Abstract
Phytohormones are key signalling molecules in developing responses in plants with various biotic and abiotic stresses. From the catalogue of well-known classical plant hormones, Jasmonic acid (JA) and its corresponding precursors and derivatives attract massive research attention by acting as potential methods for improving tolerance in diseased plants. Jasmonates are oxylipins chiefly derived from α-linolenic acids through the octadecanoid pathway. Plants synthesise this hormone in response to growth or defence-linked signs to coordinate plant development, growth or defence against numerous pathogenic microorganisms. Several reports emphasise the contribution of JAs in regulating vital physiological processes such as leaf senescence, tuber formation, photosynthesis, reproduction, seed germination and growth inhibition in plants. In response to pathogen exposure, JAs assume their operational task as a ‘master switch’, in charge of the initiation of signal transduction cascade, participating in the upregulation of genes associated with the production of alkaloids and phytoalexin, synthesis and accumulation of storage proteins, cell wall components and most importunately—stress ameliorative agents. This review focuses on recent findings connected to the structure, biosynthesis, regulation and signalling systems of JAs. In addition to this, the present article gives an essential insight into how the application of this phytohormone is involved in stress amelioration and induction of pathogenesis-resistant genes from an agriculturist, plant physiologist and biotechnologist’s point of view.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Plants persistently face a wide range of stresses which limit their productivity. These environmental stresses to which plants have been exposed can be broadly categorised as 1) abiotic and 2) biotic stress (Umar et al. 2021). Exposure of plants to stressful circumstances induces a disturbance in plant metabolism, leading to a decline in fitness and productivity (Bussotti and Pollastrini 2021). Abiotic stresses involve salinity, extremes in temperature, radiation, flood, drought, heavy metals toxicity, etc. Conversely, biotic stress incorporates pathogenic attacks by fungi, bacteria, nematodes, and herbivores. These pathogens cause several plant diseases, responsible for a significant loss in crop yield globally. Being sessile organisms, plants must face these environmental signals. To complete their life cycle, plants exhibit stress tolerance or avoidance through mechanisms like acclimation and adaptation that ultimately induce cellular or organismal homeostasis (Lamalakshmi Devi et al. 2017).
A significant component of plant defence is the appropriate and rapid perception of stress stimulus to initiate a quick and effective response. These cellular responses are executed by communicating the stimuli from sensors situated on the cell surface or inside the cytoplasm to the nucleus, where the transcriptional apparatus has been altered through the signal transduction pathway (Iqbal et al. 2021). Consequent to this, the plant’s basal defence mechanism comes into existence which leads to the activation of complex protective strategies, including kinase cascades and ion channels, generation of reactive oxygen species (ROS), biosynthesis and accumulation of phytohormones like salicylic acid (SA), abscisic acid (ABA), ethylene (ET) and jasmonic acid (JA) (Yang et al. 2021). All these changes individually or together precede the reprogramming of the plant’s genetic machinery which results in suitable defensive outcomes which ultimately increases the tolerance and minimises the biological impairment caused to plants by various kinds of stresses.
Phytohormones are plant-derived bio-chemicals which play a significant role in plant metabolism by regulating its growth and development, reproduction, endurance and death. These essential molecules are originated from secondary metabolism and are liable for the tolerance of plants to multiple environmental stimuli (Mukherjee et al. 2022). Sometimes, a single hormone controls numerous cellular or developmental activities in plants. On the other hand, the coordination of several hormones is required to regulate a single process. Higher plants have six significant phytohormones: auxins, gibberellin, cytokinin, abscisic acid, ethylene and brassinosteroids. Recently two additional categories, i.e. jasmonates and strigolactones, have also been incorporated into this list (Asif et al. 2022). By stimulating cellular growth, auxins are chiefly associated with plant stem elongation. Similarly, gibberellin mainly regulates and promotes stem extension, flowering, leaf enlargement and, most importantly, seed germination. Cytokinins are primarily located in root-tips, apical meristem, immature leaves and seeds in plants. This phytohormone is utilised in tissue culturing to induce cell division, formation of the adventitious shoot and embryogenesis. Primary physiological functions of ethylene in plants include growth retardation, fruit ripening and senescence. Abscisic acid in plants has been considered a stress hormone and plays a significant part in suppressing seed germination and budding in plants. Brassinosteroids are polyhydroxy steroidal hormones which synergistically and additively work with auxin and gibberellins, respectively, and are ultimately responsible for cellular division and elongation (Mukherjee et al. 2022). Likewise, strigolactones are endogenous signalling molecules which, by serving as root exudate constituents, facilitate plant–microbe symbiotic association in the plant’s rhizosphere.
These biological compounds regulate various physiological, morphological and biochemical functions in plants and are effective under different adverse conditions under biotic and abiotic stresses (Egamberdieva et al. 2017). During exposure to stressful circumstances, plants respond to them by operating a brilliant communication network of chemical messengers known as the hormonal signal transduction pathway. A unique characteristic of this communication system is that it can communicate over long distances through signal detecting, transmitting and responding to cells and even organelles of different tissues and organs (Klumpp and Krieglstein 2002). Among numerous phytohormones in plant spp., ABA, JA, SA and ET in adequate proportion play a significant role as stress signals. Where ABA chiefly contributes towards mitigating abiotic stress, SA, JA and ET play crucial roles in responding to biotic stress signalling (Liu et al. 2008; Cramer et al. 2011). In rare situations, ABA has also accumulated in diseased plants because of pathogen infection (Anderson et al. 2004; Fujita et al. 2006; Flors et al. 2008; Ton et al. 2009). In general, SA executes a defensive response against the pathogenicity of biotrophic pathogens, while necrotrophic-induced severe infection has been reported to be alleviated by JA/ET-mediated defence signals (Glazebrook 2005). JA and SA-induced signalling mechanisms occasionally exhibit antagonistic dealings, but certain studies also report their synergistic connections (Li et al. 2019). In addition to JA/SA, several shreds of evidence also support the involvement of effective interactions among SA/ET, JA/ABA and JA/ET, which subsequently lead to adaptive responses against adverse conditions.
Key regulatory molecular troupes of JA/SA crosstalk mainly comprise NPR1 (NONEXPRESSOR OF PR GENES 1), MAP kinase (Mitogen-activated protein kinase), GRX480 (a member of glutaredoxin family), WRKY transcription factors and many more. NPR1-encoded regulatory proteins are involved in the activation of genes which further enhance the expression and translation of pathogenesis PR proteins. In short, this regulatory sequence is involved in the induced signalling pathway (van Loon et al. 2006). On the other hand, WRKY transcription factors regulate SA- Supported protective responses and are also reported to be involved in JA/SA crosstalk. Mao et al. observed that WRKY transcription factors negatively regulate JA-dependent responses in plants (Mao et al. 2007). MAP Kinase serves as a mediator in conveying defence-related signalling information from the sensor to the responsive organ in plants. For example, as observed in Arabidopsis, MPK4 upregulate the expression of JA-induced signalling while repressing the expression of the SA-dependent defensive pathway (Brodersen et al. 2006). In plants, JA and its derivatives stimulate the plants’ responses to biotic and abiotic stresses by up/downregulating the transcription of stress-related genes, specifically antioxidative ones (Kim et al. 2021). Several previous investigations revealed that stressed-induced rapid and dynamic regulation of JA biosynthesis and signalling genes affect the transcription factors associated with promotors of JA-responsive genes. In short, the present article briefly reviews the structure, biosynthesis and physiological function of JA. It also discusses the role and signalling pathway of JA in response to stress especially biotic stress in diseased plants.
Chemical Structure and Derivatives of Jasmonic Acid (JA)
Jasmonic acid (JA) is a member of the jasmonates class of phytohormones. This organic compound has been isolated from several plants, including jasmine. Plants biosynthesise this plant hormone by using the octadecanoid pathway from linolenic acid. The methyl ester of jasmonic acid, methyl-JA (Me-JA), was isolated for the first time in 1957 by Swiss chemist Edouard Demole and his co-workers (Demole et al. 1962). JA and its derivatives, especially Me-JA, are volatile ingredients of several essential oils like jasmine, rosemary and many more flowers. Initially, Me-JA had been detected as a significant aromatic component in Jasminum grandiflorum L. (Demole et al. 1962). Later in 1971, JA was isolated from the filtrate of the fungus Lasidiplodia theobroma (Aldridge et al. 1971). Its growth controlling characteristics were identified after approximately 10 years.
JA, IUPAC name 3-oxo-2-(pent-2′-enyl) cyclopentane acetic acid, is a ubiquitous phytohormone in the plant kingdom (Lalotra et al. 2020). Its occurrence has been reported from algae ((e.g. Chlorella), fungi (Gibberella fujikuroi and Botryodiplodia theobromae), mosses, ferns, as well as from higher plants. Its presence has been reported in almost every tissue of higher plants, including stem, tuber, leaves, flower, pollens and fruits. It is a fatty acid with 12-C unsaturation and a cyclopentane ring that has a keto group (Fig. 1). In addition to this, its methyl ester, i.e. methyl jasmonate synthesised by the transfer of a methyl group from SAM (S-adenosyl-L-methionine) to the carboxyl group of JA by the action of JMT (Jasmonic acid carboxyl Methyl Transferase). JA as well as Me-JA is biologically active compounds and, along with their derivatives, is known as jasmonates (Lalotra et al. 2020).
Jasmonates displayed the phenomenon of optical activity. To its cyclopentane ring, three distinct substituents are affixed and positioned at C-3, C-6 and C-7 (Wasternack and Kombrink 2010). The subsequent stereoisomers of JA are identified as trans-(-) –(3R,7R)-JA; (-)-JA; trans-(+)-(3S,7S)-JA; (+)-JA; cis-(-)-(3S,7R)-JA; (-)-epi-JA; cis-(+)-(3R,7S)-JA; (+)-epi-JA (Han et al. 2011) (Fig. 1). Cis-isomers of JA are thermodynamically less stable as compared to trans-isomers. Therefore, to obtain a stable configuration and more biological activity, they epimerise at C-7 to achieve a more stable trans-form. The active form of jasmonates requires the presence of a cyclopentane ring along with a ketone group at the C-6 position. The presence of a pentenyl side chain at the C-7, attachment of methyl group and conjugation with amino acid increases the activity of these compounds. On the other hand, hydroxylation of C-11 or-12, reduction of carbons between 11 and 12, and the presence of a chain carrying an odd number of carbon atoms at the C-3 position decreases the activity of these molecules (Krumm et al. 1995; Wasternack 2007; Holland and Jez 2018).
Biosynthesis of Jasmonic Acid (JA)
In higher plants, the enzymes responsible for JA biosynthesis have been reported to occur in almost all plant tissue, including coleoptiles, young fruit tissue, cotyledons and leaves. Like other oxylipins, JA is synthesised by the octadecanoid pathway. The family of these defence-related compounds has been derived from the oxidation of polyunsaturated fatty acids. Biosynthesis of JA is initiated from 18 carbon fatty acids with three unsaturation, named linolenic acid (LA) (Lalotra et al. 2020). Cellular membranes, particularly esterified glycerolipids and phospholipids in chloroplast envelope, serve as a rich source of LA. Several investigations have reported that activation of the phospholipase enzyme, which releases LA from membranes, is responsible for enhancing JA concentration in wounded tissues (Canonne et al. 2011). Moreover, the plant’s enzymes, like acyl hydroxylase, can assist in the release of fatty acids from lipids. A study performed on Phytophthora parasitica var. nicotianae-infected tobacco revealed that phosphatidylcholine was reduced upon the subsequent rise in phospholipase activity (Schuck et al. 2014). Similarly, in a mutant of A. thaliana (fad3-2 fad7-2 fad8), it has been investigated that a very low level of LA cannot accumulate a sufficient level of JA because of wounding (Routaboul et al. 2012). This indicates that the availability, distribution and amount of LA directly influence JA’s biosynthesis in plants.
