Abstract
Plant growth-promoting bacteria recover the negative effects of osmotic stress on canola. For comprehending the role of bacteria in canola under osmotic stress, Sarigol and Hyola308 as drought-sensitive and -tolerant cultivars, respectively, inoculated with Enterobacter sp. S16-3 as plant growth-promoting bacteria were treated with polyethylene glycol. Bacterial inoculation increased the root volume of Hyola308 under osmotic stress. Using a gel-free/label-free proteomic technique, 115 commonly changed proteins were identified in the root between inoculated Sarigol and Hyola308 under osmotic stress. In both cultivars with bacterial inoculation, proteins related to protein metabolism, cell organization, and signaling were increased in roots under osmotic stress. On the other hand, bacterial inoculation increased and decreased the abundance of malate dehydrogenase (EC 1.1.1.37) in roots of Hyola308 and Sarigol, respectively, under severe osmotic stress. Furthermore, in leaf, bacterial inoculation increased the abundance of tricarboxylic acid cycle-related proteins in Hyola308 under severe osmotic stress. These results suggest that bacterial inoculation might increase the tolerance to severe osmotic stress by modifying proteins related to energy metabolism in both leaf and root. Enhancement of energy metabolism elicited by bacterial inoculation might provide a connection between cell metabolism and root growth, which might cause regulated growth and increased tolerance in drought-tolerant canola under osmotic stress.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Water scarcity due to drought stress induces osmotic stress (Blum 1989). Under these water fluctuations, crop plants show modifications in morphology, gas exchange, and defense mechanisms (Shao and others 2009). Application of polyethylene glycol (PEG) in a hydroponic solution results in osmotic stress, which subsequently leads to devaluation of plant growth and biomass production (Marcińska and others 2013). Canola has high sensitivity to water deficit (Bilibio and others 2014) and yield is decreased significantly under water stress (Shirani Rad and others 2013). Water stress decreases shoot/root dry weight and root volume, and increases chlorophyll content and fluorescence in canola (Nemati and others 2012). Root features are specialized for exploitation (absorption) of water from the soil and are highly sensitive to the soil water content (Lynch and Brown 2012). Aroca and others (2001) demonstrated the importance of root water uptake capacity in coping with several abiotic stresses. Seedlings with larger root volumes have a better ability to take up water (Carlson 1986). Root growth was decreased under water deficits (Hsiao 1973). These approaches indicated that drought negatively influences the growth of canola, and clarification of the response mechanisms of root and leaf separately to drought stress might help in the improvement of stress tolerance in canola.
The rhizosphere shelters many species of bacteria and fungi that have neutral, beneficial, and deleterious effects on the plant (Berendsen and others 2012). Plant growth-promoting rhizobacteria (PGPR) are aboriginal soil bacteria that colonize in the rhizosphere or plant roots and cause increase in plant growth (Kloepper and others 1989). PGPR adapt to unfavorable conditions and protect plants from the harmful effects of stresses (Marulanda and others 2007; Kasim and others 2013). Furthermore, PGPR have been reported to induce drought stress tolerance in root/shoot of canola (Heidari and others 2015), wheat (Kasim and others 2013), maize (Sandhya and others 2009), and sunflower (Sandhya and others 2010). It was demonstrated that inoculation with PGPR caused root elongation and increased water uptake from deeper soil layers, which resulted in better growth of plants under drought stress (Zahir and others 2008). Thus, it is important to comprehend the crucial mechanisms, which are involved in facilitating plant growth under drought stress with PGPR application.
Koh and others (2015) demonstrated that the number of proteins associated with metabolism, protein folding/degradation, and signaling was decreased; whereas the abundance of proteins related to energy, protein synthesis, stress, and defense was increased in canola leaf under drought stress. Cheng and others (2012) indicated that proteins related to photosynthesis, anti-oxidative processes, transportation, and pathogenesis were responsive to salt stress on canola inoculated with Pseudomonas putida UW4. Using Sarigol and Hyola308 as salt-sensitive and salt-tolerant cultivars indicated that photosynthesis-related proteins participated as a salt-tolerance factor in the adaptation of Hyola308 to stress (Bandehagh and others 2011).
Khalili and others (2012) demonstrated that Hyola308 was considered as a drought-tolerant cultivar according to the highest amount of stress tolerance index and grain yield; whereas Sarigol was considered as a drought-sensitive cultivar according to the lowest stress tolerance index and grain yield. Based on physiological and agronomic performances, Sarigol and Hyola308 were classified as drought-sensitive and drought-tolerant cultivars, respectively (Khalili and others 2012); however, their function under drought stress or bacterial inoculation is not clear. Therefore, these drought-sensitive and drought-tolerant cultivars are useful to investigate the tolerance mechanisms induced by bacterial inoculation under drought stress. Enterobacter cloacae was reported as a plant-growth enhancer with its own multiple growth-promoting activities and had the potential for solubilizing inorganic phosphate from insoluble compounds (Ramesh and others 2014).
To identify the combinational effects and impact of this bacterium on canola under osmotic stress, a gel-free/label-free proteomic technique was applied. The simultaneous analysis of root and leaf was provided to determine a complete view of the entire plant. The protein profiles of root and leaf of drought-tolerant Hyola308 and drought-sensitive Sarigol canola cultivars were compared to determine the drought-responsive mechanisms that are activated by PGPR and moderate the adverse effects of drought stress. The function and pathway mapping analyses were conducted to identify the role of the key proteins involved in the canola response to osmotic stress and the mechanisms involved in the drought tolerance of canola.
Materials and Methods
Plant Material
The seeds of canola (Brassica Napus L. cultivars Sarigol and Hyola308) were obtained from the Seed and Plant Improvement Institute of Iran. Sarigol and Hyola308 were provided as drought-sensitive and drought-tolerant canola cultivars, respectively (Khalili and others 2012). Identical homogenous seeds were sterilized in 3% sodium hypochlorite solution (Penrose and Glick 2003), sowed and kept on sand under white fluorescent light (14-h light period) in a growth chamber maintained at 27 °C.
