Abstract
Purpose
To evaluate the efficacy and safety of WX-G250, a chimeric monoclonal antibody that binds to carboxy anhydrase IX, combined with low-dose interferon-alpha (LD-IFNα) in patients with progressive metastatic renal cell carcinoma (mRCC).
Patients and methods
Thirty-one patients, nephrectomized for the primary tumor, clear cell progressive mRCC, were enrolled to receive weekly infusions of WX-G250 (20 mg i.v.; week 2–12) combined with LD-IFNα (3 MIU s.c. 3 times/week; week 1–12). At week 16, patients were evaluated for response and stratified into two groups: (a) responders into the extended treatment group for an additional 6 weeks of treatment or (b) the progressive group with no further study treatment.
Results
Of the 31 treated patients, 26 were evaluable for response to treatment. Two patients showed partial remission and 14 patients had stable disease as assessed in week 16. One patient experienced partial remission resulting in a complete remission lasting at least 17 months. Nine patients had durable stable disease of 24 weeks or longer. Clinical benefit was obtained in 42% (11/26) patients. The median overall survival achieved was 30 months and the 2-year survival was 57%. Patients receiving extended treatment showed a significantly longer 2-year survival rate than discontinued patients (79 vs. 30%; P = 0.0083). In general, treatment was well tolerated with little toxicity.
Conclusion
Treatment with the antibody WX-G250 in combination with LD-IFNα is safe, well tolerated, led to clinically meaningful disease stabilization and demonstrated clinical benefit in this progressive mRCC patient population.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Renal cell carcinoma (RCC) is the most common malignancy of the kidney and accounts for approximately 3% of adult tumors. Annual RCC incidence estimates have increased steadily, and approximately 25% of patients present with advanced disease at the time of diagnosis [1]. Several issues have focused attention on treatments that target immune manipulation, including reports of spontaneous regression of metastatic lesions, the presence of cytotoxic T lymphocytes in renal tumors, and the recent description of tumor-associated and human leukemic antigen-restricted antigens on renal cancer cells [2]. A variety of cytokines have been studied in patients with metastatic RCC (mRCC). Two of these agents, interleukin-2 (IL-2) and IFNα, have antitumor effects that are reproducible and that can be durable in some patients [3]. Numerous clinical trials evaluating these agents alone or in combination with other carcinogenic agents have been conducted over the past two decades [4–8]. However, due to high toxicity and low response rates, controversy remains regarding these therapies.
The chimeric monoclonal antibody (mAb) cG250 (RENCAREX®, WX-G250) is an immunoglobulin subtype G1 kappa light-chain antibody that binds to carbonic anhydrase IX (G250 antigen), a cell-surface antigen that is expressed in 95% of clear cell RCC. The reactivity of WX-G250 with normal tissues is restricted to the gastric epithelium and the biliary ducts in the liver, astrocytes in the brain and to the spinal cord [9, 10]. Besides efficient bio-localization in RCC, it has been demonstrated that cG250 can induce natural killer cells to kill tumor cells in vitro via antibody-dependent cellular cytotoxicity (ADCC) [11].
IFNα stimulates the expression of MHC class I molecules which are of central importance for antigen presentation on cells, for the interaction of the different populations of lymphocytes and for the target identification of cytotoxic T lymphocytes. IFNα enhances the activity of macrophages, NK and LAK cells, directly as well as indirectly via co-stimulatory cytokines. Due to these mechanisms, the tumor cells become more susceptible to ADCC effector cells.
The presented study was done to prove whether WX-G250 combined with IFNα results in additive/synergistic effects.
Patients and methods
Study design and patient population
A prospective, open-label, single arm, multicenter phase I/II study was conducted. All patients had mRCC with objective progression at study enrollment according to WHO criteria and prior nephrectomy (all with documented clear cell histology). Eligible patients had bidimensionally measurable lesions of 1–5 cm in diameter and a Karnofsky performance status (KPS) ≥80%. All patients gave written informed consent.
The study was approved by the institutional review board or ethics committee at each participating center (primary EC reference no. 837.0289.01) and was conducted in accordance with the provisions of the Declaration of Helsinki and Good Clinical Practice guidelines.
This trial was divided into part one for the evaluation of possible drug-related toxicity and safety of the combination treatment of six patients, and part two, if safety and toxicity of part one proved acceptable, to also investigate the efficacy of the combination. Thus, the primary objectives for the first part of the study included the evaluation of safety, particularly the combination of WX-G250 and IFNα, and for the second part the efficacy of treatment showing objective response (PR, CR) or disease stabilization (SD). The secondary objectives were examination of the induction of human antibody chimeric antibody (HACA), ADCC, remission as seen in bidimensional measurement of metastatic lesions and the overall survival of the enrolled patients as well as continuous safety evaluation.