At the very beginning of JA biosynthesis, LA is converted to 13-hydroperoxylinolenic acid (13-HPLA) by the action of the lipoxygenase (LOX) enzyme (Wasternack and Strnad 2018). 13-HPLA serves as a substrate for two enzymes which are Allen Oxide Synthase (Hydroperoxide dehydratase/hydroperoxide dehydrase) and Allen oxide Cyclase which converts it to an intermediate named 12-oxo-phytodienoic acid (12-oxo-PDA) (Rustgi et al. 2019). Subsequently, upon reduction and three rounds of β-oxidation, 12-oxo-PDA gives rise to JA. (+)-7-iso-JA so formed epimerises to a more stable trans configuration, i.e. (-)- JA (Liu and Park 2021) (Fig. 2). Further, it can be catabolised to methyl jasmonates (Me-JA) or various other conjugates with some biological function. Concerning the locations of enzymes involved in JA biosynthesis, it has been observed that LOX has been reported in the chloroplast. Further, enzymes such as AOS and HPL associated with the metabolism of 13-HPLA have been accompanied by chloroplast envelopes. A signal peptide associated with AOS has been reported to target it to the plastid (Ruan et al. 2019). In a deep investigation of spinach leaves, it has been carefully observed that chief enzymes associated with the synthesis of oxylipins, LOX, HPL and AOS have been isolated from the membrane fraction and not the stroma fraction of the chloroplast envelope (Srivastava 2002). Therefore, there is still uncertainty regarding the exact locations of enzymes involved in JA biosynthesis, but undoubtedly, the peroxisome is the site for β-oxidation. Entry of 12-oxo-PDA through the peroxisomal membrane is facilitated by the combined activity of membrane-localised COMATOSE (CTS), an ABC transporter. In peroxisome, it is reduced to an intermediate termed 3-oxo-2-(cis-2′-pentenyl)- cyclopentane-1-octanoic acid (OPC-8:0) by the action of OPDA reductase. Further, OPC is activated to OPC ~ CoA with the action of OPCCo-A ligase (OPCL). Subsequently, OPC-CoA undergoes three rounds of β-oxidation, mainly catalysed by three factors, i.e. 3-ketoacyl-CoA thiolase (KAT), multifunctional protein (MFP) and acyl-CoA oxidase (ACX) (Fig. 2). Consequently, JA is transported from the peroxisome to the cytoplasm at this stage. Here, it is conjugated with isoleucine and converted to biologically active (+)-7-iso-JA-Ile. In the cytoplasm, it can be transferred to an inactive form 12-hydroxy-JA-Ile by the action of CYP94B3, a form of cytochrome P450; or it can be metabolised to other inactive forms through several processes like glycosylation, methylation or sulfation (Wasternack and Strnad 2016). On the other hand, the export of 12-oxo-PDA from chloroplast to peroxisome has been observed to be assisted by a protein termed JASSY. This protein comprises a Steroid acute regulatory protein-related lipid transfer (START) domain which facilitates the binding and transport of molecules with hydrophobic properties.
JAs are synthesised from α-linolenic (LA) acid by octadecanoid pathways as a precursor molecule in chloroplast, peroxisome and cytoplasm. LOX enzyme in chloroplast catalyses the conversion of LA to 13-HPLA, which is itself converted to 12-oxo-PDA by catalytic activities of AOS and AOC. Entry of 12-oxo-PDA in peroxisome is facilitated by JASSY and CTS in chloroplast and peroxisomal membrane, respectively. Subsequently, (+)-7-iso-JA is synthesised after reduction to OPC by the action of OPDA reductase, activation to OPC ~ CoA by OPCL and three rounds of β-oxidation through ACX, MFP and KAT. JAR 1 conjures JA with Isoleucine to convert it into a biologically active form, ( +)-7-iso-JA-Ile. Transport of this JA-Ile complex through the nuclear membrane has been reported to be mediated by a JAT 1 transfer protein wherein it regulates the expression of JA-responsive genes. ASA and DIECA act as potent inhibitors in JA biosynthesis by obstructing the AOC and AOS enzymes. LA α-linolenic, LOX lipoxygenase enzyme, 13-HPLA 13-hydroperoxylinolenic acid, 12-oxo-PDA 12-oxo-phytodienoic acid, AOS Allen Oxide Synthase, AOC Allen oxide Cyclase, JA Jasmonic acid, CTS COMATOSE, OPC-3-oxo-2-(cis-2ʹ-pentenyl) cyclopentane-1-octanoic acid, OPC ~ CoA OPC ~ Co- enzyme A, OPCL OPCCo-A ligase, KAT 3-ketoacyl-CoA thiolase, MFP Multifunctional protein, ACX Acyl-CoA oxidase, JA-Ile Jasmonic acid Isoleucine, DIECA Diethyldithiocarbamic acid, ASA Acetylated salicylic Acid, JAR1 Jasmonate Resistant 1
In plants, biosynthesis of JA is prone to be blocked by many potent inhibitors. In this direction, diethyldithiocarbamic acid (DIECA) and acetylated derivative of SA, i.e. aspirin (ASA), affect its synthesis by directly inhibiting the expression of JA-induced genes (Srivastava 2002). To check which step is inhibited by which inhibitors, a precursor in JA biosynthesis is applied with an inhibitor, and its influence on gene expression is monitored. From previous studies, it has been apparent that ASA blocks the cyclisation process by blocking the activity of the cyclooxygenase enzyme. This finding exhibited that ASA obstructs the biosynthesis of JA by blocking the activity of the AOC enzyme, which is required for the conversion of 13-HPLA to 12-oxo-PDA. Similarly, DIECA obstructs by inhibiting the 13-HPLA -12-oxo-PDA conversion step. Its exact mode of action is not clear, but somehow it makes 13-HPLA unavailable by rerouting it to another derivative because of its substantial reducing agent property (Srivastava 2002) (Fig. 2).
Physiological Roles of Jasmonic Acid in Plants
In the early 1980s, Jasmonic acid and its derivatives attracted the concern of plant physiologists towards themselves when it came to their notice that they exhibited the potential to accelerate leaf senescence and reduce the growth of coleoptiles and roots. Considering this, their activities have been attested to in several bioassays. Furthermore, in multiple investigations, it has been reported that they displayed immense importance to plants in terms of induction of defence-related genes against pathogens and insects as well as responses associated with senescence and stress. The present article briefly discusses the physiological roles of JA and its ameliorative potential against abiotic stress in plants. Afterwards, it comprehensively focussed on its importance in developing disease resistance in biotically stressed plants.
Leaf Senescence
In 1980, a substance responsible for senescence was obtained from Artemisia absinthium and later identified as Me-JA (Ueda and Kato 1980). Furthermore, degradation of chlorophyll contents has been reported in the case of oat leaves floated on a medium comprising of only10 µg/ mL Me-JA. Similarly, the senescence-reducing effects of light have been observed to be reversed by Me-JA (Kim et al. 2015). If we talk about ABA to induce a similar kind of senescence in plants, a much higher concertation, approximately tenfold, is required to get the same results. Several genes related to JA biosynthetic pathway have been known to be up/downregulated during the succession of leaf senescence. For example, MYC2, MYC3 and MYC4 facilitate JA or dark-induced leaf senescence by enhancement in the expression of genes associated with the leaf senescence, for instance, senescence-associated gene 29 (SAG29) and chlorophyll catabolic genes (CCG), e.g. Pheophorbide A Oxygenase (PAO). In addition to this, they also downregulate the expression of genes related to photosynthesis (Qi et al. 2015; Zhu et al. 2015).
On the other hand, several studies suggested that during leaf senescence, JA and other oxylipins are formed as a by-product due to the destruction of biomolecules and membranes (Seltmann et al. 2010; Zhu et al. 2015). Arabidopsis mutant with a decreased level of JA displays chronological swings in the commencement of normal and dark-induced senescence (Schommer et al. 2008; Danisman et al. 2012). Furthermore, exogenous treatment of JA on wild-type Arabidopsis induces the expression of several senescence-associated genes (SAGs). In addition, an upsurge at the transcription level has been reported in genes associated with the biosynthesis of JA in Arabidopsis (He et al. 2002).
Recently, it has been observed that miR319, a micro-RNA, regulates the expression of transcription factors termed TEOSINTE BRANCHED/CYCLOIDEA/PCF (TCP) (Schommer et al. 2008). TCPs are generally considered downregulators of plant growth and development (Almeida et al. 1997; Luo et al. 1999). Further, these transcription factors are also crucial for expressing cell cycle regulators named proliferating cell nuclear antigen (PCNA). In a study, Schommer et al. demonstrated that the miR319-TCP regulatory unit controlled the biosynthesis of JA by blocking the expression of the LOX2 gene (Schommer et al. 2008). Consequently, a reduction in JA level leads to a delay in leaf senescence, and this prolonged senescence can be reversed by applying JA exogenously.
Growth Inhibition
The exogenous application of JA inhibits the elongation of seedlings and causes retardation in the growth of roots and stems. Compared to hypocotyl, roots are reported to be more sensitive to JA-mediated inhibition. By inhibiting the activity of cell wall degrading enzymes like peroxidase and polygalacturonase, Me-JA prevents softening of fruits. In addition, JA increases the content of tocopherols, linolenic acid and linoleic acid by reducing the activity of the lipoxygenase enzyme (Antognoni et al. 2009). Furthermore, exogenously applied Me-JA decreases the amount of lycopene while raising the level of β-carotene in tomatoes (Saniewski and Czapski 1983). In the case of green fruits, it quickens the degradation of chlorophyll while inhibiting anthocyanin accumulation (Horbowicz et al. 2011). Moreover, kinetin-mediated soybean callus growth has been reported to be hampered by both JA and Me-JA, even at a very low concentration. Similar findings were also observed in the case of potato meristem culture (Kamińska 2021). On the other hand, JA does not exhibit an inhibitory effect on IAA treated Avena seedlings, suggesting that JA and its derivatives insert inhibitory effects on cell division but not on cell elongation (Kamińska 2021). Applying JA reduces the expansion of leaves and cotyledons by hindering the activity of mitotic cyclin Cyc B 1;2 associated with cell division (Noir et al. 2013). Interestingly, continuous wounding or touching induces the ‘bonsai effect’ in showy bonsai plants by reducing leaf expansion due to the biosynthesis of JA (Zhang and Turner 2008; Chehab et al. 2012). In Arabidopsis, JA-induced hypocotyl growth inhibition has been reported under all tested light conditions. Similarly, applying JA to plants negatively affects the growth of their primary roots. In general, under stressful circumstances, JA-induced inhibition of plant growth ensures the more remarkable survival of plants by encouraging them to defend themselves first in the natural environment.
Tuber Formation
The role of JA in the induction of tuber formation in potatoes (Yang et al. 2004) and as a modulator of bulb stimulation in garlic is well reported in several investigations (Ravnikar et al. 1993). Contents of JA are measured to be highest at the time of tuber set in soil-grown potato plants. For short days plants, a tuber-inducing substance has been synthesised in leaves whose existence after translocation has been confirmed on stolons through grafting and other experimentations. After extraction from the potato leaves, this tuber-inducing substance has been hypothesised as tubernoic acid glucoside (TAG). Later, the chemical structure of TAG was identified to be 12-hydroxy jasmonic acid glucoside, which is, in turn, correlated to JA structurally (Yang et al. 2004). At the cellular level, JA and its derivatives are known to play a vital role in cell division and cell regulation by regulating the reorientation of cortical microtubules. Compared to JA, GA is known to inhibit tuberisation processes in potato.
