Abstract
Drought is believed to be one of the most important abiotic stresses, which drastically affects wheat production worldwide. Jasmonates, as plant growth regulators, could modify the effects of environmental stresses. Therefore, we conducted the present study to investigate the physiological responses of wheat cultivars to exogenous methyl jasmonate (MeJA) under drought stress during two crop years of 2015–2017. The results revealed that the irrigation cut-off regime at the booting decreased Fv/Fm, RWC, peroxidase activity, and grain yield; whereas it increased proline, malondialdehyde content, protein, and electrolyte leakage. The drought-tolerant cultivar, Sirvan had 13.7% higher grain yield than Pishtaz cultivar in drought stress owing to higher Fv/Fm, proline, RWC, peroxidase activity, and lower electrolyte leakage and MDA. Spraying 50 μM of MeJA increased chlorophyll and carotenoids (8.7% and 6.93%), proline (26.8%), and peroxidase activity (31.4%); whereas 100 μM of MeJA increased the RWC (2.5%), Fv/Fm (9.3%) and decreased membrane electrolyte leakage (6.7%) compared to hormone-free conditions. According to the results drought stress decreased certain physiological traits, the foliar application with 100 μM of MeJA could increase the grain yield (9.2% in irrigation cut-off at booting and 6.8% at the milk stage) and then partially compensate for the reduction caused by drought stress. Therefore, for minimizing the effect of drought stress, we could recommend the use of MeJA and drought-tolerant cultivar.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
World population growth is associated with the increase in demand for water. Global water resources are shrinking due to global warming, irregular and inadequate rainfall patterns, and irrational use of water resources. Among environmental stresses, drought stress, and water scarcity are the most critical ones that limit the production of crops in agricultural systems of arid and semi-arid regions. According to the average population growth rate, it has been estimated that the amount of required water in different areas of the world is approximately doubled every 35 years (Oraki and Aghaalikhana 2012).
Cereals play essential roles in food patterns all around the world. Wheat (Triticum aestivum L.) has a pivotal role in these patterns worldwide by providing more than 40% of calories and 50% of proteins that human body needs (Awika 2011). In warm and semi-arid regions, a large amount of rainfall is received in winter, and autumnal crops such as wheat, face drought and deficit irrigation from flowering to physiological maturity. The effect of drought stress depends on several factors, including genotypes, severity, duration of stress, climate conditions, and plant growth stages (Ahmadi et al. 2010).
Abiotic stresses reduce some of the plant growth regulators synthesis (Ashraf and Foolad 2007). Therefore, the use of growth regulators may compensate for internal deficiencies of growth regulators and reduce destructive effects of abiotic stresses (Ashraf and Foolad 2007). Exogenous applications of specific growth regulators can increase the tolerance of plants against various environmental stresses (Zhou et al. 2015). Moreover, the external use of methyl jasmonate can regulate the several plant physiological responses, which leads to the improvement of resistance against biological stresses. Methyl jasmonate also has different effects on the plant growth depending on the type of plant, studied tissue, severity, duration of drought stress, and the amount of jasmonic acid (JA). Methyl jasmonate or JA usually has a positive effect at very low concentrations and triggers stress at high levels (Karami et al. 2013).
In addition to morphological changes, water stress could reduce physiological traits, for instance, concentrations of chlorophylls and carotenoids of plant tissues. In an experiment for determination of the role of methyl jasmonate on physiological properties of soybean plants, Nafie et al. (2011) found that methyl jasmonate improved the growth of soybean plants at concentrations of 1 and 10 μM by increasing the antioxidant defense potential and reducing the oxidative stress. However, 100 and 500 μM of that increased the lipid peroxidation and decreased the soybean growth. In a study on barley genotypes, Pazirandeh et al. (2015) reported that the relative leaf humidity decreased under drought stress, yet the use of methyl jasmonate improved the reduction. Anjum et al. (2011) reported that the foliar spray of MeJA increased the total amount of sugars, phenolic compounds, total fatty acids, peroxidase activity, photosynthetic pigments, and relative water content (RWC) and improved drought tolerance and growth of soybean genotypes under drought stress.
Utilizing drought-tolerant cultivars are known as an effective method in exploiting and increasing yield in arid and semi-arid regions. Despite numerous studies on the identification of environmental stress-tolerant cultivars such as drought stress, physiological characteristics of cultivated varieties in different regions and under different weather conditions are not well recognized for proper crop decision making. Further research concerning physiological and biochemical traits, as well as the use of appropriate growth regulators could help identify ways to select tolerant cultivars and increase yield in different environmental conditions, such as drought stress. The present study aimed to study physiological traits and grain yield of two wheat cultivars as the most important crops in different irrigation regimes with the external use of methyl jasmonate.
Materials and Methods
Site of the Experiment
The experiment was carried out in two cultivation years of 2015–2016 and 2016–2017 at a research farm in Fars province, Iran, with a longitude of 52°41′E, a latitude of 29°58′N, and an altitude of 1595 m with an average annual rainfall of 365 mm, relative humidity of 41%, and maximum and minimum temperatures of 41 °C and 9 °C, respectively. Table 1 presents the meteorological data and the physicochemical properties of experimental site soil.
Experimental Design and Treatments
The seeds (Sirvan and Pishtaz cultivar) were obtained from Agricultural and Natural Resources Research Center of Fars province in Zarghan County, Iran. They were sowed on November 15, at both years, with a planting density of 400 plants m−2 in plots with 8 m2 in 8 rows. Sirvan cultivar had high grain yield and quality to be used in bakery, was suitable for warm to moderate climate, with an average yield of 4–8 tons per hectare; an average protein percentage of 12% and a hard grain quality with strong gluten are proposed for cultivation in the regions facing drought stress. On the other hand, Pishtaz wheat is a spring-growing type and characteristics such as fertility, lodging resistance, and moderate drought tolerance (Abdoli and Saeidi 2012). All the experimental plots were irrigated equally and simultaneously until the beginning of the booting. The plots for the first stress were not irrigated from the booting (code 45 of the Zadoks table) and for the second one, irrigation cut-off was carried out from the grain milking stage (code 71–72 of the Zadoks table) (Zadoks et al. 1974). At the time of stress application, the control treatment was irrigated normally. The foliar application of different concentrations of methyl jasmonate (0, 50, 100, and 150 μM) was completed at the end of April 2016 and 2017, prior to cutting irrigation, when the stipules of flag leaf appeared (code 39 of Zadoks table). For each experimental plot, 1.5 L of methyl jasmonate solution was sprayed evenly on the plants in the first hour of the morning using a hand-held sprayer with constant pressure. The booting stage was described once the head began to form inside the flag leaf and the head was fully developed and we could be easily see a swollen section of the leaf sheath below the flag leaf. The grain milking stage was seen when kernel (caryopsis) watery ripe in the milk development and when the grain was squeezed, a milky solution was apparent.
