Abstract
A common problem with vegetable production in saline areas is poor crop stand, but for black cumin (Nigella sativa L.) germination data are limited and inconsistent. The effects of chemical priming with Urmia lake salt and urea solutions for 16 h at 30 °C on seed germination and seedling growth of black cumin (Nigella sativa L.) were studied under various osmotic stress levels. For a more detailed assessment of chemical priming, the effects of hydropriming for 16 h at 30 °C were also studied. A seed lot that was not exposed to any treatment, except disinfection, was used as control. Osmotic stress levels were − 2, − 4, − 6, and − 8 bar, which were achieved with polyethylene glycol 6000 (PEG 6000). Seed germination of black cumin was reduced by 16.2%, 33.8%, 50.9%, and 74.9% under osmotic potential − 2, − 4, − 6, and − 8 bar, respectively, compared with non-stressed control. Improved germination index values, reduced mean germination time, and increased coefficients of velocity of germination were observed under osmotic stress in primed seeds compared with non-primed control. Averaged over priming treatments, priming improved the final germination percentage by 10.5%, 24.3%, 45.5%, and 74.6% under osmotic potential − 2, − 4, − 6, and − 8 bar, respectively. Post-germination growth was also inhibited under low osmotic potential compared with the non-stressed control. Nevertheless, priming improved length and weight of black cumin seedlings and enhanced peroxidase and catalase activity at all osmotic potential levels compared with non-primed seeds. Higher seedling vigor indices were recorded in seedlings from primed seeds with decreasing osmotic potential levels than non-primed seeds. Urmia lake salt priming had the greatest impact on improving seed germination and vigor indices, especially under osmotic stress conditions. Although seed priming did not completely eliminate the symptoms of osmotic stress in black cumin germination, it is an efficient method to mitigate the impact of osmotic stress on germination of this species.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Abiotic stress, such as salinity, drought, temperature extremes, heavy metal toxicity, and nutrient deficiency among others, challenge plant growth and cause adaptation problems that may result in yield losses (Hasanuzzaman et al. 2012). Increased soil salinity levels can influence plant growth and yield to a great extent. Not only some soils are naturally saline, but salinity problems are increased in the field by improper farming practices. For example, irrigation water can build salinity in soils in arid regions as well as the addition of fertilizers containing salts, when there is insufficient rain to flush the soils. In a saline environment, adaptation of plants to salinity during germination and early seedling stages is crucial for the establishment of species. In particular, seedlings are the most vulnerable stage in the life cycle of plants and germination determines when and where seedling growth begins.
The germination process constitutes the most important physiological function of seeds and is considered a precondition for successful cultivation of most crops (Tajbakhsh and Ghiyasi 2009). Good germination secures a desirable crop stand in the field (that is, final plant density with plants of uniform size per unit area) which plays an important role in the agronomical production process (Salehzade et al. 2009). The germination process is controlled by several eco-physiological factors, among which temperature, oxygen, and water availability are crucial ecological factors. In particular, the water potential of soil is an important factor in seed germination in semiarid climates, where seed is often sown into soil with inadequate water for rapid germination. Moreover, environmental stresses, such as drought and osmotic stress, negatively affect the final germination percentage, seed vigor, and seedling growth (Ghiyasi et al. 2015). The germination and seedling stages in the plant life cycle are more sensitive growth stages to salinity than the adult stage for most species (Ashraf et al. 1986). The early seedling period was noted as the most sensitive stage among different growth stages of wheat, sorghum, and cowpea, with increasing growth reduction when salinity levels increased (Shalhevet 1995).
In seeking tools to promote germination of crops, seed priming techniques can serve as effective and affordable ways for improving the speed and uniformity of germination. Generally, seed priming is the method in which seeds are soaked in water solutions under controlled conditions before planting. In seed priming techniques, such as hydropriming, osmopriming, and matricopriming, the process of water absorption by the seed is stopped prior to radicle emergence. Frequently after completing treatment, primed seeds are dried to reach near the initial moisture content under shade and then sowing is performed on a farm. The use of seed priming can improve germination and seed vigor, particularly under adverse conditions (Ghiyasi et al. 2008; Amirnia and Ghiyasi 2016). Chemical priming, that is, plant priming with chemical agents, can improve plant growth under stress conditions (Savvides et al. 2016). Various natural or synthetic compounds can be applied to plants before stress events occur, trying to enhance tolerance when plants are exposed to abiotic stresses. These compounds help primed plants to overcome the adverse effect of stress compared with non-primed plants. However, no study has explored this technique for improving seed germination in black cumin (Nigella sativa L.) under saline conditions.