Statistical methods
The study sample size was based on α ≤ 0.05 and 1-β ≥ 0.80 to detect a difference between an assumed spontaneous response rate of 3% versus an underlying true response rate of 15%. Overall survival was assessed with Kaplan–Meier curves.
Treatment schedule
Patients received weekly 20-mg WX-G250 i.v. for 11 consecutive weeks (week 2–12) in combination with a s.c. injection of 3 MIU IFNα (rHu-IFN-alpha2a; Roferon A; Roche) thrice weekly for 12 consecutive weeks (weeks 1–12). In the first week, IFNα was administered without WX-G250 in order to distinguish potential IFNα-related side effects from those of WX-G250. Patients with disease stabilization or partial response at week 16 were eligible for extended treatment, consisting of six additional infusions of WX-G250 in combination with IFNα treatment beginning with week 17.
Patient monitoring and efficacy evaluation
At baseline and at weeks 2, 6, 10, 12 and 16, medical histories were recorded, and physical examinations, urinalysis, and laboratory analysis were performed. Toxicity was evaluated according to the National Cancer Institute for Common Toxicity Criteria (Version 2.0, Apr. 1999). Computed tomography scans of the thorax and abdomen were obtained at baseline, at week 16 and 4 weeks after the extended treatment period, and at subsequent visits when appropriate. Tumor responses were centrally evaluated according to WHO criteria by an independent radiologist.
Results
Patient characteristics
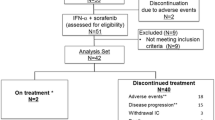
Between January 2002 and March 2003, 31 patients were enrolled at four sites in Germany. The patient characteristics are listed in Table 1. Five patients were withdrawn from the study due to early progression, or missing tumor measurements at baseline prior to enrollment.
In the present study, five out of 31 patients had a favorable risk, 23 patients were in the intermediate risk group and two patients had three risk factors and were in the poor risk group due to Motzer score [12].
Treatment results
Eighteen out of 26 evaluable patients had stable disease or response at week 16 and were eligible for an additional 6-week treatment with WX-G250 (extended treatment group), whereas eight patients received no further study treatment due to progressive disease.
Tumor response
The primary objective of a ≥15% combined CR/PR response rate was not reached; however, two out of 26 evaluable patients showed a partial response in week 16; one of these two patients developed an objective complete response assessed 48 weeks after first response detected. This response lasted 17 months. Fourteen patients achieved disease stabilization during assessment week 16. Nine patients had long durable disease stabilization ≥24 weeks.
Clinical benefit, defined as the sum of patients with partial and complete responses at a certain time point (two patients) and patients with stable disease for at least 24 weeks (nine patients), was observed in 42% (11 out of 26 evaluable patients) and in 64% of patients who had two or less risk factors.
Survival
The median overall survival was 30 months (Fig. 1) for 31 patients with 57% of patients alive after 2 years. The extended treatment group showed a median survival of 45 months compared with 10 months in the progressive group. The 2-year survival of the extended treatment group was also significantly higher than in the progressive group (79 vs. 30%; P = 0.0083).
Immunogenicity
In this study, no detectable immunological responses (HACA) during the course of WX-G250 treatment were observed.
Biological activity and ADCC
Assessment of the biological activity of the antibody was defined as a secondary objective, and ADCC was analyzed by using a 51chromium release assay (13).
Fourteen patients were analyzed for ADCC. At the beginning of the treatment, ADCC levels were low in seven patients (<10% lysis), intermediate in two patients (>10%, <20% lysis) and high in five patients (>20% lysis). Patient 23 showed no measurable ADCC activity in all blood samples at all timepoints. In eight patients, ADCC activity remained constant throughout the treatment. Total blood count was stable in all patients, and NK lysis levels remained low. All groups were compared pair wise and showed no statistically significant differences.
Safety results
No dose reduction was necessary for WX-G250. During the course of the study, the 31 treated patients experienced a total of 183 adverse events (AEs) (Table 2). All toxicity grade 3 and 4 adverse events were documented as unrelated to study medication. Almost 30% of all adverse events occurred in the first week of dosing IFNα alone.
Thirty-nine out of 183 adverse events in eight patients (25.8%) were definitely related to study medication. These adverse events mainly comprised ‘constitutional symptoms’ such as fever, rigor/chills and flu-like syndromes in 8 patients (25.8%) and ‘pain’, mostly headache in four patients (12.9%).
Five patients experienced six serious adverse events (SAE). The SAEs consisted of dyspnea (2x), lymph node swelling, dysphagia (the only grade 4 toxicity), subcutaneous lesions and pleural effusion. All six reported SAEs were unrelated to study medication and judged to be due to tumor progression.
The administration of 20-mg WX-G250 in combination with IFNα had no clinically notable effect on the hematological parameters. The mean and median values of the laboratory values reveal no general effect of the study medications on these parameters. No patient developed any detectable immunological response (HACA) during the course of cG250 treatment.