Traits Measurement
To measure the physiological traits, the samples were randomly taken from flag leaves of 10 plants in the central rows at the beginning of the dough development of grain (Zadoks code: 83) 10 days after the second irrigation cut-off.
The relative water content (RWC) of flag leaf was obtained according to the method proposed by (Mishra and Choudhuri 1999). After putting the flag leaf samples in distilled water for 24 h and weighing the saturated weight (Sw), the leaves were placed at 95–100 °C for 4 h, and their RWC was measured via Eq. 1 after measuring their dry weights (Dw).
The leaf peroxidase activity was measured using the take-out protein extract with a solution mixture of K–P buffer (pH = 7), NaOH, H2O2, and enzyme extract, which was read at 240 nm (ɛ = 0.0394 mM−1 cm−1) with a spectrophotometer (Nakano and Asada 1981). To measure carotenoids and chlorophyll content, the methods proposed by Arnon (1949) and Lichtenthaler (1987) were performed; we extracted 0.1 g of the flag leaf sample in 10 ml of acetone (80%) and the extract light absorption was read at 663, 645, and 470 nm wavelength with a spectrophotometer (Lambda EZ 210 model). The final concentration of chlorophyll and carotenoids per gram of leaf fresh weight were estimated Eqs. 2 and 3:
where V is the volume of the extracted sample (ml) and W is the fresh leaf weight of the sample (g).
The chlorophyll fluorescence trait was evaluated as a measure of drought stress effect 10 days following the second irrigation cut-off with a fluorimeter. The maximum photochemical quantum function of the photosystem II was measured as the ratio of variable fluorescence to the maximum fluorescence (Fv/Fm) after adjustment of the flag leaf to darkness for 20 min.
The soluble protein content of the leaves was measured applying the method proposed by Kar and Mishra (1976); we extracted 0.2 g of leaf fresh tissue in 0.1 mM Phosphate buffer and added 2.5 ml of the Bradford solution. The sample absorption reading was done at the wavelength of 595 nm using a spectrophotometer. The amount of leaf protein was then calculated based on mg g−1 of fresh leaf weight utilizing the Bovine Serum Albumin (BSA) standard serum albumin.
The proline content of the flag leaf was measured using the method by Paquine and Lechasseur (1979); 0.5 g of fresh leaf sample was extracted in 95% ethanol with the help of benzene and Ninhydrin reagent. Therefore, the absorbance of extract light was read at 515 nm wavelength with a spectrophotometer and the amount of proline in each sample was calculated using a standard curve of proline.
The membrane lipid peroxidation was determined by measuring malondialdehyde (MDA) applying the method proposed by Heath and Pacher (1969). The concentration of malondialdehyde was measured by extracting 0.2 g of the fresh leaf sample in 3 ml of TCA (trichloroacetic acid) 0.1% and 1 ml of TBA (thiobarbituric acid) 0.5% and the absorbance was measured at 533 and 600 nm wavelengths using a spectrophotometer. MDA were calculated according to Eq. 4.
We measured the percentage of electrolyte leakage (EL) of the leaves using the method by McKay (1992) via Eq. 5:
where EC1 and EC2 refer to the electrolyte conductivity, respectively, before and after the water bath.
The grain yield was also calculated by harvesting spikes from an area of one square meter in the middle of the experimental plots according to the observance of the marginal effect.
Data Analysis
The experiment was conducted as a split factorial in a randomized complete block design with three replications. The two-way analysis of variance of data for experimental traits was conducted using SAS software. Bartlett’s test was performed on the data of all the studied traits of both replication years. Afterward, the analysis of two years combined data was carried out once the variance of trait error was homogeneous in two consecutive years (Supplementary Table 1); otherwise, the ANOVA was done for each year separately (Supplementary Table 2). When the interaction was significant (Supplementary Table 1 and 2), the means were sliced and compared employing the LSD test (p ≤ 0.05); otherwise, the main effects were compared merely.
Results
Relative Water Content
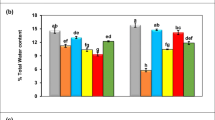
The relative leaf water content is an estimate of the water status of the plant and presents the ability of a genotype to absorb water from soil. The increase in the drought stress decreased the relative leaf water content, but the application of 50 and 100 μM methyl jasmonate improved the reduction. The comparison of the means of concentrations of methyl jasmonate indicated that under full irrigation conditions, the use of methyl jasmonate had no significant effects on the relative flag leaf water content of different wheat cultivars in 2015–2016; whereas the form of 100 μM of methyl jasmonate indicated the highest RWC which was 3% higher in Sirvan cultivar than the no hormone treatment in 2016–2017. In the cut-off irrigation regime at the beginning of the booting, the application of 100 μM of methyl jasmonate significantly increased the relative leaf water content compared to the hormone-free condition only in Sirvan cultivar in both years. In the irrigation cut-off regimen at the milk development stage, the RWC of flag leaf in both wheat cultivars increased by 5.9% and 5.3% in the first year and 4.1% and 3% in the second year by the application of 100 μM methyl jasmonate compared to the hormone-free condition (Fig. 1).
The impact of different methyl jasmonate concentration, cultivars, and irrigation regimes for RWC; means sliced by methyl jasmonate in each irrigation regime and cultivar followed by the same letters have no significant difference on the basis of the LSD test at 5% error probability; (SD = ± 0.4 and SEM = ± 0.2)
Peroxidase Enzyme Activity
The peroxidase enzyme plays a vital role in scavenging and reducing the hydrogen peroxide. Given the significant effect of treatments in ANOVA results (Supplementary Table 2), under full irrigation conditions, leaf peroxidase activities were not significantly different between both cultivars in the first year, but it significantly decreased at both stages of booting and the milk development of grain under the irrigation cut-off condition. Under both stress phases, the stages of irrigation cut-off conditions from booting and irrigation cut-off conditions from milk development of grain, in 2015–2016, Sirvan cultivar had peroxidase enzyme activity of 23.3% and 17.6%, respectively, higher than those of Pishtaz cultivar (Fig. 2a). Similar to the first year, the activity of leaf peroxidase was minimal during stress conditions at the booting in the second year, and it had a reduction of 43.8% compared to the full irrigation condition (Fig. 2b). Furthermore, the leaf peroxidase activity of Sirvan cultivar was 13.1% higher than that of Pishtaz variety in 2016–2017 (Fig. 2c). The mean comparison indicated that the application of 150 μM of methyl jasmonate increased the peroxidase activity by 31.4% compared to the hormone-free condition in the first year, but the maximum activity of peroxidase enzyme (3.2% increase) was observed applying 50 μM of hormone in the second year, and it had no statistically significant differences with 100 and 150 μM methyl jasmonate (Fig. 2d).