In vegetative storage spaces of plants, JA participates in synthesising vegetative storage proteins (VSPs). These proteins were, for the first time, isolated and purified from soybeans. According to Staswick 1994, JA and its derivatives regulate the expression of genes associated with VSPs (Staswick 1994). These proteins are formed in plants when available nutrients surpass their requirement for growth and are consequently employed either in the development of seeds in annual plants or spring growth in the case of perennials. After synthesis, VSPs accumulate in the vacuoles of paraveinal bundle sheaths and mesophyll cells in soybean leaves.
Seed Germination
Concerning seed germination, JA and its derivatives stimulate the germination of dormant seeds but are also known to inhibit germination in the case of non-dormant seeds. Exogenous applications of jasmonates to mature seeds cause inhibition in the germination of various spp. (Yildiz et al. 2008; Worrall et al. 2012). In the case of recalcitrant seeds of Quercus robur, ABA, Me-JA and ethylene were reported to inhibit their germination (Finch-Savage et al. 1996). Preceding the viability loss in these desiccation-sensitive seeds, the levels of Me-JA and JA in them were observed to have increased. In other studies, it has been demonstrated that the rise in the concentration of jasmonates leads to lipid peroxidation and thereby causing damage to the cell membrane and ultimately resulting in germination inhibition. In comparison with older seeds, the levels of JA in soybean seeds after 12 days of anthesis were observed to be exceptionally low (0.5 ng/g fresh weight (Creelman and Mullet 1997). In JA biosynthesis and signalling mutant of Arabidopsis, accumulation of OPDA and other dormancy-inducing factors like ABA cause an increase in seed dormancy (Dave et al. 2011).
Photosynthesis
Exogenous treatment of plant leaves with JA leads to decreased expression of nuclear and chloroplast genes associated with photosynthesis. In addition, the application of this hormone causes chlorophyll degradation under both in vivo and in vitro conditions. Owing to the treatment with this hormone, many alterations in gene expression have been monitored in terms of translation, transcription and mRNA levels. Further, JA and its derivatives affect the activities of plants associated with photosynthesis and antioxidants by controlling protein profile (Maserti et al. 2011). In Saxifraga longifolia, treatment with jasmonates leads to the accumulation of pigments like carotenoids and chlorophylls, as well as enhances the efficiency of Photosystem II (Cotado et al. 2018). Contrary to this, a severe decrease in the activity of ribulose-1,5-bisphosphate carboxylase/oxygenase (RuBisCO) subunit because of JA treatment has also been reported in Oryza sativa (Rakwal and Komatsu 2000). Furthermore, when applied externally, Me-JA causes stomatal closure in barley. Similarly, a reduction in oxygen evolution through thylakoids of barley has been observed due to pre-treatment with JA.
Reproduction
In addition, to regulating vegetative growth in plants, jasmonates are also associated with several processes linked to plant reproduction. As observed in the case of Arabidopsis, JA biosynthetic and signalling mutants of this plant are male sterile due to a combination of several factors like reduced pollen viability, inadequate filament elongation, faulty dehiscence of anthers etc. JA triggered the translation of two regulatory proteins, which further enhanced the process of stamens maturation in treated plants (Yan et al. 2013). Fertility-defective mutants of Arabidopsis can be protected by exogenous application of jasmonic acid in the case of biosynthetic mutants but not in the case of signalling mutants (Browse 2009). Similarly, in plants producing bisexual flowers, such as rice, JA mutant plants exhibited male sterility, which indicates that JA plays an essential role in male organ development in bisexual flowers, too (Riemann et al. 2003).
A recent study in maize which bears distinct male inflorescence (tassel) and female inflorescence (ear) on the same plant reveals that JA serves as a vital phytohormone that initiates the sex determination programme in the tassel. In the case of a mutant, ts1 (tesselseed 1) tessels become female-fertile assemblies that can be pollinated to possess seeds later (Acosta et al. 2009). A further investigation undertaken by (Yan et al. 2012) reported that JA-defective mutants exhibit 100% feminised tassel, emphasising that the JA signal is requisite for tassel development in maize. Moreover, as observed in the case of tomato, the size and mass of fruits are relatively much less in a JA signalling mutant exhibiting seed carrying sterility compared to wild-type variety of the crop. Viable seeds procured from the mutant type were only 0.1% of the seeds that were obtained from the wild cultivar under the same growth conditions (Li et al. 2004). Furthermore, as observed in the case of JA deficient and perception mutant in Arabidopsis, at the time of anthesis in comparison with wild-type plants, these exhibit large-size petals, which thereby signifies that JA influences petal expansion by restricting their growth (Reeves et al. 2012). In Arabidopsis, JA induces flowering inhibition by blocking the transition from the vegetative to the reproductive stage. Furthermore, JA delays flowering by downregulating the transcription of the florigen gene FLOWERING LOCUS T (Zhai et al. 2014).
Other Developmental Roles of Jasmonic Acid
Development of lateral and adventitious roots is also reported to be mediated by Jasmonates. In Arabidopsis, JA induces the formation of lateral roots by upregulating the expression of the ERF109 gene, which in turn unites and stimulates the auxin biosynthetic promoter genes. Climbers are the means which provide direct support to the climbing plant to coil around a supportive object. It has been reported that exogenous application of methyl derivative of an intermediate in JA biosynthetic pathway (12-oxo-PDA) induces coiling in many plants. Defects in wound /insect attack-induced perception or biosynthesis of JA cause inhibition in trichome formation in Arabidopsis. GA and JA act synergistically to promote trichome formation in plants (Nakata et al. 2013; Song et al. 2013). JA is needed for normal gravitropic responses in rice coleoptiles by influencing the lateral reallocation of auxins in them. In gravitropic response in a coleoptile, JA gathers more significantly in the upper edge than the lower flank in the same coleoptile (Gutjahr et al. 2005). Furthermore, enhancement in the biosynthesis of JA causes upregulation of the expression of genes required for the elongation of cotton fibre, and thereby treatment with jasmonates promotes the quality of cotton fibre in the textile industry (Hu et al. 2016). Me-JA influences the fruit ripening processes in plants directly by enhancing ethylene concentration. Exogenous applications of Me-JA elevate the level of ethylene precursor 1-aminocyclopropane-1- carboxylic acid (ACC) and its conversion to ethylene too.
Defensive Role of Jasmonic Acid Against Abiotic/Biotic Stresses in Plants
Jasmonic acid and its associated derivatives induce defence responses in plants by synthesising and accumulating secondary metabolites like phenolic compounds, alkaloids, terpenoids, etc. Similarly, the exogenous application of JAs leads to the development of a better oxidative defence system in stressed plants. An enhancement in the activity of antioxidative enzymes like superoxide dismutase (SOD), catalase (CAT), ascorbate peroxidase (APX), peroxidases (POX), polyphenol oxidase (PPO), lipoxygenases (LOX) and phenylalanine ammonia lyase (PAL) has also been observed. Likewise, levels of antioxidative defensive molecules such as ascorbic acid, glutathione and tocopherol contents were observed to be increased. In contrast, hydrogen peroxide (H2O2) and malondialdehyde (MDA) contents were observed to be decreased. Similarly, an enhancement in the contents of pathogenesis-related (PR)-Proteins, e.g. Chitinase, Glucanase etc., has also been reported because of JA-induced regulation of gene expression in stressed plants.
Abiotic Stress
By acting as an essential plant-signalling molecule, JA and its derivatives enhance the plant’s tolerance to multiple types of abiotic stresses (viz., drought, heavy metal-toxicity, salinity, temperature extremes etc.) (Li et al. 2018a). These stressful circumstances alter the variable metabolic processes in plants by influencing the gene expression, affecting the RNA and protein stability and transport of ions across the biological membranes (Kosová et al. 2015). In response to the exposure to different kinds of abiotic stresses, plants evolve several mechanisms to appropriately sense and execute a suitable responding strategy against a particular type of abiotic stress (Hamant and Haswell 2017). For example, physical perception of stress stimulus appears in the form of mechanical effects as the plasma membrane shrinks during drought stress. In addition, biophysical sensing involves a change in the structure of biomolecules, such as the inhibition of enzymatic activities of different enzymes under heat stress. Similarly, metabolic sensing incorporates either an increase or decrease in the content of products and reactants associated with various metabolic reactions (Ali and Baek 2020).
Jasmonates, either alone or in coordination with other phytohormones, stimulate various physiological, biochemical and molecular processes in plants which eventually assist the plants in attaining tolerance against variable abiotic stresses. In response to JA pre-treatment, the physiological reactions often involve stimulation of enzymatic and non-enzymatic antioxidative defence systems (Wasternack 2014), synthesis and accumulation of amino acids as well as soluble sugar molecules (Wasternack 2014) and regulation of opening and closing of stomatal apertures in stressed plants (Acharya and Assmann 2009). On the other hand, the molecular responses often involve a change in the expression pattern of JA-linked genes (Robson et al. 2012; Hu et al. 2017), interaction with other phytohormones and transcription factors (Ku et al. 2018; Yang et al. 2019). Exposure of the plants to low-temperature conditions upregulate the expression of genes linked with the biosynthesis of JA, such as AOC, AOS and LOX. JA-mediated signal transduction in association with C-repeat binding factor (CBF) transcriptional pathway positively regulates the expression of downstream cold-responsive genes and eventually enhances cold tolerance in the affected plants (Hu et al. 2017). Additionally, the exogenous application of JA decreases the activity of LOX and simultaneously improves the antioxidative defence system and stress tolerance in affected plants (Li et al. 2018b). Several previous investigations support the ameliorative potential of both endogenous and exogenous JA against drought stress in plants. For instance, in Arabidopsis thaliana, controlling the stomatal opening and closing of JA reduced the leaf’s water loss (Savchenko et al. 2014). Similarly, improvement in drought tolerance due to JA treatments has also been observed in Prunus armeniaca (Yun-xia et al. 2010) and soybean (Mohamed and Latif 2017) plants.
In addition to drought and cold stress, treatment of plants with JA also enhances their tolerance to salt stress (De Domenico et al. 2019). Consequently, previous investigations demonstrated the defensive role of JA under saline conditions through the effective disposal of ROS (Abouelsaad and Renault 2018), enhancement in photosynthetic rates, proline contents and ABA concentration (Bandurska et al. 2003), the establishment of enzymatic and non-enzymatic antioxidative defence system (Walia et al. 2007) as well as a decrease in the rate of Na+ accumulation in the shoots (Khan et al. 2012). Heavy metal-induced toxicity most significantly impaired plant growth and development among abiotic stresses, ultimately leading to considerable yield loss. Following Sirhindi et al., before Ni exposure, exogenous treatment with JA enhanced tolerance to Ni2+ in Glycine max seedlings (Sirhindi et al. 2015). Similarly, Azeem revealed that in Zea mays under Ni-induced toxicity, exogenous application of JA mitigates the damaging influences of ROS by increasing the activity of antioxidative enzymes in stressed plants (Azeem 2018).
Biotic Stress
Host–Pathogen Interaction in Biotically Stressed Plants
A significant factor that ascertains better vegetative and reproductive growth of plants is ‘defence’, as a well-defended plant can survive under different kinds of biotic stresses. The defensive strategies of plants are based on the rapidity in recognising a specific pathogen and mounting a signalling network to initiate the synthesis and accumulation of defence molecules. Upon exposure to a virulent pathogen, a susceptible host plant in an environment where pathogens flourish acquires disease infection. Due to interaction with these microbial pathogens, plants defend themselves by developing a signalling pathway that performs an indispensable role in sensing the stress stimulus and generating an appropriate response (Enebe and Babalola 2018). The specific surface receptors recognise pathogen invasion signals in the host plant’s cells. After successful perception of these cues, plants either initiate pattern triggered immunity (PTI) or effector triggered immunity (ETI) (Saijo et al. 2018).