Bacterial Strain
To evaluate the plant growth-promoting ability of rhizosphere strains, the following strains were used: Bacillus megaterium, Pseudomonas fluorescens, Pseudomonas putida, Enterobacter sp. C16-20, and Enterobacter sp. S16-3. Nutrient broth media were prepared for overnight culture. To determine the effect of stress on the growth of strains, overnight culture was used to inoculate new nutrient broth media containing 0, 5, and 10% stress by adding 0, 50, and 100 g/L NaCl. Triplicate flasks were used for each isolate, and the flasks were incubated at 28 °C in an incubator for 96 h. The growth density was analyzed at wavelength 600 nm. For inoculation, cells were shaken for 24 h at 28 °C on a shaker, and the density was adjusted to 108 cfu/mL using OD determination at 600 nm (Sarikhani and others 2016).
Phosphate Uptake
To specify bacterial influence on the phosphate uptake, a pot culture experiment was carried out in greenhouse conditions in the Agricultural Research Station of the University of Tabriz, Iran. Sand and muscovite (2 kg) in pots were sterilized, and the disinfected seeds were inoculated by bacterial inoculums (0.5 mL per seed) and sowed. To keep the soil water content close to field capacity and to ensure that water was not the limiting factor in nutrient uptake, Hoagland solution (Hoagland and Arnon 1950) was used for irrigation and nutrient supply. Concentration of phosphate was determined by a spectrophotometric assay according to the ammonium vanadate-molybdate method (Neves and others 2008). Phosphate uptake was calculated for each pot as the sum of nutrient contents of roots (Sarikhani and others 2016).
Polyethylene Glycol Treatment
For morphological and proteomic analyses, seven-day-old canola plants were transferred to a hydroponic system containing sterilized Hoagland solution (pH 6.5) (Hoagland and Arnon 1950). After the transplantation, 10 mL of bacterial suspension was injected to each reservoir containing 10 L of nutrient solution. Polyethylene glycol (PEG; Mr 6000) was used for induction of osmotic stress. One week after transplantation two levels of osmotic stress 0.6 and 1.2 MPa were introduced to the plants. The root volume of canola plants was measured as a morphological parameter. For all experiments, three independent experiments were performed as biological replicates. A biological replicate means that the plant sowing was performed on different days (Fig. S1). Roots and leaves were collected for proteomic analysis.
Protein Extraction, Enrichment, and Digestion for Mass Spectrometry Analysis
A portion (500 mg) of samples was ground in liquid nitrogen with a mortar and pestle. The powder was transferred to a solution containing 10% trichloroacetic acid and 0.07% 2-mercaptoethanol. Proteins were extracted based on the method by Komatsu and others (2013). Protein concentrations were determined using the Bradford assay (Bradford 1976) with bovine serum albumin as the standard. Proteins (100 µg) were enriched with methanol and chloroform to remove any detergent from the sample solutions based on the method by Nanjo and others (2012). The resulting supernatant was collected and analyzed by nano liquid chromatography (LC)-mass spectrometry (MS)/MS.
Mass Spectrometry Analysis
Peptides in 0.1% formic acid were loaded onto an Ultimate 3000 nanoLC system (Dionex, Germering, Germany) equipped with a C18 PepMap trap column (300 µm ID × 5 mm; Dionex) and were then separated by elution from the trap column using 0.1% formic acid in acetonitrile at a flow rate of 200 nL/min on a C18 Tip column (75 µm 1D × 120 mm; Nikkyo Technos, Tokyo, Japan) with a spray voltage of 1.8 kV. Peptide ions were analyzed on a nanospray LTQ Orbitrap MS (Thermo Fisher Scientific, San Jose, CA, USA) operated in data-dependent acquisition mode with Xcalibur software (version 2.1; Thermo Fisher Scientific). The operation of the MS was the same as the method by Komatsu and others (2013).
Protein Identification from Mass Spectrometry Data
Identification of proteins was performed using the Mascot search engine (version 2.5.1; Matrix Science, London, UK) and Proteome Discoverer software (version 1.4.0.288; Thermo Fisher Scientific) against an Arabidopsis peptide database (Tair10, http://www.arabidopsis.org/). The acquired raw data files were processed and converted to Mascot generic files using Proteome Discoverer software. The parameters used in the Mascot searches were as follows: cysteine carbamidomethylation/methionine oxidation was a fixed modification/variable modification; trypsin was specified as the proteolytic enzyme; 1 missed cleavage was allowed; peptide mass tolerance was 10 ppm; fragment mass tolerance was 0.8 Da; and peptide charges were + 2, + 3, and + 4. An automatic decoy database search was performed as part of the search. Mascot results were filtered with the Mascot percolator to improve the accuracy and sensitivity of peptide identification (Brosch and others 2009). False discovery rates for peptide identification of all searches were less than 1.0%. Peptides with a percolator ion score of more than 13 (p < 0.05) were used for protein identification.
Analysis of Differentially Abundant Proteins using Mass Spectrometry Data
The Mascot search results were exported in msf format for SIEVE analysis (version 2.1.49; Thermo Fisher Scientific). The relative abundances of peptides and proteins were compared between samples. For the analysis, the chromatographic peaks detected by MS were aligned, and the peptide peaks were detected as a frame using a frame time width of 5 min and a frame m/z width of 10 ppm. All produced frames for the parent ions were scanned by MS/MS. Chromatographic peak areas of each sample within a single frame were compared and the ratios between samples in each frame were determined. The frames detected in the MS/MS scan were matched to the imported Mascot search results. The ratio of peptides between samples was determined from the relative variance-weighted average of the ratios in the frames that matched the peptides in the MS/MS spectrum. The ratios of the peptides were further integrated to determine the ratios of the corresponding proteins. In the differential analysis of protein abundance, total ion current was used for normalization. The minimum requirement for the identification of a protein was 2 matched peptides and p < 0.05.