The impact of irrigation regimes with cultivar in first year (a), the main effect of irrigation regimes and cultivar in second year (b, c), and the main effect of methyl jasmonate concentrations in 2 years for leaf peroxidase; means sliced by cultivars in each irrigation regime (a, d) in other treatments followed by the same letters have no significant difference on the basis of the LSD test at 5% error probability
Chlorophyll and Carotenoids Content
According to results of combined analysis of data for these traits and their two-year mean comparison, the highest chlorophyll content and carotenoids under the full irrigation conditions, and irrigation cut-off from booting by application of 50 and 100 μM methyl jasmonate belonged to Pishtaz cultivar; there was no statistical significance between these two levels. No significant differences were observed between hormone concentrations in terms of irrigation cut-off during the milk development stage. Under the full irrigation conditions, Sirvan cultivar had 36.9% and 41.2% of, respectively, chlorophyll and carotenoids content in the application of 50 μM of methyl jasmonate higher than the hormone-free state. In the irrigation cut-off conditions from booting, the highest chlorophyll and carotenoids content were obtained after the application of 50 μM of methyl jasmonate; it had no significant differences with hormone-free conditions, but on the contrary, the irrigation cut-off conditions at the milk development stage indicated that the non-application of the hormone had the highest chlorophyll and carotenoids, which had no significant differences with the application of 50 μM hormone (Fig. 3a, b).
Maximum Photochemical Efficiency of Photosystem II (Fv/Fm)
Under the full irrigation conditions in the first and second years of the experiment, Pishtaz cultivar had the highest Fv/Fm applying 100 μM of methyl jasmonate, and they were, respectively, 21.4% and 2.5% higher than those of the non-hormone level. In Sirvan cultivar, the highest Fv/Fm was obtained at 50 μM methyl jasmonate in both years with no significant differences with the hormone-free condition. However, in all the conditions, the maximum photochemical efficiency of photosystem II in Sirvan cultivar was more than that in Pishtaz. There were no significant differences concerning the hormone concentrations between both varieties at the booting drought stress in the first year. However, by application of 100 μM of methyl jasmonate in the second year, Fv/Fm of both Pishtaz and Sirvan cultivars were 2.2% and 9.3% higher than those of the control, respectively. The highest Fv/Fm under the irrigation cut-off conditions at the milk development stage belonged to Sirvan cultivar at the first year and Pishtaz variety in the second year by applying 150 μM of methyl jasmonate, which were, respectively, 24.8% and 9.3% higher than those in the hormone-free condition. At this irrigation regime in Sirvan cultivar, no differences were observed between 100 and 150 μM methyl jasmonate regarding the increase in Fv/Fm in the first year. Overall, Sirvan cultivar had highest Fv/Fm in the all irrigation regimes and years of experiment repetition (Fig. 4).
The impact of irrigation regimes, methyl jasmonate concentrations, and cultivar in 2 years for Fv/Fm; means sliced by methyl jasmonate in each irrigation regime and cultivar followed by the same letters have no significant difference on the basis of the LSD test at 5% error probability (SD = ± 0.03 and SEM = ± 0.02)
Soluble Protein Content
The impact of different cultivars and irrigation regimes indicated that Pishtaz cultivar had, respectively, 7.4, 19.7, and 7.8% of protein content higher than Sirvan cultivar in full irrigation, irrigation cut-off in booting, and the milk development stage (Fig. 5a). The mean comparison of methyl jasmonate effect indicated the minimum amount of soluble protein at the non-hormone state and no significant differences between hormone application rates in terms of leaf protein content; meanwhile, the highest amount of soluble protein was observed at 100 μM of methyl jasmonate (Fig. 5b).
Mean comparison of irrigation regimes and cultivar impact (a) and effect of methyl jasmonate concentrations for wheat leaf protein content (b); means sliced by irrigation regimes (a) and methyl jasmonate (b) in other treatments followed by the same letters have no significant difference on the basis of the LSD test at 5% error probability
Proline Content
Proline is an amino acid that accumulates in plants in response to environmental stresses. With the irrigation cut-off at the booting stage, the amount of proline significantly increased in both wheat cultivars; the mean comparison indicated that under the full irrigation conditions and also in the irrigation cut-off at the booting stage, Sirvan cultivar had, respectively, 19.4% and 26.8% of proline more than Pishtaz cultivar (Fig. 6a). The highest amount of leaf proline was obtained using 50 μM of methyl jasmonate, and there were no significant differences with 100 and 150 μM, yet it increased the proline content by 14.8% in comparison with the control (Fig. 6b).
The impact of irrigation regimes and cultivars (a), effect of methyl jasmonate concentrations (b) in 2 years for proline content of flag leaf; means sliced by methyl jasmonate in each irrigation regimes (a) and between methyl jasmonate concentrations (b) followed by the same letters have no significant difference on the basis of the LSD test at 5% error probability
Electrolytes Leakage Rate (EL)
Preserving the integrity of cell membranes under stress conditions could be considered as a sign of control mechanisms in desiccation tolerance. The results indicated that with the increase in the stress intensity, electrolytes leakage rate enhanced in both wheat cultivars. In non-stress conditions, the application of hormones increased the EL level. The use of 100 μM of methyl jasmonate increased the EL rate by 6.7% compared to non-application of the hormone; meanwhile, under stress conditions, the use of jasmonate reduced the EL. In the stress conditions after the booting in Pishtaz cultivar, the highest level of electrolyte leakage belonged to the hormone-free levels in the first year; the EL decreased by 3.11% by application of 50 μM of methyl jasmonate compared to the non-application of the hormone. There were no significant differences in the implementation of hormone in the other two levels, but they reduced the EL compared to the level without the hormone. In Sirvan cultivar, the use of 150 μM of methyl jasmonate led to a decrease of 8.2% in the leakage of the electrolytes compared to the level without hormone. Under stress conditions following the milk development stage in Pishtaz cultivar, EL level decreased by 0.8% using 50 μM of hormone compared to the condition without hormone. No significant differences were observed among the hormone concentrations in Sirvan cultivar (Fig. 7a).