Among plants with medicinal properties, the annual species black cumin, a native to south west Asia, is receiving attention. This herb is used as a spice in the Middle East, while it has been used for several medicinal effects in traditional medicine. Black cumin germination is sensitive to salinity stress (Gholami et al. 2015; Papastylianou et al. 2018), though a good tolerance to salinity up to 150 mM (that is, osmotic potential about 3.7 bar) during germination has been reported (Hajar et al. 1996). Evidently, improving seed germination and early growth of black cumin under saline conditions would be highly useful. However, due to lack of information on this topic further research is needed to study suitable materials for improving seed germination and seedling growth of this crop under salinity conditions. Taking into consideration the above, the objective of this work was to explore the impact of different chemical priming treatments on germination and seedling growth of black cumin under osmotic stress.
Materials and Methods
Experimental Procedures
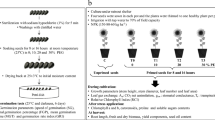
The study was conducted at the Department of Agronomy, Faculty of Agriculture, Urmia University in 2016. The influence of pre-sowing seed treatment (chemical priming) and osmotic potential (drought stress) on germination and seedling growth of black cumin were studied. The ‘Mashhad’ genotype was used. Seeds were primed with solutions of (i) Urmia lake salt and (ii) urea. These solutions were selected for the study after trying a range of diverse solutions for seed priming, which showed that Urmia lake salt and urea solutions were the most active in seed germination and seedling growth of black cumin. This is attributed to the high concentrations of Na+ and Cl− in Urmia lake water, given that sodium chloride (NaCl) is the prevailing chemical salt (over 90% of the lake’s chemical components) affecting the overall environmental and ecological characteristics of the lake water (Ahmadzadeh Kokya et al. 2011). The concentration of NaCl in Urmia lake was about 34 g L−1 in 1915, which has risen to more than 300 g L−1 (over 5 M) due to drought, the evaporation process and increased demands for agricultural water in the lake’s basin (Alipour 2006; Eimanifar and Mohebbi 2007). To prepare Urmia lake salt solution, 1 L of Urmia lake water was evaporated and the remaining salt was applied in seed priming. The remaining salt compounds after the evaporation of 1 L of Urmia lake water are shown in Table 1.
Supersaturated solutions of Urmia lake salt and urea were prepared in distilled water. These solutions were kept under room conditions in the dark and used as stock solutions. Then, 1 mL of each stock solution was poured into 100 mL of distilled water. Following this procedure, the concentration of Urmia lake salt solution used for priming was 0.45 M (concerning sodium chloride concentration of the sample used in the current study), while the concentration of urea solution used for priming was 0.10 M. Black cumin seeds were soaked in solutions for 16 h in laboratory conditions (temperature 30 °C and dark). Seed-to-solution volume ratio was 1:5 during seed priming. For a better evaluation, hydropriming effects on germination and seedling growth of black cumin seeds were also studied in addition to priming solutions with Urmia lake salt and urea. For hydropriming, treated seeds were soaked in distilled water under similar conditions to the other treatments. Seeds that did not receive any treatment, other than disinfection, were used as control. Treated seeds were dried for 24 h under shade and room temperature (22 ± 2 °C) conditions. Before germination and seedling growth tests, seed samples were sterilized with 1.5% sodium hypochlorite for 3 min and then were washed three times with distilled water.
Germination tests were conducted in a factorial randomized complete block design with four replications. Each replication consisted of 100 seeds. Seeds were placed on filter paper in sterile 9-cm Petri dishes, which were allocated for germination at 25 °C. Polyethylene glycol 6000 (PEG 6000) was used to achieve the required osmotic stress. The studied levels of osmotic stress were − 2, − 4, − 6, and − 8 bar. These osmotic potential levels were achieved by dissolution of PEG 6000 in distilled water according to Michel and Kaufmann (1973). After planting seeds, the filter paper in each Petri dish was wetted with the above solutions until saturation. In all treatments, filter paper saturated with each respective solution was maintained to the end of the experiment. Moisture of filter papers was monitored every 24 h, and if necessary, Petri dishes were watered with each respective solution for each treatment. Germination of seeds was recorded for 14 days. For the seedling growth test, 80 seeds in four replications (20 seeds per replication) in each treatment were planted in trays containing perlite. The statistical design, evaluated treatments, and other conditions of this experiment were similar to the germination test. Seedling parameters were measured 14 days after planting.