Discussion
The current prognosis in patients with mRCC is poor [13]. Although nonspecific immunotherapy has shown some evidence of inducing long-term clinical responses [8], the overall response rate is low and side effects are significant. Consequently, the main focus of new treatments for mRCC concerns anti-angiogenic therapy, e.g., sunitinib, sorafenib, temsirolimus, everolimus and bevacizumab [14–16], as well as immunotherapeutic modalities with more specificity against RCC and fewer side effects [2].
Although the WX-G250 monotherapy trial showed good results with a median survival of 15 months [17], the results of WX-G250 combination trials with IL-2 and IFNα show demonstrably better survival rates of 22 and 30 months respectively, suggestive of an additive/synergistic effect [18]. The survival data on patients treated with WX-G250 in the combination trials compare favorably with overall survival data from phase III studies of mRCC patients treated with either sunitinib or temsirolimus alone, the latter showing an OS of 26.4 and 10.9 months, respectively [14, 19, 20]. Because of the clear clinical benefit of WX-G250/IFNα in comparison with response rates of other trials with the treatment of either IL-2 or IFNα, or the combination of both cytokines (6.5, 7.5 and 18.6%, respectively), we consider it more likely that the IFNα regimen and WX-G250 act in a synergistic fashion [21].
The related side effects of the combination treatment WX-G250 and IFNα mainly comprised constitutional symptoms such as fever and flu-like symptoms, known to be inherent to the cytokine. WX-G250 monotherapy in patients with mRCC has already shown that the toxicity profile of WX-G250 is mild [17]. Interestingly, the combination of WX-G250 with IFNα showed better clinical results than the combination of WX-G250 with IL-2 and had significant fewer side effects. This may be a result of the better tolerability of IFNα in comparison with IL-2.
The antibody WX-G250 is safe and no HACA could be measured in the study samples. This compares well with a second clinical study where with a similar dosing regimen, a low-level HACA incidence of only 7.1% was observed [18]. Furthermore, no allergic reactions were observed in the phase II WX-G250 trials.
The level of ADCC was patient dependent and low in the majority of patients. A significant transient increase in ADCC activity was observed in a subgroup of patients without any evidence of correlation with clinical features. In eight patients, ADCC activity remained constant throughout the treatment, in three patients a transient increase in ADCC activity was observed (patient numbers 12, 18 and 32), and in two patients ADCC levels fluctuated throughout the treatment (patient numbers 7 and 9). Considering the small sample number, it is unclear whether the transient increase in ADCC activity was treatment related or whether the observed intra-patient variations are most likely a reflection of naturally occurring fluctuations. The combination of WX-G250 with IL-2 seems to increase activated ADCC effector cells [18]. WX-G250 alone or in combination with IFN-α did not show an enhancement of ADCC. However, in the combination therapy with IFNα a better clinical outcome could be documented.
Several anti-angiogenic therapies are being explored for the treatment of mRCC. Treatment with 10 mg/kg of the anti-VEGF bevacizumab for 2 weeks resulted in significantly longer median progression-free survival than in the placebo group (4.8 vs. 2.5 months) [16].
Subsequent to the above-mentioned combination trials of WX-G250 with cytokines, the introduction of multi-targeted drugs, such as sorafenib and sunitinib, and other anti-angiogenic substance, e.g., bevacizumab, temsirolimus and everolimus for the treatment of mRCC the paradigms of treatment have changed completely. Although response rates, progressions-free survival and overall survival are positively influenced, toxicity still remains a problem for the long-term treatment [14, 15]. As complete responses are still very rare, there is a need for combination therapies. WX-G250 has proven to be a potent combination partner with low toxicity, relevant activity and considerable survival time; thus further studies should be performed in combination with some of these newer drugs.
Conclusions
Treatment of metastatic clear cell RCC with WX-G250 in combination with IFNα is safe and very well tolerated. Adverse events were mild to moderate and reversible.
Furthermore, this treatment led to an improved clinical benefit even in second- and third line patients. With a median survival of 30 months, the combination of WX-G250 with IFNα is superior to WX-G250 monotherapy and the combination with IL-2.
These promising results should be further examined in a randomized trial evaluating the efficacy of WX-G250 in combinational regimens such as cytokines or targeted drugs such as e.g., anti-angiogenetic drugs.