Functional Annotation
Protein functions were categorized using MapMan bin codes (http://mapman.gabipd.org/) (Usadel and others 2005). Visualization of protein abundance was performed using MapMan software (Usadel and others 2009). Pathway mapping of identified proteins was performed using the Kyoto Encyclopedia of Genes and Genomes (KEGG) database (http://www.genome.jp/kegg/) (Kanehisa and Goto 2000).
Statistical Analysis
Statistical significance was evaluated by the Student’s t test when only two groups were compared or one-way ANOVA followed by Tukey’s test when multiple groups were compared. SPSS statistical software (version 22.0; IBM, Armonk, NY, USA) was used for statistical evaluation. A p value of less than 0.05 was considered to be statistically significant.
Results
Selection of Tolerant Plant Growth-Promoting Rhizobacteria
To investigate rhizosphere strains for plant growth-promoting ability, different phosphate-solubilizing bacteria were used. Phosphate uptake was analyzed by using five kinds of bacteria strains in canola root. Phosphate uptake of canola root inoculated with Enterobacter sp. S16-3 and C16-20 was significantly increased compared to inoculation by other strains (Fig. 1a). Furthermore, bacterial growth optical density was analyzed to assess osmotic stress tolerance. The evaluation of Enterobacter sp. S16-3 and C16-20 for stress tolerance indicated that growth of C16-20 significantly increased compared to S16-3 in no stress. However, the growth of S16-3 increased in density at 5 and 10% NaCl; and growth of C16-20 was dramatically reduced at 5% and 10% NaCl (Fig. 1b). Overall, high growth of Enterobacter sp. S16-3 with a large range of tolerance to osmotic stress was an important potential for usage as PGPR.
Evaluation of plant growth-promoting activity and abiotic stress tolerance of rhizosphere strains. Bacillus megaterium, Pseudomonas fluorescens, Pseudomonas putida, Enterobacter sp. C16-20, and Enterobacter sp. S16-3 were used as phosphate-solubilizing rhizobacteria. After inoculation with bacteria, phosphate uptake was measured in roots (a). Optical density was measured for potential of bacteria without or with 5 and 10% NaCl (b). Data are shown as the mean ± SD from three independent biological replicates. Different letters indicate significant changes measured by one-way ANOVA followed by Tukey’s test (p < 0.05). Asterisks indicate significant changes measured by Student’s t test (**p < 0.01)
Effect of Osmotic Stress and Bacterial Inoculation on Root of Canola Cultivars
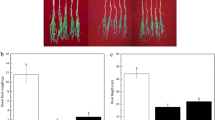
Bacterial inoculation increased root volume compared to without inoculation in both Sarigol and Hyola308 in no stress. The root volume of Sarigol did not change with bacterial inoculation under 0.6 MPa osmotic stress, whereas the root volume of Hyola308 was increased with bacterial inoculation under 0.6 MPa osmotic stress. Under 1.2 MPa osmotic stress with bacterial inoculation root volume remained unchanged. Bacterial inoculation caused an increase in root volume of Hyola308 under both 0.6 and 1.2 MPa osmotic stresses. Bacterial inoculation was efficient on Hyola308 in all conditions (Fig. 2).
Root volume of canola inoculated with bacteria under osmotic stress. Seven-day-old Sarigol and Hyola308 were inoculated without or with Enterobacter sp. S16-3 and treated without or with 0.6 or 1.2 MPa osmotic stress. The root volume was measured as a morphological parameter. Data are shown as the mean ± SD from three independent biological replicates. Asterisks indicate significant changes measured by Student′s t test (*p < 0.05)
Functional Classification of Significantly Modified Proteins in Root
A total of 344 and 258 proteins were identified in root of Sarigol with bacterial non-inoculation and inoculation, respectively (Tables S1 and S2). In addition, a total of 21 and 182 proteins were identified in Hyola308 with bacterial non-inoculation and inoculation, respectively (Tables S3 and S4). In Sarigol without bacterial inoculation, the functional categories were protein metabolism (25%), signaling (11%), and cell organization (9%) (Fig. 3a, Tables S1). However, in Hyola308 without bacterial inoculation the functional categories were cell organization (10%), secondary metabolism (14%), and stress (10%) (Fig. 3a). In Sarigol with bacterial inoculation, the main functional categories were protein metabolism (26%) and cell organization (11%) (Fig. 3b). Furthermore, in Hyola308 with bacterial inoculation, the main functional categories were protein metabolism (22%) and cell organization (14%) (Fig. 3b). The protein abundance of the tricarboxylic acid cycle was increased under 0.6 MPa osmotic stress, however, in Sarigol and Hyola308 it was clearly decreased under 1.2 MPa osmotic stress (Fig. 3b).
Functional categorization of proteins identified in non-inoculated and inoculated canola roots under osmotic stress. Seven-day-old Sarigol and Hyola308 were inoculated without (a) or with bacteria (b), and treated without or with 0.6 (M) and 1.2 MPa (S) osmotic stress. Protein functions were categorized using MapMan bin codes. The number of proteins that increased (black column) and decreased (white column) are shown. protein protein synthesis/folding/degradation/targeting, cell cell organization/vesicle transport/cycle/division, TCA tricarboxylic acid cycle, mitoETC mitochondrial electron transport chains, CHO carbohydrate, redox redox ascorbate/glutathione metabolism, and OPP oxidative pentose phosphate. Others contain biodegradation of xenobiotics, gluconeogenesis, metal handling binding, N-metabolism, nucleotide metabolism, C1 metabolism, S-assimilation, hormone metabolism, development, photosynthesis, and tetrapyrrole synthesis
Pathway Analysis of Identified Proteins of Sarigol and Hyola308
The analysis identified the main functional categories of the significantly changed proteins: tricarboxylic acid cycle and glycolysis (Fig. 4). In Sarigol with bacterial inoculation under 0.6 MPa osmotic stress, the proteins related to the tricarboxylic acid cycle and glycolysis were increased; however, they were unchanged under 1.2 MPa osmotic stress (Fig. 4a). In Hyola308 with bacterial inoculation, proteins related to the tricarboxylic acid cycle and glycolysis were increased under 0.6 MPa osmotic stress; and unchanged under 1.2 MPa osmotic stress (Fig. 4b).