Germination Parameters
Measured traits and parameters in this study were the following:
Final germination (FG) was calculated as the mean daily cumulative germination percentage of four replications on the last counting day in each treatment.
Mean germination time (MGT) was calculated according to the following equation (Ellis and Roberts 1981):
where n is the number of seeds germinated on each day and D is the day of counting.
The germination index (GI) was calculated according to the following equation (AOSA 1983):
where GT is the number of seeds germinated at the first day of counting and T is the number of days until the first day of counting.
Coefficient of Velocity of Germination (CVG)
Coefficient of velocity of germination (CVG) was calculated according to the following equation (Maguire 1962):
where G is the number of germinated seeds and n is the last day of germination.
Seedling Growth Parameters
Thirty seedlings from each treatment were randomly selected after 14 days from the start of the experiment. Seedling root and shoot length were measured with an electronic ruler with precision of 1 mm. The sum of root and shoot length per seedling was recorded as the seedling length. Seedlings were placed in the oven at 65 °C (for 48 h) to determine dry weight. After weighing, the average of 10 seedlings was considered as the seedling dry weight. Therefore, data are presented per seedling. The mean seedling length was used for the estimation of seedling vigor index (SVI) using the following formula (Abdul-Baki and Anderson 1973):
A seed lot with high seedling vigor index is considered as vigorous (Abdul-Baki and Anderson 1973).
Malondialdehyde (MDA) Assay
Malondialdehyde (MDA) content was determined using ten seedlings per treatment after 10 days of germination, following the method of Du and Bramlage (1992). Ground tissue (0.2 g) was homogenized with 2 mL of 0.1% trichloroacetic acid (TCA), and the crude extract preparation was centrifuged at 10,000 × g for 20 min. A mixture of TCA, thiobarbituric acid (TBA) (1 mL) and an aliquot of the supernatant (1 mL) was heated at 95 °C for 30 min and then quickly cooled on ice for 5 min. After cooling, the mixture was centrifuged and the absorbance of the supernatant was measured at 400, 500, and 600 nm. TBA-reactive substances (TBARS) were measured as MDA, a degraded product of lipids. The concentration of MDA was calculated from the absorbance values using the extinction coefficient of 155 mM cm−1.
Determination of Catalase (CAT) Activity
The activity of CAT was determined in the seedling stage after 10 days of germination. A quantity of 0.2 g of plant fresh tissue was crushed by using liquid nitrogen, and then, 1 mL of buffer Tris–HCl (0.05 M, pH 7.5) was added. The obtained mixture was centrifuged at 13,000 rpm for 20 min (4 °C), and then, the supernatant was used for measuring enzyme activity (Sudhakar et al. 2001). A volume of 60 µL of protein extract was added to Tris buffer (50 mM, pH 7) containing 5 mM H2O2 on the ice bath, and then, the absorbance curve was plotted at a wavelength of 240 nm. The enzyme activity was obtained for OD (optical density) mg−1 protein of fresh tissue. A quantity of 0.2 g of plant tissue was squashed with 0.6 mL extraction buffer and centrifuged at 11,500 rpm for 20 min (4 °C). The supernatant was transferred to new tubes and centrifuged at 4000 rpm for 20 min. The supernatant was then obtained. To measure the amount of protein, 10 µL of the supernatant was added to 5 mL Bradford solution and 290 µL extraction buffer. Then, the absorbance rate was read at 595 nm.
Determination of Peroxidase (POX) Activity
The assay mixture (6 mL) contained 300 µmol phosphate buffer (pH 6.8), 5 µmol pyrogallol, and 50 µmol H2O2, in which 1 mL of enzyme extract was diluted. The reaction was allowed to proceed for 5 min at 25 °C after which the reaction was stopped by adding 0.5 mL 5% (v/v) H2SO4. After centrifugation for 15 min at 14,000 × g, the amount of purpurogallin formed was determined from the A at 420 nm.