PTI
PTI is the first stage of the plant immune system, which is triggered by the perception of the pathogen by the pathogen or pattern recognition receptors (PRRs) through recognition of isolated pathogen or microbe-associated molecular patterns (PAMPs/ MAMPs) (Zhang and Zhou 2010). In addition, these plants also have the potential to detect damage-associated molecular patterns (DAMPs). DAMPs in plants may be proteins, peptides, amino acids or nucleotides formed or released by cells damaged by pathogen invasion. Moreover, some plant cell wall-derived polysaccharide oligomeric remnants released during tissue disruption by attacks of pathogens or herbivores also act as DAMPs (Choi and Klessig 2016). PRRs mainly involve leucine-rich-repeat (LRR), receptor-like kinase (RLKs), FLS (Flagellin-sensing 2) and EFR (which recognises prokaryotic protein, i.e. elongation factor thermo unstable EF-Tu) (Couto and Zipfel 2016). These receptors, bound to their corresponding ligands, further form complexes with adapter kinase of a similar class of extracellular domain and thereby initiate phosphorylation of various proteins through the assistance of RLKs and receptor-like cytoplasmic kinase. The whole of this PRR signalling cascade then brings about the eruption of cytosolic Ca2+ and apoplastic ROS outburst, CDPKs (Ca2+ dependent protein kinase), MAPK (mitogen-activated protein kinase) and extensive regulation of synthesis and metabolic reprogramming of defensive phytohormones (Yu et al. 2017). All these outputs cooperatively contribute towards PTI, which inhibits the spread of pathogen-associated infection and incorporates basal resistance in plants.
ETI
Plants can also detect the presence of pathogens through the perception and recognition of pathogen-derived molecules known as effectors. These pathogens-synthesised molecules enhance pathogens’ survival by offsetting basal resistance mechanism induction, i.e. PTI (Balint‐Kurti 2019). The plants which cannot recognise these effectors become susceptible to the pathogen, while plants that detect these effectors through the assistance of disease resistance proteins (R Proteins) can induce an immune response referred to as ETI. In contrast to PTI, ETI usually acts characteristically more strongly against pathogen-facilitated perturbations (Cui et al. 2015). Most pathogens produce apoplastic effectors such as CWDEs (cell wall degrading enzymes), hormones, toxins and cysteine-rich peptides (Schellenberger et al. 2019). CWDEs mainly include cellulase, pectinase, xylanase and protease, which assist in penetrating pathogens into plant cells, promoting cellular death and nutrient acquisition. In addition to this, the pathogen also secretes cysteine-rich proteins, which in turn protect the invading pathogen against plant-based hydrolytic enzymes such as glucanase, protease, chitinase, and so on.
Another microbial product that is toxic to the host plant is comprised of phytotoxins. Their presence in plants results in the chlorosis and necrosis of diseased host tissues (Stergiopoulos et al. 2013). Phytotoxins can be categorised into host-selective toxins (HSTs) and general toxins. Phytohormones, which regulate plant growth in minimal concentrations, may also serve as virulence effector molecules. For instance, pathogen-synthesised auxins and cytokinin can stimulate developmental and morphological alterations in plants, leading to several deformations in the shape and structure of plants, e.g. epinasty, gall, and so on. The genes that encode the specificity-determining factor of ETI are recognised as resistance (R) genes. Most of the R genes translate proteins that comprise a nucleotide-binding site (NBS) and leucine-rich repeats (LRRs) (Marone et al. 2013). NBS-LRR (NLR) proteins participate in identifying specific pathogen effectors that are believed to be responsible for virulence acts in the absence of the associated R gene (Noman et al. 2019). Furthermore, NLR activation results in the induction of an amplified type of defence, which frequently involves localised cell death, referred to as hypersensitive response (HR). After recognising pathogen-associated localised damage, long-distance signals are frequently produced and communicated through the plant (Hartmann et al. 2018; Toyota et al. 2018). This ultimately results in the induction of systemic acquired resistance (SAR), an improved state of entire-plant immunity to a wide range of pathogens (Fu and Dong 2013).
JA-mediated Stress Tolerance in Biotically Stressed Plants
JA and its derivatives play a crucial role in plants’ immune systems to defend them against pathogens (Pieterse et al. 2012). Several reports signify the importance of JA in inducing disease resistance against several biotrophic and necrotrophic pathogens (Table 1). Constitutive expression of the AOS gene, which participated in JA biosynthesis, promoted enhanced resistance to fungal pathogens by upregulating the expression of anti-pathogen-related genes in transgenic rice plants (Mei et al. 2006). Similarly, two AOS mutants, cpm2 and hebiba deficient in JA biosynthesis, were observed to be susceptible to infection by even an avirulent strain of Magnaporthe oryzae (Riemann et al. 2013). Likewise, osjar1-2 mutants of rice seemed more vulnerable to blast fungus than their wild counterpart (Shimizu et al. 2012). In addition to inducing resistance against pathogenic organisms, silencing of genes associated with JA biosynthesis downregulates the expression of genes such as polyphenol oxidase and peroxidase and makes the plant more susceptible to chewing insects (Ye et al. 2012).
Previous investigations have revealed that exogenous exposure to JA or its derivatives induces the expression of defence-related genes, thereby improving resistance against several necrotrophic pathogens (Wang et al. 2012; Ameye et al. 2015). As observed in the case of Fusarium graminearum infected wheat, significant alterations in defensive enzymes as well as secondary metabolites have been monitored, which later enhance disease resistance by directly inhibiting pathogen invasion (Moosa et al. 2019; Zhao and Li 2021). Similar findings were reported in JA-treated citrus fruits against green and blue moulds (Moosa et al. 2019). Similarly, the application of Me-JA leads to a significant reduction in the level of lipid peroxidation and H2O2 contents in Fusarium culmorum infected wheat (Motallebi et al. 2017). Furthermore, intermediates involved in JA biosynthesis also play a crucial role in inducing resistance mechanisms in diseased plants. For Example, OPDA performs a defensive function against the infection of Botrytis cinerea by initiating callose deposition in plants (Scalschi et al. 2015). Likewise, earlier experience of mechanical stress by Arabidopsis seedlings boosted JA- facilitated protection against several necrotrophic pathogens (Brenya et al. 2020).
Jasmonates are pivotal in regulating the biosynthesis of diverse compounds of specific metabolic pathways. As mentioned previously, JA assists in producing distinct types of secondary metabolites like indole alkaloids, terpenes, phenylpropanoids, nicotine, flavonoids, etc. (Wasternack and Hause 2013). For example, Camalexin in Arabidopsis and vinblastine in Catharanthus roseus are typical metabolites synthesised in dicots because of JA applications. Similarly, in the case of monocots, e.g. in rice, diterpenoid phytoalexins like momilactones and phytocsssanes and flavonoid phytoalexins such as sakuranetin have been observed (Miyamoto et al. 2014). Correspondingly, JA-prompted production of sesquiterpenoid phytoalexins, zealexin and kauralexin (diterpenoid phytoalexins) has also been revealed in maize (Schmelz et al. 2014).
In addition to the above, jasmonates also regulate biosynthesis and accumulation of secondary metabolites in plant tissue culture techniques. For instance, on the application of 0.1 mM Me-JA to the hairy root culture of Salvia miltiorrhiza, it was observed that treatment with this plant hormone upregulates the expression of genes encoding 3-hydroxy-3-methylglutaryl coenzyme A reductase (HMGR) and 1-deoxy-D-xylulose 5-phosphate reductoisomerase (DXR) responsible for producing tanshinone (Yang et al. 2012). Furthermore, the application of a similar concentration of Me-JA to cultured cells of Glycyrrhiza glabra accelerates the biosynthesis of soyasaponin by upregulating the transcription of cycloartenol synthase, oxidosqualene cyclase (OSC), squalene synthase (SQS)-related mRNA and enhancing the activities of soyasapogenol B glucuronosyl transferase enzymes (Hayashi et al. 2003). Similarly, it was observed that in Artemisia absinthium suspension cultures, the combined application of JA and GA stimulates the accumulation of total phenolic and flavonoid contents (Ali et al. 2015). Moreover, by decreasing the expression of catalase and upregulating the activity of PAL, 0.1 mM JA affects cell growth and flavonoid synthesis in the case of Hypericum perforatum (Wang et al. 2015a). Additionally, as revealed through microscopy, resin composition and chemical and terpenoid biosynthetic enzyme analysis in the Norway spruce plant, 10 mM Me-JA mediates the accumulation of mono and sesquiterpenes by provoking oxygenated linalool and (E)-b-farnesene or forming traumatic resin (TDs) ducts (Martin et al. 2002).
JA-Induced Signalling and Gene Regulation Mechanisms
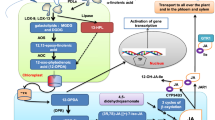
JA and its derivatives have been identified as strategic regulators that perform vital roles in defensive responses against biotrophic and necrotrophic pathogens (Pieterse et al. 2012). Upon exposure to a particular type of pathogen, two types of reactions, i.e. local and systematic, have been exhibited by infected plants. Due to pathogenic attacks, multiple invader-derived or damage-associated plant-originated signals, which may be either chemical or physical, have been reported in local responses. These signals are later recognised by PRR (pattern recognition receptor) situated on the cell membrane. Consequent to this recognition event, the de novo formation of JA and JA-Ile (JA-Isoleucine) is initiated. The cytoplasmic enzyme JASMONATE RESISTANT 1 (JAR1) is known to be responsible for the conjugation of JA with Isoleucine to convert it into a biologically active form, i.e. JA-Ile (Staswick and Tiryaki 2004) (Fig. 3 and 4). In addition, other active metabolites of JA, including Me-JA (an airborne signalling molecule) and cis-jasmone, which function as inter-plant-signalling compounds, also come into existence.
Under unstressed circumstances, a low level of JA-Ile complexes in cytoplasm leads to a closed complex of JAZ-NINJA-TPL. The stability of this complex is further enhanced by HDA-6 and HAD-19. The establishment of this complex inhibits the expression of JA-responsive genes. JAT Jasmonic acid transfer protein, JAR1 jasmonate-resistant 1, JA-Ile jasmonic acid-isoleucine, JAZ jAsmonate zim domain, NINJA novel interactors of JAZ, TPL topless, HAD histone deacetylase
A massive increase in JA-Ile concentration has been reported under stressful circumstances. On entering the nucleus, the SCF complex recognises the JA-Ile complex. After that, the JA-Ile complex is sensed by the COI1-JAZ coreceptor complex, which elicits rapid interaction of JAZ with COI1. This interaction, in turn, causes the degradation of JAZ repressor proteins in the 26S proteasome. After degradation, transcription factors linked with JA-responsive genes are activated. Furthermore, a mediator termed MED 25 modulates the gene transcription by assisting in the binding of TFs (MYC 2) with RNA II polymerase. Col-1 Coronatine insensitive-1, SCF-Skp1/Cullin/F-box, Eub E3 ubiquitin ligase complex, MED mediator
Another interesting point in JA-mediated signalling pathway associated with whole plant wide-spectrum immunity is how these cues are transported over long distances from the injured area to undamaged healthy tissue. In general, the transmission of JA can occur either systematically through vascular bundles or air in JA-dependent or independent manner. JA-independent signal transmission is exemplified by three glutamate receptor-like (GLR3) proteins facilitated by electrical signals. Owing to leaf injury in Arabidopsis, GLR3 has been reported to be rapidly transported from leaf to leaf (Mousavi et al. 2013). On the other hand, JA molecules serve as a leaf-to-leaf mobile signal in JA-dependent signal transmission (Jia 2020). On the phloem cells, two membrane-associated JA-importers, i.e. ATJAT3 and ATJAT4, mediate intercellular transportation of JA through the phloem trail (Li et al. 2020). Furthermore, ATJAT1 may be engaged in long-distance JA transport (Li et al. 2017).