References
Siebels M (2007) Epidemiology. In: Siebels M, Stief CG (eds) Neue Therapieansätze beim metastasierten Nierenzellkarzinom, 1st edn. Uni-Med Science, Bremen, pp 18–19
Bleumer I, Oosterwijk E, De Mulder P, Mulders PF (2003) Immunotherapy for renal cell carcinoma. Eur Urol 44:65–75
Figlin RA (1999) Renal cell carcinoma: management of advanced disease. J Urol 161:381–386
Negrier S, Escudier B, Lasset C et al (1998) Recombinant human interleukin-2, recombinant human interferon alpha-2a, or both in metastatic renal-cell carcinoma. Groupe Francais d’Immunotherapie. N Engl J Med 338:1272–1278
Stadler WM, Kuzel T, Dumas M, Vogelzang NJ (1998) Multicenter phase II trial of interleukin-2, interferon-alpha, and 13-cis-retinoic acid in patients with metastatic renal-cell carcinoma. J Clin Oncol 16:1820–1825
Atzpodien J, Kirchner H, Illiger HJ et al (2001) IL-2 in combination with IFN- alpha and 5-FU versus tamoxifen in metastatic renal cell carcinoma: long-term results of a controlled randomized clinical trial. Br J Cancer 85:1130–1136
Ravaud A, Delva R, Gomez F et al (2002) Subcutaneous interleukin-2 and interferon alpha in the treatment of patients with metastatic renal cell carcinoma-Less efficacy compared with intravenous interleukin-2 and interferon alpha. Results of a multicenter Phase II trial from the Groupe Francais d’Immunotherapie. Cancer 95:2324–2330
Atzpodien J, Kirchner H, Jonas U et al (2004) Prospectively randomized trial of the german cooperative renal carcinoma chemoimmunotherapy group (DGCIN). Interleukin-2- and interferon alpha-2a based immunochemotherapy in advanced renal cell carcinoma: a prospectively randomized trial of the german cooperative renal carcinoma chemoimmunotherapy group (DGCIN). J Clin Oncol 22:1188–1194
Oosterwijk E, Ruiter DJ, Hoedemaeker PJ et al (1986) Monoclonal antibody G250 recognizes a determinant present in renal-cell carcinoma and absent from normal kidney. Int J Cancer 38:489–494
Grabmeier K, Vissers JL, De Weijert MC et al (2000) Molecular cloning and immunogenicity of renal cell carcinoma-associated antigen G250. Int J Cancer 85:865–870
Surfus JE, Hank JA, Oosterwijk E et al (1996) Anti-renal-cell carcinoma chimeric antibody G250 facilitates antibody-dependent cellular cytotoxicity with in vitro and in vivo interleukin-2-activated effectors. J Immunother Emphasis Tumor Immunol 19:184–191
Motzer RJ, Bacik J, Murphy BA, Russo P, Mazumdar M (2002) Interferon alfa as a comparative treatment for clinical trials of new therapies against advanced renal cell carcinoma-2. J Clin Oncol 20:289–296
Pantuck AJ, Zisman A, Belldegrun AS (2001) The changing natural history of renal call carcinoma. J Urol 166:1611–1623
Motzer RJ, Hutson TE, Tomczak P et al (2007) Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. N Engl J Med 356:115–124
Escudier B, Eisen T, Stadler WM et al (2007) Sorafenib in advanced clear-cell renal-cell carcinoma. N Engl J Med 356:125–134
Yang JC, Haworth L, Sherry RM et al (2003) A randomized trial of bevacizumab, an anti-vascular endothelial growth factor antibody, for metastatic renal cancer. N Engl J Med 349:427–434
Bleumer I, Knuth A, Oosterwijk E et al (2004) A phase II trial of chimeric monoclonal antibody G250 for advanced renal cell carcinoma patients. Br J Cancer 90:985–990
Bleumer I, Oosterwijk E, Oosterwijk-Wakka JC et al (2006) A clinical trial with chimeric monoclonal antibody WX-G250 and low dose interleukin-2 pulsing scheme for advanced renal cell carcinoma. J Urol 175:57–62
Motzer RJ, Hutson TE, Tomczak P et al (2009) Overall survival and updated results for Sunitinib compared with Interferon alpha in patients. J Clin Oncol 27:3584–3590
Hudes G, Carducci M, Tomczak P et al (2007) Temsirolimus, Interferon alpha, or both for advanced renal-cell carcinoma. N Engl J Med 356:2271–2281
Negrier S, Escudier B, Gomez F et al (2002) Prognostic factors of survival and rapid progression in 782 patients with metastatic renal carcinomas treated by cytokines: a report from the Groupe Francais d’Immunotherapie. Ann Oncol 13:1460–1468
Acknowledgments
HACA evaluations were performed at Wilex AG, Munich, Germany, which provided the antibody.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
This research project was sponsored by Wilex AG, Munich, Germany.
Rights and permissions
About this article
Cite this article
Siebels, M., Rohrmann, K., Oberneder, R. et al. A clinical phase I/II trial with the monoclonal antibody cG250 (RENCAREX®) and interferon-alpha-2a in metastatic renal cell carcinoma patients. World J Urol 29, 121–126 (2011). https://doi.org/10.1007/s00345-010-0570-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00345-010-0570-2