Data Analysis
Data were analyzed using the Statistical Analysis System (SAS) software (v. 9.1), and treatment means were considered significantly different at P < 0.05. Mean comparison was conducted using the least significant difference (LSD) test.
Results
Germination Parameters
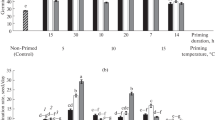
The effects of seed priming treatment, osmotic stress, and their interaction were significant on all germination traits (final germination, germination index, mean germination time, and coefficient of velocity of germination) (Table 1). Therefore, the interaction effects are presented. Values of germination index declined with low osmotic potential (Fig. 1a). However, this decline under low osmotic potential was less pronounced in primed seeds compared with non-primed controls. Urea priming had the greatest alleviating effect on the germination index under osmotic stress. Low osmotic potential increased mean germination time of seeds (Fig. 1b). However, primed seeds showed lower germination time compared with non-primed control. Urmia lake salt priming showed the lowest germination time under osmotic stress. Values of velocity of germination declined with low osmotic potential (Fig. 1c). However, this decline under low osmotic potential was less pronounced in primed seeds compared with non-primed control. Urmia lake salt priming showed the lowest germination time under osmotic stress. Germination percentage was reduced by 16.2%, 33.8%, 50.9%, and 74.9% under osmotic potential − 2, − 4, − 6, and − 8 bar, respectively, compared with non-stressed control (Fig. 2). However, this decline under low osmotic potential was less pronounced in primed seeds compared with non-primed control. Urmia lake salt priming and hydropriming showed the greatest germination percentages under osmotic stress. Evidently, seed priming treatments improved germination under low levels of osmotic stress.
Seedling Growth Parameters
The effects of seed priming treatment and osmotic stress were significant on seedling growth parameters (seedling length, seedling dry weight, and seedling vigor index) (Table 1). However, the interaction was not significant. Therefore, the main effects are presented. Seedling length was reduced linearly with decreasing osmotic potential (Fig. 3a). However, all seed priming treatments led to a significant increase in seedling length. Urmia lake salt priming had the greatest seedling lengths (Fig. 3b). Urea priming and hydropriming had the same effect on this trait. Similarly, seedling dry weight was reduced linearly with decreasing osmotic potential (Fig. 4a). However, all seed priming treatments led to a significant increase in seedling length (Fig. 4b). Similarly, Urmia lake salt priming provided the greatest seedling lengths, whereas urea priming and hydropriming had the same effect on this trait. SVI values declined with decreasing osmotic potential (Fig. 5a). However, SVI values were significantly increased under the influence of studied seed priming treatments (Fig. 5b). The greatest SVI values were obtained from Urmia lake salt priming.
Length of black cumin seedlings as affected a by osmotic potential (averaged over priming treatments) and b by priming treatment (LS Urmia lake salt priming, UR urea priming, HY hydropriming) (averaged over osmotic potentials) (different letters indicate statistically significant differences at P < 0.05)
Dry weight of black cumin seedlings as affected a by osmotic potential (averaged over priming treatments) and b by priming treatment (LS Urmia lake salt priming, UR urea priming, HY hydropriming) (averaged over osmotic potentials) (different letters indicate statistically significant differences at P < 0.05)
Vigor index of black cumin seedlings as affected a by osmotic potential (averaged over priming treatments) and b by priming treatment (LS Urmia lake salt priming, UR urea priming, HY hydropriming) (averaged over osmotic potentials) (different letters indicate statistically significant differences at P < 0.05)
MDA Content and Enzyme Activity
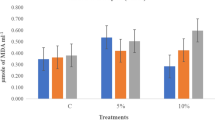
The effect of seed priming, osmotic stress, and their interaction on MDA content was significant (Table 2). Therefore, the interaction effects are presented (Fig. 6). MDA content was significantly increased with increasing osmotic stress, but priming reduced MDA content compared with non-primed control. Urmia lake salt priming had the greatest effect on reducing MDA content. The effect of seed priming and osmotic stress was significant on POX activity, but their interaction was not significant (Table 2). Therefore, the main effects are presented. POX activity increased linearly with increasing osmotic stress (Fig. 7a). POX activity in primed seeds was significantly higher than non-primed control (Fig. 7b). The highest levels of POX activity were noted in Urmia lake salt priming. The effect of seed priming, osmotic stress, and their interaction on catalase activity was significant (Table 2). Therefore, the interaction effects are presented (Fig. 8). Seed priming significantly increased CAT activity. Urmia lake salt priming had the greatest effect on the activity of this enzyme.