JA-induced signal perception and transduction pathway involve the participation of several promoters, transcription factors, repressors, activators and mediators, as well as upregulation and downregulations of various genes. Several reports emphasise that for JA signalling, ( +)-7-iso-JA-L-Ile serves as the natural and direct ligand in the plant system (Yan et al. 2016). Coronatine insensitive1 (Col-1) is a potent receptor for this ligand (Xie et al. 1998). COL-1 locus translates an F-box protein which assembles with its other companions SKP1, Cullin and Rbx proteins to create an E3 ubiquitin ligase during the signal transduction pathway (Xie et al. 1998). The Skp1/Cullin/F-box (SCF) complex is a proteinaceous ubiquitin–proteasome complex. A protein family known as jasmonate zim domain (JAZ) serves as a critical negative regulator of the JA signalling pathway and acts as a substrate for this SCFCOI1 E3 ubiquitin ligase complex (Fig. 3 and 4). In A. thaliana, about 12 JAZ proteins have been reported. These proteins constitute an extremely conserved jaz domain at the c-terminal, which facilitates interface with several transcription factors (TFS), Col-1 and ZIM (TIFY) domains participating in JAZ dimerisation and interaction with novel interactors of JAZ (NINJA). In COI1 mutants, expressions of JA-mediated pathogenesis-related genes decrease by approximately eighty per cent, which enhances the susceptibility of the mutated plants towards necrotrophic pathogens (Xie et al. 1998; Devoto et al. 2005). Similarly, a jar1-1 mutant incapable of synthesising JA-Ile displays a reduced expression of JA-responsive genes and is highly vulnerable to a soil fungus, Pythium irregulare (Staswick et al. 1998; Staswick and Tiryaki 2004).
Step-by-step mechanism of JA-induced signalling and gene expression can be broadly categorised into two types which can be summarised as follows:
Under Normal Circumstances
During non-stressful conditions, genes responsible for the biosynthesis of JA are partially activated and therefore, levels of JA-Ile complex are very low in the cytoplasm (Ali and Baek 2020). In this normal state, transcriptional repressors known as JAZ proteins suppress transcription factors associated with promotors of JA-responsive genes (Zhou and Memelink 2016). Further, JAZ protein forms an effective close complex termed JAZ-NINJA-TPL by recruiting protein topless (TPL) and adaptor protein of JAZ (NINJA). This close complex is further maintained by the additional enrolment of histone deacetylase 6 (had 6) and histone deacetylase 19 (HDA19). The establishment of this complex inhibits the expression of JA-related defensive genes (Fig. 3). (Pauwels et al. 2010; Chini et al. 2016; Wasternack and Song 2017) (Fig. 3).
Under Stressful Circumstances
The cytosolic concentration of the JA-Ile complex increases because of stressful conditions. Transport of this JA-Ile complex through plasma and the nuclear membrane has been reported to be mediated by a JA transfer protein, i.e. AtJAT1/AtABCG16 (Ali and Baek 2020). As previously mentioned, in the nucleus, the JA-Ile complex is recognised by the F-box protein of the SCF complex called COI1. Furthermore, this JA-Ile is sensed by the COI1-JAZ coreceptor complex, thereby prompting the rapid interaction of JAZ with COI1 (Mosblech et al. 2011; Zhai et al. 2015). This interaction, in turn, causes the degradation of JAZ proteins in the 26S proteasome. After this degradation of repressor protein, i.e. JAZ, activation of various transcriptional factors and regulation of JA-regulated genes occurs (Wasternack and Hause 2013; Wasternack and Song 2017). Furthermore, a subunit of the mediator complex in Arabidopsis known as a mediator 25 (MED25), which is also recognised as phytochrome and flowering time 1 (PFT1), modulates the gene transcription by assisting in the binding of TFs (MYC 2) with RNA II polymerase (Fig. 4) (Bäckström et al. 2007; Chen et al. 2012; Ali and Baek 2020). Therefore, in the context of signalling, we can conclude that under the influence of different developmental stages, kinds of stimuli, specific organs and interference with other hormones, hundreds or even thousands of genes are regulated by a vast collection of JAZ-TF blends. Further, these combinations offer particularity to this signalling system with only one chief hormonal ligand, i.e. JA-Ile (Chini et al. 2016).
Conclusion and Future Perspectives
To feed a constantly exploding population under challenging conditions like climate change, nutritional security and declining food production, there is an urgent requirement to explore the effective and remedial strategies in biotechnological, genetic, physiological and agronomical fields. Moreover, increases in incidences of biotic and abiotic stresses in field conditions further reduce soil fertility and crop production. To mitigate a particular type of stress in plants, research on phytohormones could be ascertained as a constructive approach in developing stress-tolerant plant spp. The characteristics outlined in this review significantly describe the importance of jasmonates as plant growth regulators playing critical roles throughout plant growth—from seed germination to senescence. Interestingly, the various enzymes engaged in JA biosynthesis exhibit self-activation mechanisms as a final product, i.e. JA positively regulates the biosynthetic pathways. Furthermore, JA and its derivatives upregulate the plants’ capability to deal with biotic and abiotic stresses. In general, JAs are helpful to increase stress tolerance as well defence resistance indirectly by upregulation of synthesis and accumulation of secondary metabolites like glucosinolates, phenolics and terpenoids through transcription factors such as Myb, Myc and ORCA families (Samota et al. 2017).
No doubt, the exogenous application of JA and its derivatives offers remedial influences in plants against pathogens and insects. Still, the absence of sufficient information about variation in plant physiological processes in different species must be explored. Since JAs are volatile and can be easily transported to other plant parts, therefore to improve the crop production, future research on their air transmission and disease resistance should be of great economic importance. As previously mentioned, the synthesis of these signalling molecules is prompted by activating receptors associated with the cell membrane by appropriate binding of either peptide signals, pathogens or wounding. Therefore, specific well-known molecular biology techniques like gene editing must be tried to change the regulation and expression of genes linked with JA biosynthesis to construct stress-resistant cultivars. Furthermore, innovations in nano-biotechnology will assist the advancement of nanocarriers for fast and effective delivery of exogenic JAs to directed plant cells’.
References
Abouelsaad I, Renault S (2018) Enhanced oxidative stress in the jasmonic acid-deficient tomato mutant def-1 exposed to NaCl stress. J Plant Physiol 226:136–144. https://doi.org/10.1016/J.JPLPH.2018.04.009
Acharya BR, Assmann SM (2009) Hormone interactions in stomatal function. Plant Mol Biol 69:451–462. https://doi.org/10.1007/S11103-008-9427-0
Acosta IF, Laparra H, Romero SP et al (2009) tasselseed1 is a lipoxygenase affecting jasmonic acid signaling in sex determination of maize. Science 323:262–265. https://doi.org/10.1126/SCIENCE.1164645
Aldridge DC, Galt S, Giles D, Turner WB (1971) Metabolites of Lasiodiplodia theobromae. J Chem Soci c: Org. https://doi.org/10.1039/J39710001623
Ali MS, Baek KH (2020) Jasmonic acid signaling pathway in response to abiotic stresses in plants. Int J Mol Sci 21:621. https://doi.org/10.3390/IJMS21020621
Ali M, Abbasi BH, Ali GS (2015) Elicitation of antioxidant secondary metabolites with jasmonates and gibberellic acid in cell suspension cultures of Artemisia absinthium L. Plant Cell Tissue Organ Cult 120:1099–1106. https://doi.org/10.1007/S11240-014-0666-2
Almeida J, Rocheta M, Galego L (1997) Genetic control of flower shape in Antirrhinum majus. Development 124:1387–1392. https://doi.org/10.1242/DEV.124.7.1387
Ameye M, Audenaert K, de Zutter N et al (2015) Priming of wheat with the green leaf volatile Z-3-hexenyl acetate enhances defense against Fusarium Graminearum but boosts deoxynivalenol production. Plant Physiol 167:1671–1684. https://doi.org/10.1104/PP.15.00107
Anderson JP, Badruzsaufari E, Schenk PM et al (2004) Antagonistic interaction between abscisic acid and jasmonate-ethylene signaling pathways modulates defense gene expression and disease resistance in Arabidopsis. Plant Cell 16:3460–3479. https://doi.org/10.1105/TPC.104.025833
Antognoni F, Poli F, Biondi S et al (2009) Methyl jasmonate differentially affects tocopherol content and tyrosine amino transferase activity in cultured cells of Amaranthus caudatus and Chenopodium quinoa. Wiley Online Library 11:161–169. https://doi.org/10.1111/j.1438-8677.2008.00110.x
Asif R, Yasmin R, Mustafa M et al (2022) Phytohormones as plant growth regulators and safe protectors against biotic and abiotic stress. Plant Horm Recent Adv New Perspect Appl. https://doi.org/10.5772/INTECHOPEN.102832
Azeem U (2018) Ameliorating nickel stress by jasmonic acid treatment in Zea mays L. Russian Agri Sci 44(3):209–215. https://doi.org/10.3103/S1068367418030035
Bäckström S, Elfving N, Nilsson R et al (2007) Purification of a Plant Mediator from Arabidopsis thaliana Identifies PFT1 as the Med25 Subunit. Mol Cell 26:717–729. https://doi.org/10.1016/J.MOLCEL.2007.05.007
Bali S, Kaur P, Jamwal VL et al (2020) Seed priming with jasmonic acid counteracts root knot nematode infection in tomato by modulating the activity and expression of antioxidative enzymes. Biomolecules 10(98):10–98. https://doi.org/10.3390/BIOM10010098
Balint-Kurti P (2019) The plant hypersensitive response: concepts, control and consequences. Mol Plant Pathol 20:1163. https://doi.org/10.1111/MPP.12821
Bandurska H, Stroiński A, Kubiś J (2003) The effect of jasmonic acid on the accumulation of ABA, proline and spermidine and its influence on membrane injury under water deficit in two barley genotypes. Acta Physiol Plant 25:279–285. https://doi.org/10.1007/S11738-003-0009-0
