Abstract
Purpose
To investigate the positive biopsy rate of MRI-guided biopsy (MR-GB) in a routine clinical setting, identify factors predictive for positive biopsy findings and to report about the clinical significance of the diagnosed tumors.
Methods
Patients with at least one negative trans-rectal-ultrasound-guided biopsy (TRUS-GB), persistently elevated or rising serum prostate specific antigen (PSA) and at least one lesion suspicious for PCa on diagnostic 1.5 Tesla endorectal coil MRI (eMR) were included. Biopsies were carried out using a 1.5 Tesla MRI and an 18 G biopsy gun. Clinical information and biopsy results were collected; logistic regression analysis was carried out. Definite pathology reports of patients with diagnosis of PCa and subsequent radical prostatectomy (RP) were analyzed for criteria of clinical significance.
Results
One hundred patients were included, mean number of previous biopsies was 2 (range 1–9), mean PSA at time of biopsy was 11.7 ng/ml (1.0–65.0), and mean prostate volume was 46.7 ccm (range 13–183).
In 52/100 (52.0%) patients, PCa was detected. Out of 52 patients, 27 patients with a positive biopsy underwent RP, 20 patients radiation therapy, and 5 patients active surveillance. In total, 80.8% of the patients revealed a clinically significant PCa.
In univariate regression analysis, only serum PSA levels were predictive for a positive biopsy result. Number of preceding negative biopsies was not associated with the likelihood of a positive biopsy result.
Conclusions
MR-GB shows a high detection rate of clinically significant PCa in patients with previous negative TRUS-GB and persisting suspicion for PCa.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
To date, systematic trans-rectal ultrasound-guided biopsy (TRUS-GP) of the prostate represents the gold standard for diagnosis of prostate cancer [12]. Approximately 24.1% of men in a screening population undergoing TRUS-guided biopsy (TRUS-GB) will be diagnosed with prostate cancer (PCa) [21]. Still the rate of false negative results may be as high as 35% depending on the biopsy technique used [17]. Patients with persisting suspicion of PCa after negative prostate biopsy pose a significant problem on both the patient and the treating urologist.
Conventional gray scale TRUS has a limited sensitivity and specificity for the detection of malignant intraprostatic lesions [18], and the low detection rates after secondary or tertiary biopsies demonstrate the flaws of systematic but non-targeted tissue sampling [7, 15].
Magnetic resonance imaging (MRI) for localization and staging of PCa has been well investigated over the past years [23]. The lesion-by-lesion detection rate for PCa with T2-weighted endorectal coil magnetic resonance tomography (eMRT) depending on the size and localization of the tumor reaches up to 88% [5, 20] and can be further improved by multi-modality imaging using spectroscopy and diffusion imaging [14, 23]. The technique of MRI-guided biopsy (MR-GB) has been established and implemented into clinical routine in our institution six years ago [3]. Meanwhile, several groups reported about MRI-guided biopsies [4, 8, 11].
In this study, we present the largest cohort of consecutive patients undergoing MR-GB after previous negative TRUS-GB. Goal of the present study was to demonstrate the detection rate of MR-GB and evaluate the clinical significance of the detected carcinomas. We also sought to identify factors predictive for a positive biopsy result.
Materials and methods
Patients
Between 8/2005 and 12/2009, one hundred consecutive patients with at least one prior negative TRUS-GB, persistently elevated or rising PSA values and at least one lesion suspicious for PCa in previous eMRI were submitted for MR-GB biopsy by the Department of Urology of the University Hospital Tuebingen. Patients with a history of radiation therapy of the prostate or current hormone deprivation therapy were excluded from the study.
Devices and procedures were approved by the institutional ethics committee.
Magnetic resonance imaging and MR-guided biopsy
To identify suspicious lesions and for planning of the intervention, all patients underwent eMRI (1.5 Tesla, endorectal coil, Medrad GmbH, Volkach, Germany) before the MR-GB. Primary diagnostic sequence was a T2-weighted turbo spin echo sequence (T2w TSE), since during biopsy, a T2w sequence was applied. To differentiate benign and malignant areas in the prostatic gland, additional sequences, including diffusion weighted imaging (DWI), MR-spectroscopy, and dynamic contrast enhanced imaging (DCE) were used after the first 52 patients after multi-parametric imaging that had been established in our department 2007.