Peroxidase activity (POX) of black cumin seedlings (after 10 days of germination) as affected a by osmotic potential (averaged over priming treatments) and b by priming treatment (LS Urmia lake salt priming, UR urea priming, HY hydropriming) (averaged over osmotic potentials) (different letters indicate statistically significant differences at P < 0.05)
Discussion
This study sheds some light on the effects of different chemical priming treatments on seed germination and early growth of black cumin seedlings under osmotic stress. This is the first report of using chemical priming to alleviate the effects of osmotic stress on black cumin germination. Seed germination of black cumin and post-germination growth were largely reduced under osmotic stress compared with non-stressed control. Therefore, in any case, osmotic stress led to a significant reduction in black cumin seed vigor. However, improved values of germination index, reduced values of mean germination time, and increased coefficients of germination of velocity were observed under osmotic stress in primed seeds compared with non-primed control. Moreover, priming improved final germination percentage and showed higher values of seedling vigor index with decreasing osmotic potential levels. Finally, seed priming resulted in high activity levels of catalase and peroxidase, as osmotic potential levels decreased. In the light of the above findings, seed priming can play a major role in reducing the negative effects of osmotic stress during germination and seedling growth of back cumin. In particular, the positive impact of seed priming on germination and vigor of black cumin seedlings was increased with increasing levels of osmotic stress. However, this response was not sufficient to eliminate the adverse effects of stress on the process of germination and seedling growth.
Priming promotes germination rate probably by increasing cell division and inducing metabolic activities occurring in early seed germination in primed seeds (Taylor and Harman 1990; Bose and Mishra 1992). Furthermore, the final germination percentage of primed seeds was significantly improved compared with non-primed seeds. Catalase was an important enzyme in salt stress acclimation induced by hydrogen peroxide in maize (Gondim et al. 2012). In accordance with our results, plants with higher CAT activity showed lower malondialdehyde levels, which may result from the protective function of this enzyme (Gondim et al. 2012). Higher levels of antioxidant enzymes activity were reported in tolerant species under various environmental stresses compared with sensitive species (Bor et al. 2003; Demiral and Turkan 2005; Turkan et al. 2005). High levels of CAT and POX activity cause a decrease in H2O2 concentration in cells, contribute to membrane stabilization, and increase CO2 fixation. These effects could be possibly attributed to the sensitivity of chloroplastic enzymes to H2O2, which directly inhibits CO2 fixation (Yamazaki et al. 2003). MDA content is considered an indicator of membrane damage by environmental stresses, but lipid peroxidation is also linked to the activity of antioxidant enzymes. For instance, increase in CAT and POX activity enhances tolerance to oxidative stress, but the level of MDA is decreased.
Seed priming favors accumulation of K+ and Ca2+ in the seedlings and reduces Na+ and Cl− accumulation, thereby mitigating the harmful impact of saline conditions on seed germination and early growth of seedlings (Iqbal et al. 2006; Afzal et al. 2008; Bakht et al. 2011). This response lowers plant osmotic potential and promotes water absorption (Ashraf 2004). Potassium is a critical element for activating enzymes, adjusting cell turgor, and regulating osmotic pressure in plants cells (Cherel 2004). Calcium is important for cell division and cell elongation and is included to cell walls (Patade et al. 2011). In addition, calcium regulates nutrient uptake across cell membranes and enhances water absorption (Summart et al. 2010). Concerning sodium, the negative effect of this element on growth of plants is mitigated by calcium (Gobinathan et al. 2009). Therefore, seed priming with various inorganic salts promotes enzyme activity and alters the mobilization and distribution of organic substances in the embryo.