Brenya E, Chen ZH, Tissue D et al (2020) Prior exposure of Arabidopsis seedlings to mechanical stress heightens jasmonic acid-mediated defense against necrotrophic pathogens. BMC Plant Biol. https://doi.org/10.1186/S12870-020-02759-9
Brodersen P, Petersen M, Nielsen HB et al (2006) Arabidopsis MAP kinase 4 regulates salicylic acid- and jasmonic acid/ethylene-dependent responses via EDS1 and PAD4. Plant J 47:532–546. https://doi.org/10.1111/J.1365-313X.2006.02806.X
Browse J (2009) The power of mutants for investigating jasmonate biosynthesis and signaling. Phytochemistry 70:1539–1546. https://doi.org/10.1016/J.PHYTOCHEM.2009.08.004
Bussotti F, Pollastrini M (2021) Revisiting the concept of stress in forest trees at the time of global change and issues for stress monitoring. Plant Stress. https://doi.org/10.1016/J.STRESS.2021.100013
Canonne J, Froidure-Nicolas S, Rivas S (2011) Phospholipases in action during plant defense signaling. Plant Signal Behav 6:13. https://doi.org/10.4161/PSB.6.1.14037
Chehab EW, Yao C, Henderson Z et al (2012) Arabidopsis touch-induced morphogenesis is jasmonate mediated and protects against pests. Curr Biol 22:701–706. https://doi.org/10.1016/J.CUB.2012.02.061
Chen R, Jiang H, Li L et al (2012) The Arabidopsis Mediator subunit MED25 differentially regulates jasmonate and abscisic acid signaling through interacting with the MYC2 and ABI5 transcription factors. Plant Cell 24:2898–2916. https://doi.org/10.1105/TPC.112.098277
Chen L, Zhang L, Xiang S et al (2021) The transcription factor WRKY75 positively regulates jasmonate-mediated plant defense to necrotrophic fungal pathogens. J Exp Bot 72:1473–1489. https://doi.org/10.1093/JXB/ERAA529
Chini A, Gimenez-Ibanez S, Goossens A, Solano R (2016) Redundancy and specificity in jasmonate signalling. Curr Opin Plant Biol 33:147–156. https://doi.org/10.1016/J.PBI.2016.07.005
Choi HW, Klessig DF (2016) DAMPs, MAMPs, and NAMPs in plant innate immunity. BMC Plant Biol 16:232. https://doi.org/10.1186/S12870-016-0921-2
Cotado A, Müller M, Morales M, Munné-Bosch S (2018) Linking jasmonates with pigment accumulation and photoprotection in a high-mountain endemic plant, Saxifraga longifolia. Environ Exp Bot 154:56–65. https://doi.org/10.1016/J.ENVEXPBOT.2017.12.018
Couto D, Zipfel C (2016) Regulation of pattern recognition receptor signalling in plants. Nat Rev Immunol 16:537–552. https://doi.org/10.1038/NRI.2016.77
Cramer GR, Urano K, Delrot S et al (2011) Effects of abiotic stress on plants: a systems biology perspective. BMC Plant Biol 11:163. https://doi.org/10.1186/1471-2229-11-163
Creelman RA, Mullet JE (1997) Oligosaccharins, brassinolides, and jasmonates: nontraditional regulators of plant growth, development, and gene expression. Plant Cell 9:1211–1223. https://doi.org/10.1105/TPC.9.7.1211
Cui H, Tsuda K, Parker JE (2015) Effector-triggered immunity: from pathogen perception to robust defense. Annu Rev Plant Biol 66:487–511. https://doi.org/10.1146/ANNUREV-ARPLANT-050213-040012
Danisman S, van der Wal F, Dhondt S et al (2012) Arabidopsis Class I and Class II TCP transcription factors regulate jasmonic acid metabolism and leaf development antagonistically. Plant Physiol 159:1511–1523. https://doi.org/10.1104/PP.112.200303
Dave A, Hernández ML, He Z et al (2011) 12-Oxo-Phytodienoic acid accumulation during seed development represses seed germination in Arabidopsis. Plant Cell 23:583–599. https://doi.org/10.1105/TPC.110.081489
De Domenico S, Taurino M, Gallo A et al (2019) Oxylipin dynamics in Medicago truncatula in response to salt and wounding stresses. Physiol Plant 165:198–208. https://doi.org/10.1111/PPL.12810
Demole E, Lederer E, Mercier D (1962) Isolation and determination of the structure of methyl jasmonate, the characteristic fragrant constituent of jasmine essence. Helv Chim Acta 45:675–685
Desmond OJ, Edgar CI, Manners JM et al (2006) Methyl jasmonate induced gene expression in wheat delays symptom development by the crown rot pathogen Fusarium pseudograminearum. Physiol Mol Plant Pathol 67:171–179. https://doi.org/10.1016/J.PMPP.2005.12.007
Devoto A, Ellis C, Magusin A et al (2005) Expression profiling reveals COI1 to be a key regulator of genes involved in wound- and methyl jasmonate-induced secondary metabolism, defence, and hormone interactions. Plant Mol Biol 58:497–513. https://doi.org/10.1007/S11103-005-7306-5
Egamberdieva D, Wirth SJ, Alqarawi AA et al (2017) Phytohormones and beneficial microbes: essential components for plants to balance stress and fitness. Front Microbiol. https://doi.org/10.3389/FMICB.2017.02104
Enebe MC, Babalola OO (2018) The impact of microbes in the orchestration of plants’ resistance to biotic stress: a disease management approach. Appl Microbiol Biotechnol 103:9–25. https://doi.org/10.1007/S00253-018-9433-3
Finch-Savage WE, Blake PS, Clay HA (1996) Desiccation stress in recalcitrant Quercus robur L. seeds results in lipid peroxidation and increased synthesis of jasmonates and abscisic acid. J Exp Bot 47:661–667
Flors V, Ton J, van Doorn R et al (2008) Interplay between JA, SA and ABA signalling during basal and induced resistance against Pseudomonas syringae and Alternaria brassicicola. Plant J 54:81–92. https://doi.org/10.1111/J.1365-313X.2007.03397.X
Fu ZQ, Dong X (2013) Systemic acquired resistance: turning local infection into global defense. Annu Rev Plant Biol 64:839–863. https://doi.org/10.1146/ANNUREV-ARPLANT-042811-105606
Fujita M, Fujita Y, Noutoshi Y et al (2006) Crosstalk between abiotic and biotic stress responses: a current view from the points of convergence in the stress signaling networks. Curr Opin Plant Biol 9:436–442. https://doi.org/10.1016/J.PBI.2006.05.014
Glazebrook J (2005) Contrasting mechanisms of defense against biotrophic and necrotrophic pathogens. Annu Rev Phytopathol 43:205–227. https://doi.org/10.1146/ANNUREV.PHYTO.43.040204.135923
Gutjahr C, Riemann M, Müller A et al (2005) Cholodny-Went revisited: a role for jasmonate in gravitropism of rice coleoptiles. Planta 222:575–585. https://doi.org/10.1007/S00425-005-0001-6
Hamant O, Haswell ES (2017) Life behind the wall: sensing mechanical cues in plants. BMC Biol 15:1–9. https://doi.org/10.1186/S12915-017-0403-5
Han Y, Bai Y, Xiao Y et al (2011) Simultaneous discrimination of jasmonic acid stereoisomers by CE-QTOF-MS employing the partial filling technique. Electrophoresis 32:2693–2699. https://doi.org/10.1002/ELPS.201100043
Hartmann M, Zeier T, Bernsdorff F et al (2018) Flavin monooxygenase-generated N-Hydroxypipecolic acid is a critical element of plant systemic immunity. Cell 173:456-469.e16. https://doi.org/10.1016/J.CELL.2018.02.049
Hayashi H, Huang P, Inoue K (2003) Up-regulation of soyasaponin biosynthesis by methyl jasmonate in cultured cells of Glycyrrhiza glabra. Plant Cell Physiol 44:404–411. https://doi.org/10.1093/PCP/PCG054
He Y, Fukushige H, Hildebrand DF, Gan S (2002) Evidence supporting a role of jasmonic acid in Arabidopsis leaf senescence. Plant Physiol 128:876–884. https://doi.org/10.1104/PP.010843
Holland CK, Jez JM (2018) Structural biology of jasmonic acid metabolism and responses in plants. Plant Struct Biol Horm Regul. https://doi.org/10.1007/978-3-319-91352-0_5
Horbowicz M, Kosson R, Wiczkowski W et al (2011) The effect of methyl jasmonate on accumulation of 2-phenylethylamine and putrescine in seedlings of common buckwheat (Fagopyrum esculentum). Acta Physiol Plant 33:897–903. https://doi.org/10.1007/s11738-010-0616-5
Hu H, He X, Tu L et al (2016) GhJAZ2 negatively regulates cotton fiber initiation by interacting with the R2R3-MYB transcription factor GhMYB25-like. Plant J 88:921–935. https://doi.org/10.1111/TPJ.13273
Hu Y, Jiang Y, Han X et al (2017) Jasmonate regulates leaf senescence and tolerance to cold stress: crosstalk with other phytohormones. J Exp Bot 68:1361–1369. https://doi.org/10.1093/JXB/ERX004
Hu J, Huang J, Xu H et al (2020) Rice stripe virus suppresses jasmonic acid-mediated resistance by hijacking brassinosteroid signaling pathway in rice. PLoS Pathog. https://doi.org/10.1371/JOURNAL.PPAT.1008801
Iqbal Z, Iqbal MS, Hashem A et al (2021) Plant defense responses to biotic stress and its interplay with fluctuating dark/light conditions. Front Plant Sci 12:297. https://doi.org/10.3389/FPLS.2021.631810/BIBTEX
Jaiti F, Verdeil JL, el Hadrami I (2009) Effect of jasmonic acid on the induction of polyphenoloxidase and peroxidase activities in relation to date palm resistance against Fusarium oxysporum f. sp. albedinis. Physiol Mol Plant Pathol 74:84–90. https://doi.org/10.1016/J.PMPP.2009.09.005
Jia M (2020) Jasmonic acid transport in wound-induced systemic immunity. Mol Plant 13:1673. https://doi.org/10.1016/J.MOLP.2020.10.005
Jin P, Zheng Y, Tang S et al (2009) Enhancing disease resistance in peach fruit with methyl jasmonate. J Sci Food Agric 89:802–808. https://doi.org/10.1002/JSFA.3516
Kamińska M (2021) Role and activity of jasmonates in plants under in vitro conditions. Plant Cell Tissue Organ Cult 146:425–447. https://doi.org/10.1007/S11240-021-02091-6
Khan NA, Nazar R, Iqbal N, Anjum NA (2012) Phytohormones and abiotic stress tolerance in plants. Springer, Berlin
Kim J, Chang C, Tucker ML (2015) To grow old: regulatory role of ethylene and jasmonic acid in senescence. Front Plant Sci 6:1–7. https://doi.org/10.3389/FPLS.2015.00020
Kim H, Seomun S, Yoon Y, Jang G (2021) Jasmonic acid in plant abiotic stress tolerance and interaction with abscisic acid. Agronomy. https://doi.org/10.3390/AGRONOMY11091886
Klumpp S, Krieglstein J (2002) Phosphorylation and dephosphorylation of histidine residues in proteins. Eur J Biochem 269:1067–1071. https://doi.org/10.1046/J.1432-1033.2002.02755.X
Kosová K, Vítámvás P, Urban MO et al (2015) Biological networks underlying abiotic stress tolerance in temperate crops—a proteomic perspective. Int J Mol Sci 16:20913–20942. https://doi.org/10.3390/IJMS160920913
Krumm T, Bandemer K, Boland W (1995) Induction of volatile biosynthesis in the Lima bean (Phaseolus lunatus) by leucine- and isoleucine conjugates of 1-oxo- and 1-hydroxyindan-4-carboxylic acid: evidence for amino acid conjugates of jasmonic acid as intermediates in the octadecanoid signalling pathway. FEBS Lett 377:523–529. https://doi.org/10.1016/0014-5793(95)01398-9