MRI-guided biopsies were performed on a 1.5 Tesla scanner (Siemens Magnetom Avanto or Espree, Erlangen, Germany); images were acquired with combined body- and spine-phased array coils. Patients were placed in a prone position, a needle guide filled with a Gd-chelate dotted gel for visualization fixed to a portable biopsy device was introduced rectally (Invivo GmbH, Schwerin, Germany). Biopsies were taken with an MRI-compatible 18-gauge, fully automatic, core needle, double-shot biopsy gun (needle length 150 mm, total probe feed 25 mm, TSK Laboratory, Japan/Invivo GmbH, Schwerin, Germany).
A transversal and coronal T2w TSE (FOV 230 × 230, matrix size 230 × 256/346 × 384, TR 4000/5560, TE 121/121, 4 mm slices) and a final axial T1w TSE sequence for detection of hemorrhage (FOV 270 × 270, matrix 230 × 320, TR 500, TE 9.4, 3 mm slices) were obtained. Three-dimensional adjustments of the needle guide were based on transversal T2w images, adapted in an oblique orientation parallel to the needle guide. Interpretation of MRI, placement of the needle guide, and biopsy were conducted in cooperation of a urologist and a radiologist. At least two specimens were taken from a suspicious area in case of multifocality all suspicious lesions were biopsied.
Criteria for clinical significance
To evaluate the tumors for clinical significance, the criteria reported for MRI-GB by Hambrock et al. were applied [11]: In patients undergoing RP after positive MR-GB, PCa was considered clinically significant if at least one of the following criteria was present: (a) Gleason pattern ≥ 4 in the definite pathology report, (b) final T stage ≥ pT3a and/or pN1 and (c) tumor volume > 0.5 cc. In patients undergoing radiation therapy or active surveillance criteria for clinical significance were either one of the following: (a) biopsy Gleason pattern ≥ 4 (b) serum PSA > 10 ng/ml and (c) PSA density > 0.15 ng/ml/cc.
Statistical analysis
The data were analyzed using the statistical software package JMP® (SAS Institute, Cary, NC, USA). Contingency tables/chi-square tests were used to compare ordinal variables, and univariate linear regression analysis was performed with Fisher’s exact test. If possible, Pearson’s correlation coefficient was calculated. A p value of p < 0.05 was considered as statistically significant.
Results
Patients
A total of 100 patients underwent MRI-guided biopsy of the prostate. Mean patient age was 64.9 years (median 66, range 48–81). Mean PSA level at time of biopsy was 12.3 ng/ml (median 8.7, range 3.9–65.0). Average prostate volume in diagnostic eMRI estimated by the ellipsoid formula was 46.7 ccm (median 41, range 13–183). The median number of previous TRUS-guided biopsies was 2 (range 1–9). Since the majority of previous biopsies had been performed outside our institution, information on biopsy technique and number of cores was not available for most patients.
Biopsy results
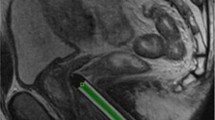
Mean number of suspicious areas identified by eMRI was 1.16. A median of 4 biopsy cores per patient was obtained (range 2–8) and average time of the MR-GB procedure was 48 min (range 28–95). All procedures were well tolerated by the patients. Figure 1 shows an example of T2w-MRI with the needle guide positioned transrectally.
65-year-old patient with PSA 8 ng/ml and 5 previous negative TRUS-GB. Poorly defined hypointense area (*) in the ventral transitional zone (A, oblique T2w TSE before biopsy) with suspicious correlate (*) in DWI. Histopathology reported a Gleason score 3 + 4, final histopathologic result after radical prostatectomy revealed Gleason Score 4 + 5
In 52 (52.0%) patients, PCa was detected by MR-GB. The median biopsy Gleason score was 7 (range 5–9). In 33 (63.5%) patients, PCa was localized in the peripheral zone and in 18 (34.6%) in the transitional zone.
In 48 patients, no PCa was detected. In 14/48 (29.2%) patients without evidence of PCa, histology revealed prostatitis; PIN or ASAP was reported in 9 (18.8%).
Clinical significance of PCa
Of the 52 patients with PCa, 27 subsequently underwent RP. The definite pathology report showed PCa confined to the prostate (pT2) in 12 and non-organconfined PCa in 15 patients (pT3a: 6, pT3b: 7, pT4: 2; see Fig. 2a for stage distribution). Eight of the 17 patients with Gleason sum ≤6 showed an upgrading to a Gleason score ≥ 7. In total, 20/27 patients undergoing RP had a Gleason pattern of 4 or 5. Estimated tumor volume was >0.5 cc in 18 patients. In summary, 23 patients after RP met the criteria for clinically significant PCa.