Abundance of proteins related to primary metabolism identified in canola root with bacterial inoculation under osmotic stress. Seven-day-old Sarigol and Hyola308 were inoculated without or with bacteria; and treated without or with 0.6 and 1.2 MPa osmotic stress. The abundance changes of proteins grouped into the functional categories were visualized using MapMan software to obtain an overview of affected metabolic processes. Each square and color indicates the fold change value of protein abundance in comparison to no stress in Sarigol (a) and Hyola308 (b). Red, green, and white colors indicate an increase, decrease, and no change, respectively
Glycolysis and Tricarboxylic Acid Cycle Pathway Differences in Root of Canola Cultivars under Osmotic Stress
In Sarigol with bacterial inoculation, 0.6 MPa osmotic stress increased the abundance of proteins related to glycolysis and tricarboxylic acid cycle pathways (Fig. 5a; Table 1). Furthermore, the abundance of fructose-bisphosphate aldolase, glyceraldehyde-3-phosphate dehydrogenase, phosphoglycerate kinase, dihydrolipoamide dehydrogenase, phosphoenolpyruvate carboxylase, and oxoglutarate dehydrogenase was decreased under 1.2 MPa osmotic stress (Fig. 5a). The abundance of ATP citrate (pro-S)-lyase remained unchanged under osmotic stress in Sarigol.
Metabolic pathway of proteins identified in canola root with bacterial inoculation under osmotic stress. Seven-day-old Sarigol and Hyola308 were inoculated with bacteria; and treated without or with 0.6 and 1.2 MPa osmotic stress. Glycolysis and tricarboxylic acid cycle pathways were analyzed using KEGG database for identified proteins in canola root. The changes of protein abundance are shown in Sarigol (a) and Hyola308 (b) with bacterial inoculation exposed to 0.6 and 1.2 MPa osmotic stress. Black and white colors indicate an increase and a decrease, respectively. Left and right side colored boxes refer to 0.6 and 1.2 MPa osmotic stress, respectively
The abundance of fructose-bisphosphate aldolase, glyceraldehyde-3-phosphate dehydrogenase, phosphoglycerate kinase, malate dehydrogenase, aconitate hydratase, isocitrate dehydrogenase, oxoglutarate dehydrogenase, and dihydrolipoamide dehydrogenase was increased in glycolysis and tricarboxylic acid cycle pathways in Hyola308 under 0.6 MPa osmotic stress (Fig. 5b; Table 1). The abundance of glyceraldehyde-3-phosphate dehydrogenase, phosphoglycerate kinase, malate dehydrogenase, isocitrate dehydrogenase, and succinate dehydrogenase was increased under 1.2 MPa osmotic stress. The abundance of fructose-bisphosphate aldolase, ATP citrate (pro-S)-lyase, dihydrolipoamide dehydrogenase, and oxoglutarate dehydrogenase was decreased in Hyola308 under 1.2 MPa osmotic stress. In contrast with Sarigol, the abundance of glyceraldehyde-3-phosphate dehydrogenase, malate dehydrogenase, and phosphoglycerate kinase was increased in Hyola308 (Fig. 5b).
Functional Classification of Significantly Modified Proteins in Leaf
A total of 22 and 210 proteins were identified in leaf of Sarigol and Hyola308 with bacterial inoculation, respectively (Tables S5 and S6). Under osmotic stress, 10 and 198 proteins were specific to inoculated Sarigol and Hyola308, respectively; and 12 proteins were commonly detected between Sarigol and Hyola308 with bacterial inoculation (Fig. 6). To determine the biological processes involved in tolerance and gain further insight into the effects of bacterial inoculation on canola cultivars exposed to osmotic stress, the identified proteins were functionally classified using MapMan bin codes (Fig. 6). In leaf of Sarigol with bacterial inoculation, the main functional categories were photosynthesis (25%), glycolysis (11%), and redox (9%) (Fig. 6, Tables S5); whereas in Hyola308 the main functional categories were protein metabolism (10%), photosynthesis (14%), and signaling (10%) (Fig. 6, Tables S6). In leaf of Sarigol with bacterial inoculation, 0.6 and 1.2 MPa osmotic stresses changed the abundance of proteins related to glycolysis and tricarboxylic acid cycle pathways (Fig. 7). The abundance of glyceraldehyde-3-phosphate dehydrogenase, transaldolase, ATP citrate (pro-S)-lyase, malate dehydrogenase, and phosphoenolpyruvate carboxylase was decreased under 1.2 MPa osmotic stress (Fig. 7a).