Ku YS, Sintaha M, Cheung MY, Lam HM (2018) Plant hormone signaling crosstalks between biotic and abiotic stress responses. Int J Mol Sci 19:3206. https://doi.org/10.3390/IJMS19103206
Lalotra S, Hemantaranjan A, Yashu BR et al (2020) Jasmonates: an emerging approach in biotic and abiotic stress tolerance. Plant Sci Struct Anatomy Physio Plants Cult Vivo Vitro. https://doi.org/10.5772/INTECHOPEN.84608
Lamalakshmi Devi E, Kumar S, Basanta Singh T et al (2017) Adaptation strategies and defence mechanisms of plants during environmental stress. Med Plants Environ Chall. https://doi.org/10.1007/978-3-319-68717-9_20
Li L, Zhao Y, McCaig BC et al (2004) The tomato homolog of CORONATINE-INSENSITIVE1 is required for the maternal control of seed maturation, jasmonate-signaled defense responses, and glandular trichome development. Plant Cell 16:126–143. https://doi.org/10.1105/TPC.017954
Li Q, Zheng J, Li S et al (2017) Transporter-mediated nuclear entry of jasmonoyl-isoleucine is essential for jasmonate signaling. Mol Plant 10:695–708. https://doi.org/10.1016/J.MOLP.2017.01.010
Li J, Zhang K, Meng Y et al (2018a) Jasmonic acid/ethylene signaling coordinates hydroxycinnamic acid amides biosynthesis through ORA59 transcription factor. Plant J 95:444–457. https://doi.org/10.1111/TPJ.13960
Li S, Yang Y, Zhang Q et al (2018b) Differential physiological and metabolic response to low temperature in two zoysiagrass genotypes native to high and low latitude. PLoS ONE. https://doi.org/10.1371/JOURNAL.PONE.0198885
Li N, Han X, Feng D et al (2019) Signaling crosstalk between salicylic acid and ethylene/jasmonate in plant defense: do we understand what they are whispering? Int J Mol Sci 20(3):671. https://doi.org/10.3390/IJMS20030671
Li M, Wang F, Li S et al (2020) Importers drive leaf-to-leaf jasmonic acid transmission in wound-induced systemic immunity. Mol Plant 13:1485–1498. https://doi.org/10.1016/J.MOLP.2020.08.017
Liu W, Park SW (2021) 12-oxo-Phytodienoic acid: a fuse and/or switch of plant growth and defense responses? Front Plant Sci 12:1687. https://doi.org/10.3389/FPLS.2021.724079/BIBTEX
Liu C, Ruan Y, Lin Z et al (2008) Antagonism between acibenzolar-S-methyl-induced systemic acquired resistance and jasmonic acid-induced systemic acquired susceptibility to Colletotrichum orbiculare infection in cucumber. Physiol Mol Plant Pathol 72:141–145. https://doi.org/10.1016/J.PMPP.2008.08.001
Lu Z-X, Gaudet D, Puchalski B et al (2006) Inducers of resistance reduce common bunt infection in wheat seedlings while differentially regulating defence-gene expression. Physiol Mol Plant Pathol 67:138–148. https://doi.org/10.1016/j.pmpp.2005.12.001
Luo D, Carpenter R, Copsey L et al (1999) Control of Organ Asymmetry in Flowers of Antirrhinum. Cell 99:367–376. https://doi.org/10.1016/S0092-8674(00)81523-8
Manavella PA, Dezar CA, Bonaventure G et al (2008) HAHB4, a sunflower HD-Zip protein, integrates signals from the jasmonic acid and ethylene pathways during wounding and biotic stress responses. Plant J 56:376–388. https://doi.org/10.1111/J.1365-313X.2008.03604.X
Mao P, Duan M, Wei C, Li Y (2007) WRKY62 transcription factor acts downstream of cytosolic NPR1 and negatively regulates jasmonate-responsive gene expression. Plant Cell Physiol 48:833–842. https://doi.org/10.1093/PCP/PCM058
Marone D, Russo MA, Laidò G et al (2013) Plant nucleotide binding site-leucine-rich repeat (NBS-LRR) genes: active guardians in host defense responses. Int J Mol Sci 14:7302–7326. https://doi.org/10.3390/IJMS14047302
Martin D, Tholl D, Gershenzon J, Bohlmann J (2002) Methyl jasmonate induces traumatic resin ducts, terpenoid resin biosynthesis, and terpenoid accumulation in developing xylem of Norway spruce stems. Plant Physiol 129:1003–1018. https://doi.org/10.1104/PP.011001
Maserti B, del Carratore R, Maria C et al (2011) Comparative analysis of proteome changes induced by the two spotted spider mite Tetranychus urticae and methyl jasmonate in citrus leaves. J Plant Physiol 168:392–402. https://doi.org/10.1016/j.jplph.2010.07.026
Mei C, Qi M, Sheng G, Yang Y (2006) Inducible overexpression of a rice allene oxide synthase gene increases the endogenous jasmonic acid level, PR gene expression, and host resistance to fungal infection. Mol Plant Microbe Interact 19:1127–1137. https://doi.org/10.1094/MPMI-19-1127
Miyamoto K, Shimizu T, Okada K (2014) Transcriptional regulation of the biosynthesis of phytoalexin: a lesson from specialized metabolites in rice. Plant Biotechnology 31:377–388. https://doi.org/10.5511/PLANTBIOTECHNOLOGY.14.0730A
Mohamed HI, Latif HH (2017) Improvement of drought tolerance of soybean plants by using methyl jasmonate. Physiol Mol Biol Plants 23:545–556. https://doi.org/10.1007/S12298-017-0451-X/TABLES/7
Moosa A, Sahi ST, Khan SA, Malik AU (2019) Salicylic acid and jasmonic acid can suppress green and blue moulds of citrus fruit and induce the activity of polyphenol oxidase and peroxidase. Folia Hortic 31:195–204. https://doi.org/10.2478/FHORT-2019-0014
Mosblech A, Thurow C, Gatz C et al (2011) Jasmonic acid perception by COI1 involves inositol polyphosphates in Arabidopsis thaliana. Plant J 65:949–957. https://doi.org/10.1111/j.1365-313X.2011.04480.x
Motallebi P, Niknam V, Ebrahimzadeh H et al (2017) Exogenous methyl jasmonate treatment induces defense response against Fusarium culmorum in wheat seedlings. J Plant Growth Regul 36:71–82. https://doi.org/10.1007/S00344-016-9620-3
Mousavi SAR, Chauvin A, Pascaud F et al (2013) GLUTAMATE RECEPTOR-LIKE genes mediate leaf-to-leaf wound signalling. Nature 500:422–426. https://doi.org/10.1038/nature12478
Mukherjee A, Gaurav AK, Singh S et al (2022) The bioactive potential of phytohormones: a review. Biotechnol Rep. https://doi.org/10.1016/J.BTRE.2022.E00748
Nakata M, Mitsuda N, Herde M et al (2013) A bHLH-type transcription factor, ABA-inducible BHLH-type transcription factor/ja-associated MYC2-LIKE1, acts as a repressor to negatively regulate jasmonate signaling in Arabidopsis. Plant Cell 25:1641–1656. https://doi.org/10.1105/TPC.113.111112
Noir S, Bömer M, Takahashi N et al (2013) Jasmonate controls leaf growth by repressing cell proliferation and the onset of endoreduplication while maintaining a potential stand-by mode. Plant Physiol 161:1930–1951. https://doi.org/10.1104/PP.113.214908
Noman A, Aqeel M, Lou Y (2019) PRRs and NB-LRRs: from signal perception to activation of plant innate immunity. Int J Mol Sci 20(8):1882. https://doi.org/10.3390/IJMS20081882
Pauwels L, Barbero GF, Geerinck J et al (2010) NINJA connects the co-repressor TOPLESS to jasmonate signalling. Nature 464:788–791. https://doi.org/10.1038/NATURE08854
Pieterse CMJ, van der Does D, Zamioudis C et al (2012) Hormonal modulation of plant immunity. Annu Rev Cell Dev Biol 28:489–521. https://doi.org/10.1146/ANNUREV-CELLBIO-092910-154055
Qi T, Wang J, Huang H et al (2015) Regulation of jasmonate-induced leaf senescence by antagonism between bHLH subgroup IIIe and IIId factors in Arabidopsis. Plant Cell 27:1634–1649. https://doi.org/10.1105/TPC.15.00110
Rakwal R, Komatsu S (2000) Role of jasmonate in the rice (Oryza sativa L.) self-defense mechanism using proteome analysis. Electrophoresis 21:2492–2500
Ravnikar M, Žel J, Plaper I, Špacapan A (1993) Jasmonic acid stimulates shoot and bulb formation of garlic in vitro. J Plant Growth Regul 12(2):73–77. https://doi.org/10.1007/BF00193236
Reeves PH, Ellis CM, Ploense SE et al (2012) A regulatory network for coordinated flower maturation. PLoS Genet 8(2):e1002506. https://doi.org/10.1371/JOURNAL.PGEN.1002506
Riemann M, Müller A, Korte A et al (2003) Impaired induction of the jasmonate pathway in the rice mutant hebiba. Plant Physiol 133:1820–1830. https://doi.org/10.1104/PP.103.027490
Riemann M, Haga K, Shimizu T et al (2013) Identification of rice allene oxide cyclase mutants and the function of jasmonate for defence against Magnaporthe oryzae. Plant J 74:226–238. https://doi.org/10.1111/TPJ.12115
Robson F, Okamoto H, Patrick E et al (2012) Jasmonate and phytochrome a signaling in arabidopsis wound and shade responses are integrated through JAZ1 stability. Plant Cell 22:1143–1160. https://doi.org/10.1105/TPC.109.067728
Routaboul JM, Skidmore C, Wallis JG, Browse J (2012) Arabidopsis mutants reveal that short- and long-term thermotolerance have different requirements for trienoic fatty acids. J Exp Bot 63:1435. https://doi.org/10.1093/JXB/ERR381
Ruan J, Zhou Y, Zhou M et al (2019) Jasmonic acid signaling pathway in plants. Int J Mol Sci 20(10):2479. https://doi.org/10.3390/IJMS20102479
Rustgi S, Springer A, Kang C et al (2019) Allene oxide synthase and hydroperoxide lyase, two non-canonical cytochrome P450s in Arabidopsis thaliana and their different roles in plant defense. Int J Mol Sci. https://doi.org/10.3390/IJMS20123064
Saijo Y, Loo EP, Yasuda S (2018) Pattern recognition receptors and signaling in plant–microbe interactions. Plant J 93(4):592–613. https://doi.org/10.1111/TPJ.13808
Samota MK, Bhatt L, Garg N, Geat N (2017) Defense induced by jasmonic acid: a review. Int J Curr Microbiol Appl Sci 6:2467–2474. https://doi.org/10.20546/IJCMAS.2017.605.276
Saniewski M, Czapski J (1983) The effect of methyl jasmonate on lycopene and β-carotene accumulation in ripening red tomatoes. Experientia 39:1373–1374. https://doi.org/10.1007/BF01990110
Savchenko T, Kolla VA, Wang CQ et al (2014) Functional convergence of oxylipin and abscisic acid pathways controls stomatal closure in response to drought. Plant Physiol 164:1151–1160. https://doi.org/10.1104/PP.113.234310
Scalschi L, Sanmartín M, Camañes G et al (2015) Silencing of OPR3 in tomato reveals the role of OPDA in callose deposition during the activation of defense responses against Botrytis cinerea. Plant J 81:304–315. https://doi.org/10.1111/TPJ.12728
Schellenberger R, Touchard M, Clément C et al (2019) Apoplastic invasion patterns triggering plant immunity: plasma membrane sensing at the frontline. Mol Plant Pathol 20:1602. https://doi.org/10.1111/MPP.12857
Schmelz EA, Huffaker A, Sims JW et al (2014) Biosynthesis, elicitation and roles of monocot terpenoid phytoalexins. Plant J 79:659–678. https://doi.org/10.1111/TPJ.12436
Schommer C, Palatnik JF, Aggarwal P et al (2008) Control of jasmonate biosynthesis and senescence by miR319 Targets. PLoS Biol. https://doi.org/10.1371/JOURNAL.PBIO.0060230