The impact of irrigation regimes, cultivar, and methyl jasmonate concentrations in first year (a), effect of irrigation regimes in second year (b) and effect of methyl jasmonate concentrations in second year (c) for electrolyte leakage of flag leaf; means sliced by methyl jasmonate in each irrigation regime and cultivar (a), between irrigation regimes (b) and methyl jasmonate concentrations (c) followed by the same letters have no significant difference on the basis of the LSD test at 5% error probability
Under stress conditions at the booting, the highest electrolyte leakage was seen in comparison with other conditions, which was 16.1% more than that in the full irrigation regime in the second year (Fig. 7b). The mean comparison of the effects of different concentrations of methyl jasmonate on the amount of electrolytes leakage in the second year indicated that the application of 50 μM methyl jasmonate reduced the amount of electrolytes leakage, yet there were no statistically significant differences with non-application of hormone (Fig. 7c).
Malondialdehyde Content
In the full irrigation conditions, Sirvan cultivar had 25.25% higher MDA content than Pishtaz cultivar in 2015–2016. Moreover, in terms of stress in booting, no significant differences were there between varieties of wheat in terms of MDA content while in the milk development stage, Pishtaz cultivar had an MDA content of 26.26% higher than that of Sirvan (Fig. 8a). Furthermore, the effects of different concentrations of methyl jasmonate on the MDA content were not significant in the first year. However, in the second year, 150 μM of methyl jasmonate decreased the MDA content in Pishtaz cultivar only in irrigation cut-off regime in the milk development stage of grain and only under full irrigation conditions in Sirvan variety with no significant differences with the non-application of the hormone. In terms of stress at the booting stage, none of the levels of methyl jasmonate in the varieties had a considerable effect on the MDA content of the leaves (Fig. 8b).
The impact of irrigation regimes and cultivar in first year (a), and The impact of irrigation regimes, methyl jasmonate concentration, and cultivar in second year (b) for MDA; means sliced in each irrigation regimes (a) or each irrigation regimes and cultivar (b) followed by the same letters have no significant difference on the basis of the LSD test at 5% error probability
Grain Yield
Irrigation cut-off regimes significantly reduced the grain yield in both years. The irrigation cut-off led to a reduction of 41.7% and 35.9% in grain yield at the booting stage, and a reduction of 34.3% and 14.3% in grain yield at the milk development stage compared to those in full irrigation conditions in both years, respectively. In both years, the use of 100 μM of methyl jasmonate increased the grain yield compared to non-hormone levels in all the irrigation regimes, but there were no significant differences between 50 and 100 μM of hormone regarding the improvement of the grain yield under irrigation cut-off conditions in the second year (Fig. 9a). Sirvan cultivar had 11.0%, 14.8%, and 15.3% higher yields than Pishtaz cultivar in all the three irrigation regimes (Fig. 9b). The application of 100 μM of methyl jasmonate in 2015–2016 increased the grain yield by 6.9% and 1.8%, in Pishtaz and Sirvan cultivars, respectively, compared to that in hormone-free conditions. Like the first year, the application of 100 μM of methyl jasmonate in the second year increased the grain yield compared to the hormone-free state, but no significant differences existed between 50 and 100 μM of methyl jasmonate in Pishtaz cultivar (Fig. 9c).
The impact of irrigation regimes and methyl jasmonate levels (a), the impact of irrigation regimes and variety (b), and the impact of variety and methyl jasmonate levels (c) for grain yield. Means sliced in each irrigation regime (a, b), or cultivar (c) followed by the same letters have no significant difference on the basis of the LSD test at 5% error probability
Discussion
Drought stress occurs in numerous wheat growth regions at last stages of growth (Dehghan et al. 2020a). Investigating physiological responses of plants under vegetative growth conditions could perfectly reveal the response mechanisms of grain yield to drought stress (Dehghan et al. 2020b, Sallam et al. 2019). The results of research on physiological responses of wheat cultivars to different drought stresses are contradictory on a number of occasions (Osmolovskaya et al. 2018). The differences might vary in view of the differences in plant materials and experimental conditions (Sallam et al. 2019). The observed difference herein between the grain yields under stress at the booting stage, in the two years of the experiment, could be attributed to the difference in weather conditions and the higher rainfall and lower temperature at the time of the spike formation and during the growth and development of the tiller in the second year.
The relative water content of leaves is a useful index to determine the plant water status. Even though drought stress reduced the relative leaf water content in both cultivars in our experiment, the application of 100 μM of MeJA at different irrigation regimes prevented the decrease in RWC in both years, and the application of higher concentrations of MeJA decreased it at some levels. RWC in plants is an indicator of drought stress sensitivity. Based on protoplasmic permeability, plants with higher RWC are more drought resistant (Raja et al. 2020). During the present study, the plants had less RWC (about 50%) under higher stress conditions from the booting. Sohag et al. (2020), Kaya et al. (2020), and Raja et al. (2020) reported a decrease in RWC in rice, corn, and tomato under drought stress, respectively. Plants under drought stress seem to minimize their intercellular spaces and water content in their bodies by increasing the osmotic content within tissues. Therefore, water enters through the soil texture with a higher force and decreases the relative water content under drought stress conditions. Pazirandeh et al. (2015) reported that the RWC of barley genotypes leaves reduced under drought stress, but the use of methyl jasmonate improved this decline. This result is consistent with the findings of Ahmad et al. (2018) and Mir et al. (2018a) who reported that exogenous jasmonic acid restored the RWC accumulation in Solanum lycopersicum L. and Zea mays L. seedlings under salt and alkaline stress. In addition, Bali et al. (2019a) and Ahmad et al. (2017) revealed that the relative water content of tomato and faba bean seedlings increased by 16.24% and 32.01%, respectively, with the application of jasmonic acid under heavy metal stress. Methyl jasmonate increases the RWC of the leaf with effect on the plant stoma cells and recovers the water status, membrane constancy, and water transport system of plants under stress. On the other hand, it has no adverse impacts on the plant's photosynthesis, and even helps it grow. The increase in RWC is probably due to the closure of stoma, so that less water is wasted from plant cells.