MR-GB detects clinically significant tumors in the majority of patients with a positive biopsy result (n = 52). PCa Prostate carcinoma, GS Gleason Score, MR-GB MRI-guided biopsy. a Distribution of serum PSA values in patients with a positive MR-GB (n = 52). b Distribution of Gleason Score (GS). About 59.7% of all patients diagnosed with PCa harbored tumors with GS 7 or greater. Biopsy GS in patients undergoing radiotherapy or active surveillance (n = 23), definite GS in patients after RP (n = 29). c Distribution of pathologic T stage in patients undergoing radical prostatectomy (RP, n = 29) after positive MR-GB
Of the 25 patients with PCa not undergoing RP, 20 underwent external beam radiation and 5 active surveillance. In 11 of these patients, biopsy showed a Gleason pattern of 4 or 5, in 12 PSA was ≥10 ng/ml. Sixteen patients had a PSA density > 0.15 ng/ml/cc. In this group, 19 patients were considered to harbor clinically significant disease.
Adhering to these criteria, forty-two of the 52 patients (80.8%) diagnosed with PCa exhibited clinically significant PCa.
Logistic regression analysis
Statistical analysis of clinical factors to predict a positive biopsy result was carried out. Neither patient age, prostate volume, PSA levels, number of preceding biopsies nor anatomical location of the suspicious lesion on eMRI correlated significantly with findings of PCa in the biopsy specimen (Table 1). In a second step, only serum PSA levels >10 ng/ml correlated positively with a positive biopsy result in univariate analysis of subcohorts (Table 2).
Discussion
The dilemma of repeated negative biopsies in men with persistently elevated or even rising serum PSA is well known. Although saturation biopsies improve the detection rate of PCa, solely increasing the number of biopsy cores may also contribute to the detection of clinically insignificant disease [26]. Several groups have shown that considering suspicious lesions detected on eMRI or M-M eMRI on TRUS-guided re-biopsies significantly improves the detection rate [2, 25]. In the following years, several groups established direct MR-GB of the prostate and reported promising results [4, 10, 28].
In our institution, the technique has been implemented into clinical routine more than five years ago. In the current study, fifty-two percent of patients with at least one negative TRUS-GB and elevated or rising PSA can be diagnosed with PCa using MR-GB. Although most men in our cohort underwent more than one TRUS-biopsy before MR-GB, in 69.2% of our patients clinically significant carcinomas were detected. This is in concordance with Hambrock et al. who found clinically significant disease in 37 of 40 patients diagnosed with PCa by MR-GB [11]. There is evidence that the sensitivity to PCa detection in eMRI correlates with histological features of the tumor. In a study of 123 patients undergoing MRI before RP, the sensitivity to predicting tumor foci was 44% in patients with Gleason 3 + 3 tumors and 89% in patients with Gleason 4 + 4 and higher [27]. The histopathologic grading of PCa diagnosed after biopsy of suspicious lesions in MRI seems to reflect the definite pathological grading of the surgical specimen: In a series of 70 consecutive patients with diagnosis of PCa after MRI-directed TRUS-GB, the biopsy Gleason score was confirmed in 90% of the cases. Downgrading only occurred in 1.4% of these patients [13]. This emphasizes the capability of MR-GB to diagnose clinically significant tumors, but also opens new perspectives for patients under active surveillance [9]: The scheduled targeted re-biopsy of a defined dominant intraprostatic lesion/index lesion may aid to monitor patients more accurately than by repeated random TRUS-biopsy.
Several anatomical factors influence the detection rate of PCa in TRUS-GB: localization of the tumor and prostate volume. Although histopathological studies have demonstrated that the majority of PCa lesions are situated in the peripheral zone [16], the analysis of the biopsy cores in our study revealed that about 34.6% of the tumors were localized in the transitional zone. Similarly, this has been observed for rebiopsy populations in other investigations where up to 18.1% of the patients with prior negative TRUS-GB were diagnosed with cancer in the transitional zone only [6]. In a study reporting about MRI-guided, diffusion weighted TRUS-rebiopsy, even 13 of 17 patients with a positive biopsy had cancer in the transitional zone [19]. Routine random TRUS-GB with 10 or 12 biopsy cores will not include tissue samples from this region and part of the tumors might have been missed in previous biopsy rounds. This might explain the substantially higher number of positive transitional zone biopsies in the targeted re-biopsy setting.