Schuck S, Kallenbach M, Baldwin IT, Bonaventure G (2014) The Nicotiana attenuata GLA1 lipase controls the accumulation of Phytophthora parasitica-induced oxylipins and defensive secondary metabolites. Plant Cell Environ 37:1703. https://doi.org/10.1111/PCE.12281
Seltmann MA, Stingl NE, Lautenschlaeger JK et al (2010) Differential impact of Lipoxygenase 2 and jasmonates on natural and stress-induced senescence in Arabidopsis. Plant Physiol 152:1940–1950. https://doi.org/10.1104/PP.110.153114
Shimizu T, Lin F, Hasegawa M et al (2012) Purification and identification of Naringenin 7-O-methyltransferase, a key enzyme in biosynthesis of flavonoid phytoalexin sakuranetin in rice. J Biol Chem 287:19315. https://doi.org/10.1074/JBC.M112.351270
Sirhindi G, Mir MA, Sharma P et al (2015) Modulatory role of jasmonic acid on photosynthetic pigments, antioxidants and stress markers of Glycine max L. under nickel stress. Physiol Mol Biol Plants 21:559–565. https://doi.org/10.1007/S12298-015-0320-4/FIGURES/2
Song S, Qi T, Huang H, Xie D (2013) Regulation of stamen development by coordinated actions of jasmonate, auxin, and gibberellin in Arabidopsis. Mol Plant 6:1065–1073. https://doi.org/10.1093/MP/SST054
Srivastava LM (2002) Jasmonates and other defense-related compounds. Plant Growth Dev. https://doi.org/10.1016/B978-012660570-9/50153-2
Staswick PE (1994) Storage proteins of vegetative plant tissues. Annu Rev Plant Physiol Plant Mol Biol 45:303–322. https://doi.org/10.1146/ANNUREV.PP.45.060194.001511
Staswick PE, Tiryaki I (2004) the oxylipin signal jasmonic acid is activated by an enzyme that conjugates it to isoleucine in Arabidopsis. Plant Cell 16:2117–2127. https://doi.org/10.1105/TPC.104.023549
Staswick PE, Yuen GY, Lehman CC (1998) Jasmonate signaling mutants of Arabidopsis are susceptible to the soil fungus Pythium irregulare. Plant J 15:747–754. https://doi.org/10.1046/J.1365-313X.1998.00265.X
Stergiopoulos I, Collemare J, Mehrabi R, de Wit PJGM (2013) Phytotoxic secondary metabolites and peptides produced by plant pathogenic Dothideomycete fungi. FEMS Microbiol Rev 37:67–93. https://doi.org/10.1111/J.1574-6976.2012.00349.X
Ton J, Flors V, Mauch-Mani B (2009) The multifaceted role of ABA in disease resistance. Trends Plant Sci 14:310–317. https://doi.org/10.1016/J.TPLANTS.2009.03.006
Toyota M, Spencer D, Sawai-Toyota S et al (2018) Glutamate triggers long-distance, calcium-based plant defense signaling. Science 361:1112–1115. https://doi.org/10.1126/SCIENCE.AAT7744
Ueda J, Kato J (1980) Isolation and identification of a senescence-promoting substance from wormwood (Artemisia absinthium L.). Plant Physiol. https://doi.org/10.1104/PP.66.2.246
Umar OB, Ranti LA, Abdulbaki AS, et al (2021) Stresses in Plants: Biotic and Abiotic. In: Current Trends in Wheat Research. IntechOpen
van Loon LC, Rep M, Pieterse CMJ (2006) Significance of inducible defense-related proteins in infected plants. Annu Rev Phytopathol 44:135–162. https://doi.org/10.1146/ANNUREV.PHYTO.44.070505.143425
Walia H, Wilson C, Condamine P et al (2007) Large-scale expression profiling and physiological characterization of jasmonic acid-mediated adaptation of barley to salinity stress. Plant Cell Environ 30:410–421. https://doi.org/10.1111/J.1365-3040.2006.01628.X
Wang Z, Tan X, Zhang Z et al (2012) Defense to Sclerotinia sclerotiorum in oilseed rape is associated with the sequential activations of salicylic acid signaling and jasmonic acid signaling. Plant Sci 184:75–82. https://doi.org/10.1016/J.PLANTSCI.2011.12.013
Wang J, Qian J, Yao L, Lu Y (2015a) Enhanced production of flavonoids by methyl jasmonate elicitation in cell suspension culture of Hypericum perforatum. Bioresour Bioprocess 2:5. https://doi.org/10.1186/S40643-014-0033-5
Wang L, Jin P, Wang J et al (2015b) Methyl jasmonate primed defense responses against Penicillium expansum in sweet cherry fruit. Plant Mol Biol Report 33:1464–1471. https://doi.org/10.1007/S11105-014-0844-8/FIGURES/5
Wasternack C (2007) Jasmonates: an update on biosynthesis, signal transduction and action in plant stress response, growth and development. Ann Bot 100:681–697. https://doi.org/10.1093/AOB/MCM079
Wasternack C (2014) Action of jasmonates in plant stress responses and development — applied aspects. Biotechnol Adv 32:31–39. https://doi.org/10.1016/J.BIOTECHADV.2013.09.009
Wasternack C, Hause B (2013) jasmonates: biosynthesis, perception, signal transduction and action in plant stress response, growth and development. an update to the 2007 review in annals of botany. Ann Bot 111:1021–1058. https://doi.org/10.1093/AOB/MCT067
Wasternack C, Kombrink E (2010) Jasmonates: Structural requirements for lipid-derived signals active in plant stress responses and development. ACS Chem Biol 5:63–77. https://doi.org/10.1021/CB900269U
Wasternack C, Song S (2017) Jasmonates: Biosynthesis, metabolism, and signaling by proteins activating and repressing transcription. J Exp Bot 68:1303–1321. https://doi.org/10.1093/JXB/ERW443
Wasternack C, Strnad M (2016) Jasmonate signaling in plant stress responses and development - active and inactive compounds. N Biotechnol 33:604–613. https://doi.org/10.1016/J.NBT.2015.11.001
Wasternack C, Strnad M (2018) Jasmonates: news on occurrence biosynthesis metabolism and action of an ancient group of signaling compounds. Int J Mol Sci. https://doi.org/10.3390/IJMS19092539
Worrall D, Holroyd GH, Moore JP et al (2012) Treating seeds with activators of plant defence generates long-lasting priming of resistance to pests and pathogens. New Phytol 193:770–778. https://doi.org/10.1111/J.1469-8137.2011.03987.X
Xie DX, Feys BF, James S et al (1998) COI1: an Arabidopsis gene required for jasmonate-regulated defense and fertility. Science 280:1091–1094. https://doi.org/10.1126/SCIENCE.280.5366.1091
Yan Y, Christensen S, Isakeit T et al (2012) Disruption of OPR7 and OPR8 reveals the versatile functions of jasmonic acid in maize development and defense. Plant Cell 24:1420–1436. https://doi.org/10.1105/TPC.111.094151/DC1
Yan Y, Borrego E, V. M, (2013) Jasmonate biosynthesis perception and function in plant development and stress responses. Lipid Metabo. https://doi.org/10.5772/52675
Yan J, Li S, Gu M et al (2016) Endogenous bioactive jasmonate is composed of a Set of (+)-7-iso-JA-amino acid conjugates. Plant Physiol 172:2154. https://doi.org/10.1104/PP.16.00906
Yang Q, Gao X, Fujino Y et al (2004) Effects of theobroxide, a natural product, on the level of endogenous jasmonoids. Zeitschrift Fur Naturforschung Sect C J Biosci 59:828–834. https://doi.org/10.1515/ZNC-2004-11-1211
Yang D, Ma P, Liang X et al (2012) PEG and ABA trigger methyl jasmonate accumulation to induce the MEP pathway and increase tanshinone production in Salvia miltiorrhiza hairy roots. Physiol Plant 146:173–183. https://doi.org/10.1111/J.1399-3054.2012.01603.X
Yang J, Duan G, Li C et al (2019) The crosstalks between jasmonic acid and other plant hormone signaling highlight the involvement of jasmonic acid as a core component in plant response to biotic and abiotic stresses. Front Plant Sci 10:1349. https://doi.org/10.3389/FPLS.2019.01349/BIBTEX
Yang C, Dolatabadian A, Fernando WGD (2021) The wonderful world of intrinsic and intricate immunity responses in plants against pathogens. Can J Plant Path 44:1–20. https://doi.org/10.1080/07060661.2021.1960610
Ye M, Luo SM, Xie JF et al (2012) Silencing COI1 in rice increases susceptibility to chewing insects and impairs inducible defense. PLoS ONE. https://doi.org/10.1371/JOURNAL.PONE.0036214
Yildiz K, Muradoglu F, Yilmaz H (2008) The effect of jasmonic acid on germination of dormant and nondormant pear (Pyrus communis L.) seeds. Seed Sci Technol 36:569–574. https://doi.org/10.15258/SST.2008.36.3.06
Yu X, Feng B, He P, Shan L (2017) From chaos to harmony: responses and signaling upon microbial pattern recognition. Annu Rev Phytopathol 55:109–137. https://doi.org/10.1146/ANNUREV-PHYTO-080516-035649
Yun-xia G, Li-jun Z, Feng-hai L et al (2010) Relationship between jasmonic acid accumulation and senescence in drought-stress. Afr J Agric Res 5:1978–1983. https://doi.org/10.5897/AJAR.9000585
Zhai H, Lü S, Liang S et al (2014) GmFT4 a homolog of flowering locus T, is positively regulated by E1 and functions as a flowering repressor in soybean. PLoS ONE 9(2):e89030. https://doi.org/10.1371/JOURNAL.PONE.0089030
Zhai Q, Zhang X, Wu F et al (2015) Transcriptional mechanism of jasmonate receptor COI1-mediated delay of flowering time in Aarabidopsis. Plant Cell 27:2814–2828. https://doi.org/10.1105/TPC.15.00619
Zhang Y, Turner JG (2008) Wound-induced endogenous jasmonates stunt plant growth by inhibiting mitosis. PLoS ONE 3(11):e3699. https://doi.org/10.1371/JOURNAL.PONE.0003699
Zhang J, Zhou JM (2010) Plant immunity triggered by microbial molecular signatures. Mol Plant 3:783–793. https://doi.org/10.1093/MP/SSQ035
Zhao S, Li Y (2021) Current understanding of the interplays between host hormones and plant viral infections. PLoS Pathog 17(2):e1009242. https://doi.org/10.1371/JOURNAL.PPAT.1009242
Zhou M, Memelink J (2016) Jasmonate-responsive transcription factors regulating plant secondary metabolism. Biotechnol Adv 34:441–449. https://doi.org/10.1016/J.BIOTECHADV.2016.02.004
Zhu Z, Tian S (2012) Resistant responses of tomato fruit treated with exogenous methyl jasmonate to Botrytis cinerea infection. Sci Hortic 142:38–43. https://doi.org/10.1016/j.scienta.2012.05.002
Zhu X, Chen J, Xie Z et al (2015) Jasmonic acid promotes degreening via MYC2/3/4- and ANAC019/055/072-mediated regulation of major chlorophyll catabolic genes. Plant J 84:597–610. https://doi.org/10.1111/TPJ.13030
Funding
This research received no external funding.
Author information
Authors and Affiliations
Contributions
MS drafted and revised the manuscript.
Corresponding author
Ethics declarations
Conflicts of Interest
The author declares no conflict of interest.
Additional information
Handling Editor: Parvaiz Ahmad.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sood, M. Jasmonates: “The Master Switch” for Regulation of Developmental and Stress Responses in Plants. J Plant Growth Regul 42, 5247–5265 (2023). https://doi.org/10.1007/s00344-023-11047-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00344-023-11047-3