Enzymes are mostly catalysts of physiological reactions in plants and among the most sensitive factors causing physiological changes in plants under environmental stresses (Foyer and Shigeoka 2011). Oxidative stress or ROS is initiated by generation of O2· − produced from oxygen in response to abiotic stress. It eventually reduces O2 to H2O or enzymatically dismutates to H2O2 and causes transient alterations in the cellular redox state in cells under stress (Ahmad et al. 2010; Kohli et al. 2019). In the current research, the highest activity of peroxidase was observed during full irrigation conditions in Sirvan cultivar. Higher stresses decreased peroxides, whereas increasing concentration of methyl jasmonate enhanced the activity of leaf peroxidase. The maximum peroxidase activity was obtained by application of 150 μM methyl jasmonate. The relative reduction of peroxidase may be due to increased stress to the low potential of these cultivars to eliminate free radicals of superoxide caused by drought stress, and the potential increased using methyl jasmonate. This is indicative of the higher sensitivity of Pishtaz cultivar to oxidative stress. Bali et al. (2019b, 2020) reported that the treatment with 100 nM JA elevated the activities of POD by 1.91-fold, modulated the transcript levels of antioxidative enzymes, and declined oxidative damage by decreasing O2· − content, nuclear, and membrane damage in tomato seedlings under stress. The improvement in the peroxidase activity might be owing to the improved discharge of peroxidases localized in the cell walls. Comparot et al. (2002) observed an increase in peroxidase activity in the treatment with 150 μM methyl jasmonate in rapeseed. The exogenous application of MeJA and JA to plants under abiotic stresses improves the activities of antioxidants, facilitates the neutralization of ROS, and provides protection against oxidative stress (Mir et al. 2018). Farooq et al. (2016) reported that the application of MeJA could minimize the oxidative stress, as revealed via a lower level of ROS synthesis in the leaves of Brassica napus. Their study also indicated that MeJA plays an effective role in the regulation of multiple transcriptional pathways involved in oxidative stress responses, thereby enhancing the enzymatic activities and gene expression of important antioxidants (SOD, APX, CAT, POD) and secondary metabolites. However, Tayyab et al. (2020) found that exogenous applications of MeJA + SA approximately doubled the activities of the antioxidant enzymes catalase, peroxidase, and superoxide dismutase. Although pre-treatment with MeJA alone was not found to be effective in maize for drought tolerance, it imposed the highest increase in drought-induced production of endogenous abscisic acid (ABA) (Tayyab et al. 2020).
Drought stress inhibits plant photosynthesis, causes changes in chlorophyll content, and damages the photosynthetic structures. Environmental stresses, such as drought, can reduce the plant growth and photosynthesis potential by striking the balance between the production of free oxygen radicals and the protective mechanisms that repel these radicals, which leads to the accumulation of active oxygenates, the induction of oxidative stress, damage to proteins, membrane lipids, and other cellular components (Dąbrowski et al. 2019). The results of the present study indicated that the amounts of photosynthetic pigments decreased under drought stress. The reduction in the chlorophyll a and b contents due to water stress has been reported for more crops (Parveen et al. 2019; Hussain et al. 2019; Kosar et al. 2021), which was confirmed in the present study. This reduction in photosynthetic pigments could be because of the improved activities of chlorophyllase and peroxidase complicated in the interruption of chlorophyll under drought stress (Farooq et al. 2020). The 50 and 100 μM of hormone increased the total carotenoids and chlorophyll content, but an increase in the concentration of methyl jasmonate (150 μM) triggered a sharp decrease in them. As the photosynthetic capacity of chlorophyll decreases under stress, plants seek to compensate for this deficiency by the chlorophyll accumulation because of methyl jasmonate (Kang et al. 2005). In the present study, methyl jasmonate prevented chlorophyll degradation and reduced photosynthesis by increasing the activity of peroxidase in stress conditions and caused the plant growth and activity. Sirhindi et al. (2016) reported that application of JA restored the total chlorophyll content by 38.70% in soybean under heavy metal stress. JA improved the chlorophyll content by 71.5% in tomato under nematodes stress (Bali et al. 2018). Improved chlorophyll content by exogenous application of JA might be attributed to the increase in the CO2 fixation; thus, it helps enhancing photosynthetic rate. Jasmonates lead to the accumulation of chlorophyll, carotenoids, and other pigments as well as the increased PSII efficiency in Brassica oleracea L. (Sirhindi et al. 2020). Me-JA, at low concentrations, has further potential in making the PSII structure more stable and is actively more efficient than JA (Sirhindi et al. 2020).
The reduced Fv/Fm ratio in drought stress conditions indicates the efficiency of photosystem II due to the reduction of electron transfer from photosystem II–I under the influence of drought stress. It has indicated the fact that PSII gets damaged/photochemically inactive under water stress (Akhter et al. 2021). An 11% reduction in Fv/Fm ratio by applying tension from the booting stage and 10% in the stress from the milk development stage indicate a decrease in light protection and a reason that the drought stress had a significant effect on the efficiency of photosynthesis (Li et al., 2006). Drought stress reduces the reception and transmission capacity of electrons, thus, the system rapidly reaches Fm, resulting in decreases variable fluorescence (Fv). Therefore, due to this variability, environmental stresses decrease the Fv/Fm ratio by an impact on photosystem II (Mirakhori et al. 2011). Oxidative stress damages the antenna molecules, thereby deteriorating the chlorophyll fluorescence and eventually causing either complete or partial blockage of electron transport from PSII to PSI stress (Alyemeni et al. 2018). Our results confirmed previously reported findings in other studies indicating that JA increases Fv/Fm and has the maximum quantum efficiency of PSII (Sirhindi et al. 2016, 2020).
The amount of soluble proteins increased with cut-off irrigation regimes, and the use of methyl jasmonate increased the soluble protein content. Farooq et al. (2020) reported that drought significantly decreased seedling fresh and dry weights and chlorophyll contents in safflower while increasing the proline, total soluble proteins, and activities of peroxidase. Proteins are hydrolyzed by proteases to enhance amino acids for storage, transfer, and osmotic regulation. Osmotic regulation, protection of cell macromolecules, storage of nitrogen, keeping cell pH constant, cell detoxification, and free radical control are the proposed measures for the accumulation of amino acids released from protein under stress (Parida et al. 2004). Methyl jasmonate may protect the structure of proteins, maintain functions of them, and prevent their decomposition under stress conditions by reducing free radicals (Tarchevsky et al. 2001). JA enhanced the protein content in the current study and the findings were in line with the reports of Poonam et al. (2013) on Cajanus cajan under copper stress, and Sirhindi et al. (2016) on soybean (Glycine max L.) subjected to nickel stress. Hussain et al. (2018) found that the chilling tolerance in wheat improved by exogenous application of methyl jasmonate due to modifying antioxidant defense system and soluble protein production.
Proline as an osmo-protectant allows plant functions to tolerate stress (Parveen et al. 2019). The accumulation of compatible osmolytes, such as proline, is a common reaction to stress in plants and protects cells against stress damages (Farooq et al. 2020). Raja et al. (2020) evaluated a previous finding concerning proline, which reported that tomato plants accrued greater proline content under drought stress in comparison to heat stress. Additionally, several recent studies have supported our findings regarding the fact that proline accumulation occurs in plants exposed to stress conditions (Hussain et al. 2019; Parveen et al. 2019; Sohag et al. 2020; Kosar et al. 2021) because of its property to stabilize subcellular structures, scavenging free radicals, and buffer cellular redox potential. Certain crops, wheat for instance, have been characterized by low levels of these compounds and indicated significantly increased proline accumulation with water-deficit tolerance (Nayyar and Walia 2003). In this study, due to the increase in stress, proline was significantly accumulated in the leaves and the use of methyl jasmonate increased the amount of proline. The highest amount of proline was in irrigation cut-off from the booting. Bali et al. (2018, 2019a) and Ahmad et al. (2017) reported that the JA (100 nM) increased the proline content in the metal-treated plants. Similarly, in a study on soybean plants under drought stress, Anjum et al. (2011) reported that the use of methyl jasmonate would further increase the proline content and help maintain relative humidity in soybean plants under stress compared with the control plants. The application of JA further increased the proline content, suggesting that the production of proline biosynthesizing enzymes was induced by JA (Ahmad et al. 2017).