In the current study, Urmia lake salt priming had the greatest impact on improving seed germination and vigor indices, especially under osmotic stress conditions. This effect could be attributed to the high capacity for osmotic adjustment caused by seed priming with Urmia lake salt, probably through the promotion of K+ and Ca2+ accumulation in the primed seeds, a response which may further reflect the high tolerance of seedlings from primed seeds to salinity (Farhoudi and Sharifzadeh 2006). Nevertheless, further studies are required to shed light on the exact mechanisms underlying seedling tolerance to salinity from primed seeds and examine whether the beneficial effects of seed priming persist beyond the seedling stage. In the current study, seed priming did not completely eliminate the symptoms of osmotic stress in black cumin germination, yet it appeared as a quite efficient method to mitigate the impact of osmotic stress on germination of this species. In the light of the findings of this study, salt priming may be effective for successful and economic cultivation of black cumin and perhaps of other sensitive field crops under saline or drought stress regimes.
References
Abdul-Baki A, Anderson JD (1973) Vigor determination in soybean seed by multiple criteria. Crop Sci 13:630–633
Afzal I, Rauf S, Barsa SMA, Murtaza G (2008) Halopriming improves vigour, metabolism of reserves and ionic contents in wheat seedlings under salt stress. Plant Soil Environ 54:382–388
Ahmadzadeh Kokya T, Pejman AH, Mahin Abdollahzadeh E, Ahmadzadeh Kokya B, Nazariha M (2011) Evaluation of salt effects on some thermodynamic properties of Urmia Lake water. Int J Environ Res 5:343–348
Alipour S (2006) Hydrogeochemistry of seasonal variation of Urmia salt lake, Iran. Saline Syst 2:1–9
Amirnia R, Ghiyasi M (2016) Reducing drought stress effects in germination and establishment stage of cumin (Cuminum cyminum L.) by seed priming. J Appl Biol Sci 10:24–26
AOSA (1983) Seed vigour testing handbook. Association of Official Seed Analysts. Ithaca
Ashraf M (2004) Some important physiological selection criteria for salt tolerance in plants. Flora 199:361–376
Ashraf M, McNeilly T, Bradshaw AD (1986) The response of selected salt-tolerant and normal lines of four grass species to NaCl in sand culture. New Phytol 104:453–461
Bakht J, Shafi M, Jamal Y, Sher H (2011) Response of maize (Zea mays L.) to seed priming with NaCl and salinity stress. Span J Agric Res 9:252–261
Bor M, Ozdemir F, Turkan I (2003) The effect of salt stress on lipid peroxidation and antioxidants in leaves of sugar beet Beta vulgaris L. and wild beet Beta maritime L. Plant Sci 164:77–84
Bose B, Mishra T (1992) Response of wheat seed to pre sowing seed treatments with Mg(NO3). Ann Agric Res 13:132–136
Cherel L (2004) Regulation of K+ channel activities in plant from physiological to molecular aspects. J Exp Bot 55:337–351
Demiral T, Turkan I (2005) Comparative lipid peroxidation, antioxidant systems and proline content in roots of two rice cultivars differing in salt tolerance. Environ Exp Bot 53:247–257
Du Z, Bramlage WJ (1992) Modified thiobarbituric acid assay for measuring lipid oxidation in sugar-rich plant tissue extracts. J Agric Food Chem 40:1566–1570
Eimanifar A, Mohebbi F (2007) Urmia Lake (northwest Iran): a brief review. Saline Syst 3:1–5
Ellis RH, Roberts EH (1981) The quantification of aging and survival in orthodox seeds. Seed Sci Technol 9:373–409
Farhoudi R, Sharifzadeh F (2006) The effects of NaCl priming on salt tolerance in canola (Brassica napus L.) seedlings grown under saline conditions. Indian J Crop Sci 1:74–78
Ghiyasi M, Seyahjani AA, Tajbakhsh M, Amirnia R, Salehzadeh H (2008) Effect of osmopriming with polyethylene glycol (8000) on germination and seedling growth of wheat (Triticum aestivum L.) seeds under salt stress. Res J Biol Sci 3:1249–1251