Functional categorization of proteins identified in inoculated canola leaf under osmotic stress. Seven-day-old Sarigol and Hyola308 were inoculated with bacteria, and treated without or with 0.6 and 1.2 MPa osmotic stress. Protein functions were categorized using MapMan bin codes. The number of proteins that increased (black column) and decreased (white column) are shown. protein protein synthesis/folding/degradation/targeting, cell cell organization/vesicle transport/cycle/division, redox redox ascorbate/glutathione metabolism. Others contain fermentation, gluconeogenesis, C1-metabolism, DNA, RNA, mitochondrial electron transport, major CHO metabolism, tetrapyrrole synthesis, and secondary metabolism
Metabolic pathway of proteins identified in canola leaf with bacterial inoculation under osmotic stress. Seven-day-old Sarigol and Hyola308 were inoculated with bacteria; and treated without or with 0.6 and 1.2 MPa osmotic stress. Leaves of canola cultivars were collected, proteins were extracted, and then analyzed using nanoLC-MS/MS. Glycolysis and tricarboxylic acid cycle pathways were analyzed using KEGG database for proteins identified in canola leaf. The changes of protein abundance are shown in Sarigol (a) and Hyola308 (b) with bacterial inoculation exposed to 0.6 and 1.2 MPa osmotic stress in compared to no stress. Black and white colors indicate an increase and a decrease, respectively. Left and right side colored boxes refer to 0.6 and 1.2 MPa osmotic stress, respectively
Furthermore, the abundance of glyceraldehyde-3-phosphate dehydrogenase, phosphoglycerate kinase, enolase, malate dehydrogenase, and isocitrate dehydrogenase was increased in glycolysis and tricarboxylic acid cycle pathways in leaf of Hyola308 under 0.6 and 1.2 MPa osmotic stress. The abundance of succinate dehydrogenase was decreased in Hyola308 under osmotic stress (Fig. 7b).
Glyceraldehyde-3-phosphate dehydrogenase was decreased in leaf of Sarigol under 0.6 and 1.2 MPa osmotic stresses, whereas in root it just decreased under 1.2 MPa osmotic stress. Glyceraldehyde-3-phosphate dehydrogenase was increased in both leaf and root of Hyola308, under osmotic stress (Figs. 5, 7). The abundance of ATP citrate (pro-S)-lyase was decreased in leaf of Sarigol and remained unchanged in root under stress. However, in Hyola308, the abundance of ATP citrate (pro-S)-lyase was not changed in leaf and the majority was decreased in root under osmotic stress (Figs. 5, 7).
Discussion
Abiotic Stress Tolerance and Phosphate Uptake Potential of Enterobacter sp. S16-3
For alleviation of abiotic stress effects on plants, selection of bacteria with multi-functional traits such as tolerance to abiotic stresses and PGPR characteristics is important. Root phosphate uptake of canola inoculated by different phosphate-solubilizing bacteria was measured, and Enterobacter sp. S16-3 with the highest effect on phosphate uptake was selected (Fig. 1). The high activity of Enterobacter sp. S16-3 led to higher availability of phosphate content in the soil, consequently, resulting in increased nutrient uptake and ultimately reflected on the growth. The present results are analogues with the approach of Kucey and others (1989) who demonstrated that inoculation with Penicillium bilaji as a PGPR increased phosphate uptake and subsequently enhanced the growth of canola roots. By inoculation with phosphate solubilizers, a similar occurrence of increasing phosphate uptake in pepper and cucumber (Han and others 2006), rice (Stephen and others 2015), and maize (Abdelmoneim and others 2014) was observed.
Bacterial tolerance for survival, multiplication, and spread of bacterial strains is considerable in abiotic stresses in agricultural soils. Garcia and Hernandez (1996) reported that salinity negatively affected biological activity by high osmotic stress which caused toxic effects on microbial growth with the exception of salt-tolerant bacteria. The present results demonstrated that Enterobacter sp. S16-3 was tolerant to higher concentrations of salt (10%) in comparison to Enterobacter sp. C16-20.
Nautiyal and others (2000) reported that among the four strains of phosphate-solubilizing bacteria, NBRI2601 was the most efficient strain in terms of its capability to solubilize phosphorus in the presence of 10% salt. In another investigation, Bacillus sp. strains showed optimum phosphate solubilization just at 2.5% salt concentration (Banerjee and others 2010). The present results are consistent with the findings of Bernard and others (1986), who indicated that tolerance of different bacteria to salt varied from 0·1 to 0·75 mol and the response of bacteria to salt was strain dependent (Cherif-Silini and others 2013). These results suggested that analysis of bacterial-isolates’ ability to increase phosphate uptake in plants and their abiotic stress tolerance might be a valuable basis for the usage of bacteria isolates as PGPR to increase crop production under stress.
Enterobacter sp. was reported as a plant-growth enhancer because of its multiple growth-promoting activities (Deepa and others 2010). In the present study, inoculation by Enterobacter sp. caused an increase in root volume of drought-tolerant canola under stress (Fig. 2). Kim and others (2014) reported that the newly isolated Enterobacter sp. EJ01 was a PGPR and alleviated salt stress in Arabidopsis thaliana. Plants inoculation by PGPR altered root proliferation/metabolism and improved mineral/water uptake (Vacheron and others 2013). Creus and others (2005) indicated that after inoculation with Azospirillum brasilense, tomato root displayed a significant increase in number/length of root hairs, and rate of appearance and root surface area were analogous with the present results. Taken together, it is suggested that the positive effects of PGPR might be derived from roots with an enhanced capacity for mineral and water uptake under osmotic stress.
Bacterial Inoculation Effects on Cell and Energy Metabolisms
Plant growth-promoting rhizobacteria utilize several mechanisms to induce abiotic stress tolerance in plants (Dimkpa and others 2009). To investigate the stress tolerance inducted by bacterial inoculation and its beneficial effect on canola under osmotic stress, drought-sensitive and drought-tolerant canola cultivar responses were explored using a proteomic approach. In both Sarigol and Hyola308 roots with bacterial inoculation, the main functional categories were related to protein metabolism, cell organization, and signaling under osmotic stress (Fig. 3). The abundance of cell wall synthesis-related proteins was increased in roots of Pearl millet as a stress-tolerant plant (Ghatak and others 2016). In wheat, most of the modified proteins related to cell metabolism/elongation caused a regulated root growth under water deficit (Zhu and others 2006). The present results were consistent with the findings of Banaei-Asl and others (2015), who indicated that bacterial inoculation of roots increased the tolerance to abiotic stress by alteration proteins related to energy metabolism and cell division. Taken together, it is suggested that PGPR might improve the endurance to stress and support root growth in canola by increasing abundance of protein and cell metabolism-related proteins.