Under drought stress conditions, the plasma membrane is damaged, which leads to increased permeability of the cell membrane. Herein, increasing the stress intensity could raise the electrolytes leakage rate in both wheat cultivars, particularly in Pishtaz cultivar. Application of 50 μM of methyl jasmonate reduced the amount of electrolytes leakage and higher concentrations increased the leakage levels. Since the ionic leakage and cytoplasmic membrane stability are inversely correlated, Pishtaz variety had a lower membrane stability due to its higher ion leakage. As a result, it has less drought tolerance. Ahmad et al. (2018) reported that osmotic stress increased the electrolyte leakage in S. lycopersicum L. by 424.88%; however, the electrolyte leakage reduced by 26.72% applying JA compared to the control. Similarly, in a study on faba bean (Vicia faba L.) under Cd stress, the plants treated with a combination of JA + Cd showed less electrolyte leakage of 4.06-fold in comparison with the control (Ahmad et al. 2017). The alteration of membrane structure caused by oxidative stress in the water deficiency could increase the permeability of the cell membrane and lead to the leakage of intracellular electrolytes to the outside; hence, the measurement of the amount of electrolytes leakage from the plasma membrane is considered as an index of damage to the cell membrane. More cellular membrane stability and less leakage of electrolytes in water stress conditions are the main characteristics of drought-resistant genotypes and indicate the presence of control mechanisms in drought tolerance (Kocheva and Georgiev 2003).
The amount of malondialdehyde (MDA), as a chemical biomarker in plants, increases in drought stress condition which is an indicator of lipid peroxidation and reflected the degree of damage at stress conditions (Yang and Deng 2015). The increasing stress maximized MDA in both our cultivars under the irrigation cut-off condition from the booting. However, the application of methyl jasmonate resulted into a reduction in MDA accompanied by an increase in antioxidant activity due to its essential role in the decomposition and detoxification of free oxygen radicals (Reddy et al. 2004). JA treatment reduced the MDA, O2· − , and H2O2 levels in the plants exposed to different stress, which scavenges ROS by increasing the activity and transcript levels of antioxidative enzymes under stress conditions (Bali et al. 2019b).
The irrigation cut-off from the booting caused a further decrease in the grain yield. Therefore, the stress at the pollination stage reduced yield by decreasing the amount of available plant water that made grains small and thin. Earlier mature Sirvan cultivar had a higher yield than Pishtaz cultivar due to mechanisms of escape from the moisture and heat stress in the late growth period of wheat under stress conditions. Methyl jasmonate also helped to improve the yield by decreasing the amount of malondialdehyde and electrolytes leakage and increasing the activity of the peroxidase enzyme under stress conditions. Even though drought stress decreased certain physiological traits, the effect of methyl jasmonate could partially compensate for the drought stress by increasing the grain yield (9.2% in the irrigation cut-off of from booting and 6.8% in the irrigation cut-off from the milk development stage in Sirvan cultivar). In this regard, Anjum et al. (2011) reported that soaking soybeans in 1 μM of methyl jasmonate increased the grain yield per plant by 17% compared to the control treatment. The positive influence of growth hormones, such as JA (Sirhindi et al. 2016), has been previously reported. Sirhindi et al. (2020) stated that the biomass of B. oleracea L. increased owing to the application of JA or MeJA. Mir et al. (2018a) recorded similar consequences in maize plants, in which the seeds affected by salinity were pre-treated with JA, after which plant growth and biomass increased.
Conclusion
Irrigation cut-off regimes caused pessimistic effects and changes in physiological traits affecting the grain yield in both wheat cultivars. However, the application of 100 μM of methyl jasmonate in both wheat cultivars had a constructive effect on increasing relative water content traits of the leaves, peroxidase activity, protein content, carotenoids, and chlorophyll content, Fv/Fm. It also reduced Malondialdehyde and electrolyte leakage, and further improved the yield of Sirvan cultivar by 13.7% in the case of irrigation cut-off from the booting. The improvement of physiological traits in irrigation cut-off from the booting in Sirvan cultivar was more affected by the hormone, which indicates that Sirvan is making Sirvan more tolerant with a higher yield than Pishtaz cultivar. Drought stress may occur at any stage of wheat growth; hence, it is necessary to recognize the responses of different stages of wheat growth to drought. Therefore, the use of 100 μM of methyl jasmonate is recommended as a practical way to modify ravages of drought stress or deficit irrigation along with the selection of appropriate cultivars. There might also be certain beneficial effects of MeJA on biomass accumulation, the number of grains per spike, and grain weight, which in turn may explain the biological yield of plants treated with MeJA under drought as well as well-watered conditions.