The mean prostate volume in our patient cohort was 46.7 ccm, suggesting a substantial number of patients with enlarged prostate glands. The likelihood of a positive biopsy result decreases in prostates with larger volumes [24]. The high tumor detection rate of over 50% emphasizes the advantages of targeted biopsy techniques in this selected population.
The most prevalent histological classification in negative biopsy specimens was prostatitis. In fact, currently, the specificity of MR-imaging is mainly limited by the differentiation of malignant and inflamed lesions. Advances in multimodality imaging and improved resolution with 3 Tesla MR-devices might lead to a further improvement in diagnostic accuracy [1].
Analysis of clinical factors in our cohort revealed that only PSA was associated with a positive biopsy outcome. Interestingly, the number of preceding biopsies did not affect the probability of finding PCa on MR-GB. With increasing number of biopsies, the likelihood of detecting PCa on TRUS-GB decreases from 23% after the first round to 17.6% and 11.7% on the second and third re-biopsy respectively[26]. The positive biopsy rate of >50% in our cohort of patients underlines the advantage of a targeted biopsy in this selected patient cohort.
Some limitations of our study have to be considered. First, the cohort is not homogeneous, including patients with varying number of previous negative biopsies. Second, there is a pre-selection bias: All patients undergoing MR-GB demonstrated a suspicious lesion on previous diagnostic eMRI. Since sensitivity to eMRI correlates with Gleason Score and tumor size [13, 20, 27], it can be hypothesized that MR-GB might contribute in selecting patients with clinically significant cancer. Last, follow-up information for the patients with negative biopsies was not available for the majority of the patients. Therefore, the true false-negative rate of MR-GB cannot be assessed. This issue should be addressed in further studies concerning biopsy techniques.
Considering the technical complexity, incremental costs and still limited sensitivity to eMRI for cancer detection, MR-GB will not replace systematic TRUS. Therefore, in our institution, the method is restricted to patients with persisting suspicion of PCa after previous negative TRUS-GB. MRI-directed TRUS biopsies might overcome the aforementioned drawbacks [22]. However, translation of the findings in eMRI to gray scale ultrasound may not always be accurate. Fusion imaging might overcome these obstacles in the future. In this context, the potential not only for primary cancer detection but also for monitoring patients under active surveillance with scheduled targeted rebiopsies of index lesions has to be kept in mind.
Conclusions
The results of our investigation demonstrate the effectiveness of MR-GB to diagnose clinically significant PCa in patients with persistently elevated PSA levels after negative TRUS-GB. The increased cancer detection rate justifies the use of this elaborate biopsy technique in this particular subset of men with detectable lesions on eMRI. Further prospective studies will have to elucidate whether this method might be advantageous in differentiating clinically significant from insignificant disease. The possibility of reproducibly biopsy target lesions (e.g., dominant intraprostatic lesions) may open new perspectives for the follow-up of patients with PCa opting for active surveillance. In future, fusion imaging might lead the way for a more widespread application.
References
Ahmed HU, Kirkham A, Arya M et al (2009) Is it time to consider a role for MRI before prostate biopsy? Nat Rev Clin Oncol 6:197–206
Amsellem-Ouazana D, Younes P, Conquy S et al (2005) Negative prostatic biopsies in patients with a high risk of prostate cancer. Is the combination of endorectal MRI and magnetic resonance spectroscopy imaging (MRSI) a useful tool? A preliminary study. Eur Urol 47:582–586
Anastasiadis AG, Lichy MP, Nagele U et al (2006) MRI-guided biopsy of the prostate increases diagnostic performance in men with elevated or increasing PSA levels after previous negative TRUS biopsies. Eur Urol 50:738–748
Beyersdorff D, Winkel A, Hamm B et al (2005) MR imaging-guided prostate biopsy with a closed MR unit at 1.5 T: initial results. Radiology 234:576–581
Colleselli D, Schilling D, Lichy MP et al (2010) Topographical sensitivity and specificity of endorectal coil magnetic resonance imaging for prostate cancer detection. Urol Int 84:388–394
Deliveliotis C, Varkarakis J, Albanis S et al (2002) Biopsies of the transitional zone of the prostate. Should it be done on a routine basis, when and why? Urol Int 68:113–117