Although the response mechanism of inoculated canola was identified under salt stress (Banaei-Asl and others 2015, 2016), under drought stress the response mechanism of inoculated canola was not clarified. The functional categorization depicted that proteins related to energy metabolism were the most abundantly changed pathways under bacterial inoculation in both salt-stressed canola cultivars (Banaei-Asl and others 2015), which was in common with the present results (Fig. 5). Rizhsky and others (2002) indicated that transcripts encoding glycolysis and pentose phosphate pathway enzymes were induced under drought stress. Botha and Small (1985) also reported that glycolysis, pentose phosphate, and tricarboxylic acid cycle pathways were not inhibited in the water-stressed plant. It is suggested that adequate energy is a prerequisite for roots to deal with osmotic stress with increasing levels of metabolism in glycolysis and the tricarboxylic acid cycle.
Induction of Tricarboxylic Acid Cycle by Osmotic Stress
Cramer and others (2013) indicated that protein abundance related to the tricarboxylic acid cycle was increased by water deficit. Several enzymes related to ATP-generating pathways were induced on osmotic stress in cultured cells of rice (Umeda and others 1994). Malate dehydrogenase reversibly catalyzes the incorporation of malate to oxaloacetate (Musrati and others 1998). The expression levels of the malate dehydrogenases isoforms changes under abiotic stresses (Scheibe 2004). Zhou and others (2009) reported that malate dehydrogenase was suppressed in sensitive tomato root under aluminum stress. Banaei-Asl and others (2015) also demonstrated that malate dehydrogenase decreased in the root of sensitive canola inoculated with bacteria under salt stress. Furthermore, Soussi and others (1998) reported that malate dehydrogenase increased in salt-tolerant chickpea leaves inoculated with a Mesorhizobium ciceri strain under salt stress. Consistent with these approaches, the abundance of malate dehydrogenase decreased in leaf and root of the drought-sensitive cultivar and increased in the drought-tolerant cultivar under osmotic stress (Figs. 5, 7). It is suggested that malate dehydrogenase might be considered as a key enzyme to evaluate drought tolerance in canola cultivars.
ATP citrate lyase harvested the fixed carbon as acetyl-CoA, and regenerated the oxaloacetate required to continue the tricarboxylic acid cycle (Fatland and others 2002). ATP citrate lyase suppression affected plant phenotypes and inhibited root elongation (Fatland and others 2005). In the present study, the abundance of ATP citrate lyase decreased in leaves of Sarigol, whereas it did not change in roots under osmotic stress. In Hyola308, the abundance of ATP citrate lyase was not changed in leaves, whereas it changed in roots under osmotic stress (Figs. 5, 7). In contrast, reports by Suh and others (2001) demonstrated that fungal infection of pepper induced the accumulation of an ATP citrate lyase homolog. Wang and others (2016) also reported that the ATP citrate lyase gene was up-regulated during heavy metal stress of radish root. It is suggested that unchanged ATP citrate lyase might keep the activity of tricarboxylic acid cycle for adaptation to osmotic stress in leaf of drought-tolerant canola with bacterial inoculation.
The Alternative Changes in Glycolysis-Related Proteins
Glycolysis is a complex network containing alternative enzymatic reactions, which facilitates plant development and acclimation to environmental stress (Pu and others 2015). The present results indicated that the abundance of fructose-bisphosphate aldolase and glyceraldehyde-3-phosphate dehydrogenase decreased and increased in roots of Sarigol and Hyola308, respectively; whereas they remained unchanged in leaves of Sarigol and Hyola308 (Figs. 5, 7). Fructose-bisphosphate aldolase is a key enzyme in the pathways of glycolysis. It was reported that fructose-bisphosphate aldolase abundance was decreased in root and shoot of drought-sensitive cultivars of rice (Ghaffari and others 2014) and creeping bent grass (Xu and others 2010), but was increased in tolerant cultivars (Gong and others 2010), which was consistent with our present results. It is suggested that bacterial inoculation induced fructose-bisphosphate aldolase in leaves of drought-tolerant canola and energy was maintained for cell metabolism, changing its abundance in root and leaf.
Glyceraldehyde 3-phosphate dehydrogenase catalyzes a key step in glycolysis that breaks down glucose into carbon and energy. Glyceraldehyde-3-phosphate dehydrogenase had a significantly positive correlation with drought tolerance (Degenkolbe and others 2013): overexpression of glyceraldehyde-3-phosphate dehydrogenase in potato resulted in the improvement of drought tolerance (Kappachery and others 2015). Merewitz and others (2011) reported that in root and leaf of drought-tolerant creeping bent grass, glyceraldehyde-3-phosphate dehydrogenase abundance increased under drought stress, which was in common with the present results. The current study with previous findings suggests that bacterial inoculation keeps energy metabolism stable in root compared to leaf by increasing the abundance of glyceraldehyde-3-phosphate dehydrogenase and improves endurance to osmotic stress.
Conclusions
Osmotic stress reduces growth and productivity of plants (Farooq and others 2009). Root water uptake capacity plays a critical role in coping with abiotic stresses (Aroca and others 2001). PGPR are beneficial native soil bacteria that colonize plant roots and result in increased plant growth (Kloepper and others 1989). To investigate the effect of bacterial inoculation on canola cultivars under osmotic stress, a proteomic approach was carried out in roots and leaves of drought-sensitive and drought-tolerant canola. The main findings of present study are as follows: (i) Enterobacter sp. S16-3 as a phosphate-solubilizing PGPR improved the capacity for phosphor uptake of canola; (ii) Root volume of drought-tolerant canola did not change under osmotic stress and was improved by bacterial inoculation; (iii) Bacterial inoculation affected proteins related to energy and cell metabolism in roots of canola under osmotic stress; and (iv) Severe osmotic stress induced proteins related to the tricarboxylic acid cycle and glycolysis in leaves and roots of drought-tolerant canola with bacteria inoculation. Taken together, it is suggested that bacterial inoculation might improve a synchrony in drought-tolerant canola by improving the abundance of proteins related to energy metabolism in roots and leaves. Enhancement of energy metabolism elicited by bacterial inoculation might provide a connection between cell metabolism and root growth, which might cause a regulated growth and increased tolerance under osmotic stress.