References
Abdoli M, Saeidi M (2012) Using different indices for selection of resistant wheat cultivars to post anthesis water deficit in the west of Iran. Ann Biol Res 3:1322–1333
Ahmad P, Jaleel CA, Salem MA, Nabi G, Sharma S (2010) Roles of enzymatic and nonenzymatic antioxidants in plants during abiotic stress. Crit Rev Biotechnol 30(3):161–175
Ahmad P, Alyemeni MN, Wijaya L, Alam P, Ahanger MA, Alamri SA (2017) Jasmonic acid alleviates negative impacts of cadmium stress by modifying osmolytes and antioxidants in faba bean (Vicia faba L.). Arch Agron Soil Sci 63:1889–1899
Ahmad P, Ahanger MA, Alyemeni MN, Wijaya L, Alam P, Ashraf M (2018) Mitigation of sodium chloride toxicity in Solanum lycopersicum L. by supplementation of jasmonic acid and nitric oxide. J Plant Interact 13(1):64–72
Ahmadi ME, Kalantari KM, Jafari R, Hasibi N, Mahdavian K (2010) Study of the effects of 24-epibrassinolid and water stress on some physiological parameters in canola (Brassica napus L.) seedling. Iran J Biol 23:275–286
Akhter MS, Noreen S, Mahmood S, Ashraf M, Alsahli AA, Ahmad P (2021) Influence of salinity stress on PSII in barley (Hordeum vulgare L.) genotypes, probed by chlorophyll-a fluorescence. J King Saud Univ Sci 33(1):101239
Alyemeni MN, Ahanger MA, Wijaya L, Alam P, Bhardwaj R, Ahmad P (2018) Selenium mitigates cadmium-induced oxidative stress in tomato (Solanum lycopersicum L.) plants by modulating chlorophyll fluorescence, osmolyte accumulation, and antioxidant system. Protoplasma 255(2):459–469
Anjum S, Wang L, Farooq M, Khan I, Xue L (2011) Methyl jasmonate-induced alteration in lipid peroxidation, antioxidative defence system and yield in soybean under drought. J Agron Crop Sci 197:296–301
Arnon DI (1949) Copper enzymes in isolated chloroplasts. Polyphenoloxidase in Beta vulgaris. Plant Physiol 24:1
Ashraf M, Foolad M (2007) Roles of glycine betaine and proline in improving plant abiotic stress resistance. Environ Exp Bot 59:206–216
Awika JM (2011) Major cereal grains production and use around the world, advances in cereal science: implications to food processing and health promotion. ACS Publications, Washington, pp 1–13
Bali S, Kaur P, Kohli SK, Ohri P, Thukral AK, Bhardwaj R, Wijaya L, Alyemeni MN, Ahmad P (2018) Jasmonic acid induced changes in physio-biochemical attributes and ascorbate-glutathione pathway in Lycopersicon esculentum under lead stress at different growth stages. Sci Total Environ 645:1344–1360
Bali S, Jamwal VL, Kohli SK, Kaur P, Tejpal R, Bhalla V, Ohri P, Gandhi SG, Bhardwaj R, Al-Huqail AA, Siddiqui MH, Ali HM, Ahmad P (2019a) Jasmonic acid application triggers detoxification of lead (Pb) toxicity in tomato through the modifications of secondary metabolites and gene expression. Chemosphere 235:734–748
Bali S, Jamwal VL, Kaur P, Kohli SK, Ohri P, Gandhi SG, Bhardwaj R, Al-Huqail AA, Siddiqui MH, Ahmad P (2019b) Role of P-type ATPase metal transporters and plant immunity induced by jasmonic acid against lead (Pb) toxicity in tomato. Ecotoxicol Environ Saf 174:283–294
Bali S, Kaur P, Jamwal VL, Gandhi SG, Sharma A, Ohri P, Bhardwaj R, Ali MA, Ahmad P (2020) Seed priming with jasmonic acid counteracts root knot nematode infection in tomato by modulating the activity and expression of antioxidative enzymes. Biomolecules 10:98
Comparot SM, Graham CM, Reid DM (2002) Methyl jasmonate elicits a differential antioxidant response in light-and dark-grown canola (Brassica napus) roots and shoots. Plant Growth Regul 38:21–30
Dąbrowski P, Baczewska-Dąbrowska AH, Kalaji HM, Goltsev V, Paunov M, Rapacz M, Wójcik-Jagła M, Pawluśkiewicz B, Bąba W, Brestic M (2019) Exploration of chlorophyll a fluorescence and plant gas exchange parameters as indicators of drought tolerance in perennial ryegrass. Sensors 19:2736
Dehghan M, Balouchi H, Yadavi A (2020a) Improvement of seed quality of wheat (Triticum aestivum) as affected by brassinolide under different irrigation regimes. J Crop Sci Biotechnol 23:137–148
Dehghan M, Balouchi H, Yadavi A, Zare E (2020b) Improve wheat (Triticum aestivum) performance by brassinolide application under different irrigation regimes. South Afr J Bot 130:259–267
Farooq MA, Gill RA, Islam F, Ali B, Liu H, Xu J, He S, Zhou W (2016) Methyl jasmonate regulates antioxidant defense and suppresses arsenic uptake in Brassica napus L. Front Plant Sci 7:468
Farooq A, Bukhari SA, Akram NA, Ashraf M, Wijaya L, Alyemeni MN, Ahmad P (2020) Exogenously applied ascorbic acid-mediated changes in osmoprotection and oxidative defense system enhanced water stress tolerance in different cultivars of safflower (Carthamus tinctorious L.). Plants 9:104
Foyer CH, Shigeoka S (2011) Understanding oxidative stress and antioxidant functions to enhance photosynthesis. Plant Physiol 155:93–100
Heath RL, Pacher L (1969) Photo per oxidation in isolated chloroplast. I. Kinetics and stoichiometry of fatty acid per oxidation. Arch Biochem Biophys 125:189–198
Hussain HA, Hussain S, Khaliq A, Ashraf U, Anjum SA, Men S, Wang L (2018) Chilling and drought stresses in crop plants: implications, cross talk, and potential management opportunities. Front Plant Sci 9:393
Hussain HA, Men S, Hussain S, Yinglong C, Ali S, Sai Z, Zhang K, Yan L, Qiwen X, Liao C, Wang L (2019) Interactive effects of drought and heat stresses on morphophysiological attributes, yield, nutrient uptake and oxidative status in maize hybrids. Sci Rep 9(1):3890
Kang DJ, Seo YJ, Lee JD, Ishii R, Kim K, Shin D, Park S, Jang S, Lee IJ (2005) Jasmonic acid differentially affects growth, ion uptake and abscisic acid concentration in salt-tolerant and salt-sensitive rice cultivars. J Agron Crop Sci 191:273–282
Kar M, Mishra D (1976) Catalase, peroxidase, and polyphenoloxidase activities during rice leaf senescence. Plant Physiol 57:315–319
Karami A, Shahbazi M, Niknam V, Shobbar ZS, Tafreshi RS, Abedini R, Mabood HE (2013) Expression analysis of dehydrin multigene family across tolerant and susceptible barley (Hordeum vulgare L.) genotypes in response to terminal drought stress. Acta Physiol Plant 35:2289–2297
Kaya C, Şenbayram M, Akram NA, Ashraf M, Alyemeni MN, Ahmad P (2020) Sulfur-enriched leonardite and humic acid soil amendments enhance tolerance to drought and phosphorus deficiency stress in maize (Zea mays L.). Sci Rep 10:6432
Kocheva K, Georgiev G (2003) Evaluation of the reaction of two contrasting barley (Hordeum vulgare L.) cultivars in response to osmotic stress with PEG 6000. Bulg J Plant Physiol 49:290–294