Djavan B, Milani S, Remzi M (2005) Prostate biopsy: who, how and when. An update. Can J Urol 12(Suppl 1):44–48
Engelhard K, Hollenbach HP, Kiefer B et al (2006) Prostate biopsy in the supine position in a standard 1.5-T scanner under real time MR-imaging control using a MR-compatible endorectal biopsy device. Eur Radiol 16:1237–1243
Fradet V, Kurhanewicz J, Cowan JE et al (2010) Prostate cancer managed with active surveillance: role of anatomic MR imaging and MR spectroscopic imaging. Radiology 256:176–183
Hambrock T, Futterer JJ, Huisman HJ et al (2008) Thirty-two-channel coil 3T magnetic resonance-guided biopsies of prostate tumor suspicious regions identified on multimodality 3T magnetic resonance imaging: technique and feasibility. Invest Radiol 43:686–694
Hambrock T, Somford DM, Hoeks C et al (2010) Magnetic resonance imaging guided prostate biopsy in men with repeat negative biopsies and increased prostate specific antigen. J Urol 183:520–527
Heidenreich A, Aus G, Bolla M et al (2008) EAU guidelines on prostate cancer. Eur Urol 53:68–80
Labanaris AP, Zugor V, Smiszek R et al (2010) Guided e-MRI prostate biopsy can solve the discordance between Gleason score biopsy and radical prostatectomy pathology. Magn Reson Imaging 28:943–946
Lichy MP, Pintaske J, Kottke R et al (2005) 3D proton MR spectroscopic imaging of prostate cancer using a standard spine coil at 1.5 T in clinical routine: a feasibility study. Eur Radiol 15:653–660
Lujan M, Paez A, Santonja C et al (2004) Prostate cancer detection and tumor characteristics in men with multiple biopsy sessions. Prostate Cancer Prostatic Dis 7:238–242
McNeal JE (1969) Origin and development of carcinoma in the prostate. Cancer 23:24–34
Norberg M, Egevad L, Holmberg L et al (1997) The sextant protocol for ultrasound-guided core biopsies of the prostate underestimates the presence of cancer. Urology 50:562–566
Pallwein L, Mitterberger M, Pelzer A et al (2008) Ultrasound of prostate cancer: recent advances. Eur Radiol 18:707–715
Park BK, Lee HM, Kim CK et al (2008) Lesion localization in patients with a previous negative transrectal ultrasound biopsy and persistently elevated prostate specific antigen level using diffusion-weighted imaging at three Tesla before rebiopsy. Invest Radiol 43:789–793
Roethke MC, Lichy MP, Jurgschat L, et al. (2010) Tumorsize dependent detection rate of endorectal MRI of prostate cancer—A histopathologic correlation with whole-mount sections in 70 patients with prostate cancer. Eur J Radiol
Schroder FH, Hugosson J, Roobol MJ et al (2009) Screening and prostate-cancer mortality in a randomized European study. N Engl J Med 360:1320–1328
Sciarra A, Panebianco V, Ciccariello M et al (2010) Value of magnetic resonance spectroscopy imaging and dynamic contrast-enhanced imaging for detecting prostate cancer foci in men with prior negative biopsy. Clin Cancer Res 16:1875–1883
Umbehr M, Bachmann LM, Held U et al (2009) Combined magnetic resonance imaging and magnetic resonance spectroscopy imaging in the diagnosis of prostate cancer: a systematic review and meta-analysis. Eur Urol 55:575–590
Uzzo RG, Wei JT, Waldbaum RS et al (1995) The influence of prostate size on cancer detection. Urology 46:831–836
Yuen JS, Thng CH, Tan PH et al (2004) Endorectal magnetic resonance imaging and spectroscopy for the detection of tumor foci in men with prior negative transrectal ultrasound prostate biopsy. J Urol 171:1482–1486
Zackrisson B, Aus G, Bergdahl S et al (2004) The risk of finding focal cancer (less than 3 mm) remains high on re-biopsy of patients with persistently increased prostate specific antigen but the clinical significance is questionable. J Urol 171:1500–1503
Zakian KL, Sircar K, Hricak H et al (2005) Correlation of proton MR spectroscopic imaging with gleason score based on step-section pathologic analysis after radical prostatectomy. Radiology 234:804–814
Zangos S, Eichler K, Engelmann K et al (2005) MR-guided transgluteal biopsies with an open low-field system in patients with clinically suspected prostate cancer: technique and preliminary results. Eur Radiol 15:174–182
Acknowledgments
The authors thank Miriam Germann for critically proof reading and reviewing the manuscript.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Roethke, M., Anastasiadis, A.G., Lichy, M. et al. MRI-guided prostate biopsy detects clinically significant cancer: analysis of a cohort of 100 patients after previous negative TRUS biopsy. World J Urol 30, 213–218 (2012). https://doi.org/10.1007/s00345-011-0675-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00345-011-0675-2