Abbreviations
- LC:
-
Liquid chromatography
- MS:
-
Mass spectrometry
- PGPR:
-
Plant growth-promoting rhizobacteria
- PEG:
-
Polyethylene glycol
- ROS:
-
Reactive oxygen species
References
Abdelmoneim TS, Tarek Moussa AA, Almaghrabi OA, Alzahrani HS, Abdelbag I (2014) Increasing plant tolerance to drought stress by inoculation with arbuscular mycorrhizal fungi. Life Sci J 11:10–17
Aroca R, Tognoni F, Irigoyen JJ, Sànchez-Díaz M, Pardossi A (2001) Different root low temperature response of two maize genotypes differing in chilling sensitivity. Plant Physiol Biochem 39:1067–1073
Banaei-Asl F, Bandehagh A, Uliaei ED, Farajzadeh D, Sakata K, Mustafa G, Komatsu S (2015) Proteomic analysis of canola root inoculated with bacteria under salt stress. J Proteom 124:88–111
Banaei-Asl F, Farajzadeh D, Bandehagh A, Komatsu S (2016) Comprehensive proteomic analysis of canola leaf inoculated with a plant growth-promoting bacterium, Pseudomonas fluorescens, under salt stress. Biochim Biophys Acta 1864:1222–1236
Bandehagh A, Salekdeh GH, Toorchi M, Mohammadi A, Komatsu S (2011) Comparative proteomic analysis of canola leaves under salinity stress. Proteomics 11:1965–1975
Banerjee S, Palit R, Sengupta C, Standing D (2010) Stress induced phosphate solubilization by Arthrobacter sp. and Bacillus sp. isolated from tomato rhizosphere. Aus J Crop Sci 4:378–383
Berendsen RL, Pieterse CM, Bakker PAHM (2012) The rhizosphere microbiome and plant health. Trends Plant Sci 17:78–486
Bernard T, Pocard J, Perroud B, Le Rudulier D (1986) Variation in the response of salt stressed Rhizobium strains to betaines. Arch Microbiol 143:359–364
Bilibio C, Carvalho JDA, Hensel O, Fraga AC, Richter U, Rezende F (2014) Effects of different soil water tensions on rapeseed crops (Brassica napus L.). Agric Eng Int CIGR J 16:1–11
Blum A (1989) Osmotic adjustment and growth of barley genotypes under drought stress. Crop Sci 29:230–233
Botha FC, Small JGC (1985) Effect of water stress on the carbohydrate metabolism of Citrullus lanatus seeds during germination. Plant Physiol 77:79–82
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Brosch M, Yu L, Hubbard T, Choudhary J (2009) Accurate and sensitive peptide identification with mascot percolator. J Proteome Res 8:3176–3181
Carlson WC (1986) Root system considerations in the quality of loblolly pine seedlings. S J Appl For 10:87–92
Cheng Z, Woody OZ, McConkey BJ, Glick BR (2012) Combined effects of the plant growth-promoting bacterium Pseudomonas putida UW4 and salinity stress on the Brassica napus proteome. Appl Soil Ecol 61:255–263
Cherif-Silini H, Silini A, Ghoul M, Yahiaoui B, Arif F (2013) Solubilization of phosphate by the Bacillus under salt stress and in the presence of osmoprotectant compounds. Afr J Microbiol Res 7:4562–4571
Cramer GR, van Sluyter SC, Hopper DW, Pascovici D, Keighley T, Haynes PA (2013) Proteomic analysis indicates massive changes in metabolism prior to the inhibition of growth and photosynthesis of grapevine (Vitis vinifera L.) in response to water deficit. BMC Plant Biol 21:13–49
Creus CM, Graziano M, Casanovas EM, Pereyra MA, Simontacchi M, Puntarulo S, Barassi CA, Lamattina L (2005) Nitric oxide is involved in the Azospirillum brasilense-induced lateral root formation in tomato. Planta 221:297–303
Deepa CK, Dastager SG, Pande A (2010) Isolation and characterization of plant growth promoting bacteria from non-rhizospheric soil and their effect on cowpea Vigna unguiculata L. Walp. seedling growth. World J Microbiol Biotech 26:1233–1240
Degenkolbe T, Do PT, Kopka J, Zuther E, Hincha DK, Köhl KI (2013) Identification of drought tolerance markers in a diverse population of rice cultivars by expression and metabolite profiling. PLoS ONE 8:e63637
Dimkpa CO, Merten D, Svatoš A, Büchel G, Kothe E (2009) Metal-induced oxidative stress impacting plant growth in contaminated soil is alleviated by microbial siderophores. Soil Biol Biochem 41:154–162
Farooq MA, Wahid N, Kobayashi D, Fujita SM, Basra A (2009) Plant drought stress: effects, mechanisms and management. Agron Sustain Dev 29:185–212
Fatland BL, Ke J, Anderson MD, Mentzen WI, Cui LW, Allred CC, Johnston JL, Nikolau BJ, Wurtele ES (2002) Molecular characterization of a heteromeric ATP-citrate lyase that generates cytosolic acetyl-coenzyme A in Arabidopsis. Plant Physiol 130:740–756
Fatland BL, Nikolau BJ, Wurtele ES (2005) Reverse genetic characterization of cytosolic acetyl-CoA generation by ATP-citrate lyase in Arabidopsis. Plant Cell 17:182–203
Garcia C, Hernandez T (1996) Influence of salinity on the biological and biochemical activity of a calciorthird soil. Plant Soil 178:255–263
Ghaffari A, Gharechahi J, Nakhoda B, Salekdeh GH (2014) Physiology and proteome responses of two contrasting rice mutants and their wild type parent under salt stress conditions at the vegetative stage. J Plant Physiol 171:31–44
