nickel JC, Gilling P, Tammela TL et al (2011) Comparison of dutasteride and finasteride for treating benign prostatic hyperplasia: the Enlarged Prostate International Comparator Study (EPICS). BJU Int 108(3):388–394
Barry MJ, Williford WO, Chang Y et al (1995) Benign prostatic hyperplasia specific health status measures in clinical research: how much change in the American urological association symptom index and the benign prostatic hyperplasia impact index is perceptible to patients? J Urol 154(5):1770–1774
McVary KT (2003) Clinical evaluation of benign prostatic hyperplasia. Rev Urol 5(5 Suppl):S3–S11
Jacobsen SJ, Jacobson DJ, Girman CJ et al (1997) Natural history of prostatism: risk factors for acute urinary retention. J Urol 158(2):481–487
Joo KJ, Sung WS, Park SH et al (2012) Comparison of alpha-blocker monotherapy and alpha-blocker plus 5 alpha-reductase inhibitor combination therapy based on prostate volume for treatment of benign prostatic hyperplasia. J Int Med Res 40(3):899–908
Als-Nielsen B, Chen W, Gluud C et al (2003) Association of funding and conclusions in randomized drug trials: a reflection of treatment effect or adverse events? JAMA 290(7):921–928
Tsukamoto T, Endo Y, Narita M (2009) Efficacy and safety of dutasteride in Japanese men with benign prostatic hyperplasia. Int J Urol 16(9):745–750
Roehrborn CG, Siami P, Barkin J et al (2008) The effects of dutasteride, tamsulosin and combination therapy on lower urinary tract symptoms in men with benign prostatic hyperplasia and prostatic enlargement: 2-year results from the CombAT study. J Urol 179(2):616–621
Roehrborn CG, Siami P, Barkin J et al (2010) The effects of combination therapy with dutasteride and tamsulosin on clinical outcomes in men with symptomatic benign prostatic hyperplasia: 4-year results from the CombAT study. Eur Urol 57(1):123–131
Conflict of interest
There is no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Park, T., Choi, JY. Efficacy and safety of dutasteride for the treatment of symptomatic benign prostatic hyperplasia (BPH): a systematic review and meta-analysis. World J Urol 32, 1093–1105 (2014). https://doi.org/10.1007/s00345-014-1258-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00345-014-1258-9