Kohli SK, Khanna K, Bhardwaj R, Abd-Allah EF, Ahmad P, Corpas FJ (2019) Assessment of subcellular ROS and NO metabolism in higher plants: multifunctional signaling molecules. Antioxidants 8:641
Kosar F, Akram NA, Ashraf M, Ahmad A, Alyemeni MN, Ahmad P (2021) Impact of exogenously applied trehalose on leaf biochemistry, achene yield and oil composition of sunflower under drought stress. Physiol Plant. https://doi.org/10.1111/ppl.13155
Li R-H, Guo P-G, Michael B, Stefania G, Salvatore C (2006) Evaluation of chlorophyll content and fluorescence parameters as indicators of drought tolerance in barley. Agric Sci China 5:751–757
Lichtenthaler HK (1987) Chlorophylls and carotenoids: pigments of photosynthetic biomembranes. Methods Enzymol 148:350–382
McKay H (1992) Electrolyte leakage from fine roots of conifer seedlings: a rapid index of plant vitality following cold storage. Can J for Res 22:1371–1377
Mir MA, Sirhindi G, Alyemeni MN, Alam P, Ahmad P (2018) Jasmonic acid improves growth performance of soybean under nickel toxicity by regulating nickel uptake, redox balance, and oxidative stress metabolism. J Plant Growth Regul 37:1195–1209
Mirakhori M, Moradi F, Ardakani M, Nazeri P, Jahromi ME (2011) Effect of drought stress and methanol on chlorophyll parameters, chlorophyll content and relative water content of soybean (Glycine max L., var. L 17). Iran J Field Crops Res 8:531–541
Mishra A, Choudhuri M (1999) Effects of salicylic acid on heavy metal-induced membrane deterioration mediated by lipoxygenase in rice. Biol Plant 42:409–415
Nafie E, Hathout T, Mokadem A, Shyma A (2011) Jasmonic acid elicits oxidative defense and detoxification systems in Cucumis melo L. cells. Braz J Plant Physiol 23:161–174
Nakano Y, Asada K (1981) Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol 22:867–880
Nayyar H, Walia D (2003) Water stress induced proline accumulation in contrasting wheat genotypes as affected by calcium and abscisic acid. Biol Plant 46:275–279
Oraki H, Aghaalikhana M (2012) Effect of water deficit stress on proline contents, soluble sugars, chlorophyll and grain yield of sunflower (Helianthus annuus L.) hybrids. Afr J Biotech 11:164–168
Osmolovskaya N, Shumilina J, Kim A, Didio A, Grishina T, Bilova T, Keltsieva OA, Zhukov V, Tikhonovich I, Tarakhovskaya E, Frolov A, Wessjohann LA (2018) Methodology of drought stress research: experimental setup and physiological characterization. Int J Mol Sci 19(12):4089
Paquine R, Lechasseur P (1979) Observations sur one method dosage la libra dans les de planets. Can J Bot 57:1851–1854
Parida AK, Das AB, Mittra B, Mohanty P (2004) Salt-stress induced alterations in protein profile and protease activity in the mangrove Bruguiera parviflora. Zeitschrift Für Naturforschung C 59:408–414
Parveen A, Liu W, Hussain S, Asghar J, Perveen S, Xiong Y (2019) Silicon priming regulates morpho-physiological growth and oxidative metabolism in maize under drought stress. Plants 8:431
Pazirandeh MS, Hasanloo T, Shahbazi M, Niknam V, Moradi-Payam A (2015) Effect of methyl jasmonate in alleviating adversities of water stress in barley genotypes. Int J Farming Allied Sci 4:111–118
Poonam S, Kaur H, Geetika S (2013) Effect of jasmonic acid on photosynthetic pigments and stress markers in Cajanus cajan (L.) Millsp. Seedlings under copper stress. Am J Plant Sci 4:817–823
Raja V, Qadir SU, Alyemeni MN, Ahmad P (2020) Impact of drought and heat stress individually and in combination on physio-biochemical parameters, antioxidant responses, and gene expression in Solanum lycopersicum. 3 Biotech 10:208
Reddy AR, Chaitanya KV, Vivekanandan M (2004) Drought-induced responses of photosynthesis and antioxidant metabolism in higher plants. J Plant Physiol 161:1189–1202
Sallam A, Alqudah AM, Dawood M, Baenziger PS, Börner A (2019) Drought stress tolerance in wheat and barley: advances in physiology, breeding and genetics research. Int J Mol Sci 20(13):3137
Sirhindi G, Mir MA, Abd-Allah EF, Ahmad P, Gucel S (2016) Jasmonic acid modulates the physio-biochemical attributes, antioxidant enzyme activity, and gene expression in Glycine max under nickel toxicity. Front Plant Sci 7:591
Sirhindi G, Mushtaq R, Gill SS, Sharma P, Abd-Allah EF, Ahmad P (2020) Jasmonic acid and methyl jasmonate modulate growth, photosynthetic activity and expression of photosystem II subunit genes in Brassica oleracea L. Sci Rep 10(1):1–14
Sohag AAM, Tahjib-Ul-Arif M, Brestic M, Afrin S, Sakil MA, Hossain MT, Hossain MA, Hossain MA (2020) Exogenous salicylic acid and hydrogen peroxide attenuate drought stress in rice. Plant, Soil Environ 66:7–13
Tarchevsky IA, Maksyutova NN, Yakovleva VG (2001) Effect of jasmonic, salicylic, and abscisic acids on [14C] leucine incorporation into proteins of pea leaves. Biochem Mosc 66:68–71
Tayyab N, Naz R, Yasmin H, Nosheen A, Keyani R, Sajjad M, Hassan MN, Roberts TH (2020) Combined seed and foliar pre-treatments with exogenous methyl jasmonate and salicylic acid mitigate drought-induced stress in maize. Plos One 15:e0232269
Yang S, Deng X (2015) Effects of drought stress on antioxidant enzymes in seedlings of different wheat genotypes. Pak J Bot 47:49–56
Zadoks JC, Chang TT, Konzak CF (1974) A decimal code for the growth stages of cereals. Weed Res 14:415–421
Zhou Y, Zhang M, Li J, Li Z, Tian X, Duan L (2015) Phytotoxin coronatine enhances heat tolerance via maintaining photosynthetic performance in wheat based on electrophoresis and TOF-MS analysis. Sci Rep 5:1–13
Acknowledgements
The authors would like to thank their colleagues in the laboratory of agronomy in agricultural faculty, Yasouj University.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
ZJ performed the experimental work and wrote the draft manuscript as a part of dissertation for PhD in field of crop physiology in Yasouj University. HB supervised the research and finalized the manuscript, and MMD and AY helped as advisor the research and dissertation.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical Approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Handling Editor: Parvaiz Ahmad.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Javadipour, Z., Balouchi, H., Movahhedi Dehnavi, M. et al. Physiological Responses of Bread Wheat (Triticum aestivum) Cultivars to Drought Stress and Exogenous Methyl Jasmonate. J Plant Growth Regul 41, 3433–3448 (2022). https://doi.org/10.1007/s00344-021-10525-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00344-021-10525-w