Ghiyasi M, Amirnia R, Fazelimanesh M (2015) The effect of seed priming with different concentrations of ascorbic acid on germination and vigor of cumin (Cumin cyminum L.) under saline conditions. Thai J Agric Sci 48:179–785
Gholami M, Mokhtarian F, Baninasab B (2015) Seed halopriming improves the germination performance of black seed (Nigella sativa) under salinity stress conditions. J Crop Sci Biotechnol 18:21–26
Gobinathan P, Sankar B, Murali PV, Panneerselvam RN (2009) Effect of calcium chloride on salinity—induced oxidative stress in Pennisetum typoidies. Bot Res Int 2:143–148
Gondim FA, Gomes-Filho E, Costa JH, Mendes Alencar NL, Prisco JT (2012) Catalase plays a key role in salt stress acclimation induced by hydrogen peroxide pretreatment in maize. Plant Physiol Biochem 56:62–71
Hajar AS, Zidan MA, Al-Zahrani HS (1996) Effect of salinity stress on the germination, growth and some physiological activities of black cumin (Nigella sativa L.). Arab Gulf J Sci Res 14:445–454
Hasanuzzaman M, Nahar K, Fujita M (2012) Plant response to salt stress and role of exogenous protectants to mitigate salt-induced damages. In: Ahmad P et al. (eds) Ecophysiology and responses of plants under salt stress. Springer Science + Business Media, Berlin, pp 25–87
Iqbal M, Ashraf M, Jamil A, Ur-Rehman S (2006) Does seed priming induce changes in the levels of some endogenous plant hormones in hexaploid wheat plants under salt stress. J Integr Plant Biol 48:181–189
Maguire JD (1962) Speed of germination—aid in selection and evaluation for seedling emergence and vigour. Crop Sci 2:176–177
Michel BE, Kaufmann MR (1973) The osmotic potential of polyethylene glycol 6000. Plant Physiol 51:914–916
Papastylianou P, Bakogianni NN, Travlos I, Roussis I (2018) Sensitivity of seed germination to salt stress in black cumin (Nigella sativa L.). Not Bot Horti Agrobot Cluj-Napoca 46:202–205
Patade VY, Maya K, Zakwan A (2011) Seed priming mediated germination improvement and tolerance to subsequent exposure to cold and salt stress in capsicum. Res J Seed Sci 4:125–136
Salehzade H, Shishvan MI, Ghiyasi M, Forouzin F, Siyahjani AA (2009) Effect of seed priming on germination and seedling growth of wheat (Triticum aestivum L.). Res J of Biol Sci 4:629–631
Savvides A, Ali S, Tester M, Fotopoulos V (2016) Chemical priming of plants against multiple abiotic stresses: mission possible? Trends Plant Sci 21:329–340
Shalhevet J (1995) Using marginal quality water for crop production. Int Water Irrig Rev 15:5–10
Sudhakar C, Lakshmi A, Giridarakumar S (2001) Changes in the antioxidant enzyme efficacy in two high yielding genotypes of mulberry (Morus alba L.) under NaCl salinity. Plant Sci 161:613–619
Summart J, Thanonkeo P, Panichajakul S, Prathepha P, McManus MT (2010) Effect of salt stress on growth, inorganic ion and proline accumulation in Thai aromatic rice, Khao Dawk Mail 105, callus culture. Afr J Biotechnol 9:145–152
Tajbakhsh M, Ghiyasi M (2009) Seed ecology. Jahde-Daneshghahi. Urmia University, Urmia, Iran
Taylor AG, Harman GE (1990) Concepts and technologies of selected seed treatments. Ann Rev Phytopathol 28:321–339
Turkan I, Bor M, Ozdemir F, Koca H (2005) Differencial responses of lipid peroxidation and antioxidants in the leaves of drought-tolerant P. acutifolius gray and drought sensitive P. vulgaris L. subjected to polyethylene glycol mediated water stress. Plant Sci 168:223–231
Yamazaki J, Ohashi A, Hashimoto Y, Negishi E, Kumagai S, Kubo T, Oikawa T, Maruta E, Kamimura Y (2003) Effects of high light and low temperature during harsh winter on needle photodamage of Abies mariesii growing at the forest limit on Mt. Norikura in Central Japan. Plant Sci 165:257–264
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Ghiyasi, M., Siavash Moghaddam, S., Amirnia, R. et al. Chemical Priming with Salt and Urea Improves Germination and Seedling Growth of Black Cumin (Nigella sativa L.) under Osmotic Stress. J Plant Growth Regul 38, 1170–1178 (2019). https://doi.org/10.1007/s00344-019-09922-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00344-019-09922-z