Ghatak A, Chaturvedi P, Nagler M, Roustan V, Lyon D, Bachmann G, Postl W, Schröfl A, Desai N, Varshney RK, Weckwerth W (2016) Comprehensive tissue-specific proteome analysis of drought stress responses in Pennisetum glaucum (L.) R. Br. (Pearl millet). J Proteomics 143:122–135
Gong P, Zhang J, Li H, Yang C, Zhang C, Zhang X, Khurram Z, Zhang Y, Wang T, Fei Z, Ye Z (2010) Transcriptional profiles of drought-responsive genes in modulating transcription signal transduction, and biochemical pathways in tomato. J Exp Bot 61:3563–3575
Han HS, Supanjani K, Lee D (2006) Effect of co-inoculation with phosphate and potassium solubilizing bacteria on mineral uptake and growth of pepper and cucumber. Plant Soil Environ 52:130–136
Heidari F, Bandehagh A, Farajzadeh D, Kazemi Oskuei B, Motie Noparvar P (2015) Response of spring canola (Brassica napus L.) cultivars inoculated with P. fluorescens FY 32 to drought stress. Crop Res 50:55–62
Hoagland DR, Arnon DI (1950) The water culture method for growing plants without soil. Circular: California Agricultural Experiment Station 347
Hsiao TC (1973) Plant response to water stress. Ann Rev Plant Physiol 24:519–570
Kanehisa M, Goto S (2000) KEGG: Kyoto encyclopedia of genes and genomes. Nucleic Acids Res 28:27–30
Kappachery S, Baniekal Hiremath G, Yu JW, Park SW (2015) Effect of over-and under-expression of glyceraldehyde3-phosphate dehydrogenase on tolerance of plants to water-deficit stress. Plant Cell Tissue Organ Cult 121:97–107
Kasim WA, Osman ME, Omar MN, Abd El-Daim IA, Bejai S, Meijer J (2013) Control of drought stress in wheat using plant-growth-promoting bacteria. J Plant Growth Regul 32:122–130
Khalili M, Naghavi MR, Pour Aboughadareh A, Talebzadeh SJ (2012) Evaluating of drought stress tolerance based on selection indices in spring canola cultivars (Brassica napus L.). J Agri Sci 4:11
Kim K, Jang YJ, Lee SM, Oh BT, Chae JC, Lee KJ (2014) Alleviation of salt stress by Enterobacter sp. EJ01 in tomato and Arabidopsis is accompanied by up-regulation of conserved salinity responsive factors in plants. Mol Cells 37:109–117
Kloepper JW, Lifshitz R, Zablotowicz RM (1989) Free-living bacterial inocula for enhancing crop productivity. Trends Biotechnol 7:39–43
Koh J, Chen G, Yoo MJ, Zhu N, Dufresne D, Erickson JE, Chen S (2015) Comparative proteomic analysis of Brassica napus in response to drought stress. J Proteome Res 14:3068–3081
Komatsu S, Han C, Nanjo Y, Altaf-Un-Nahar M, Wang K, He D, Yang P (2013) Label-free quantitative proteomic analysis of abscisic acid effect in early-stage soybean under flooding. J Proteome Res 12:4769–4784
Kucey RMN, Janzen HH, Leggett ME (1989) Microbially mediated increases in plant-available phosphorus. Adv Agron 42:199–228
Lynch JP, Brown KM (2012) New roots for agriculture: exploiting the root phenome. Phil Trans R Soc B 367:1598–1604
Marcińska I, Czyczyło-Mysza I, Skrzypek E, Grzesiak MT, Janowiak F, Filek M, Dziurka M, Dziurka K, Waligórski P, Juzoń K, Cyganek K, Grzesiak S (2013) Alleviation of osmotic stress effects by exogenous application of salicylic or abscisic acid on wheat seedlings. Int J Mol Sci 14:13171–13193
Marulanda A, Porcel R, Barea JM, Azcon R (2007) Drought tolerance and antioxidant activities in lavender plants colonized by native drought-tolerant or drought-sensitive Glomus species. Microb Ecol 54:543–552
Merewitz EB, Gianfagna T, Huang B (2011) Protein accumulation in leaves and roots associated with improved drought tolerance in creeping bentgrass expressing an ipt gene for cytokinin synthesis. J Exp Bot 62:5311–5333
Musrati RA, Kollarova M, Mernik N, Mikulasova D (1998) Malate dehydrogenase: distribution, function and properties. Gen Physiol Biophys 17:193–210
Nanjo Y, Skultety L, Uváčková L, Klubicová K, Hajduch M, Komatsu S (2012) Mass spectrometry-based analysis of proteomic changes in the root tips of flooded soybean seedlings. J Proteom Res 11:372–385
Nautiyal CS, Bhaduria S, Kumar P, Lal H, Mondal R, Verma D (2000) Stress induced phosphate solubilization in bacteria isolated from alkaline soils. FEMS Microbiol Lett 182:291–296
Nemati M, Asghari A, Sofalian O, Rasoulzadeh A, Mohamaddoust Chamanabad H (2012) Effect of water stress on rapeseed cultivars using morpho-physiological traits and their relations with ISSR markers. J Plant Physiol Breed 2:55–66
Neves MSAC, Souto MRS, Tóth IV, Victal SMA, Drumond MC, Rangel AOSS (2008) Spectrophotometric flow system using vanadomolybdophosphate detection chemistry and a liquid waveguide capillary cell for the determination of phosphate with improved sensitivity in surface and ground water samples. Talanta 77:527–532
Penrose DM, Glick BR (2003) Methods for isolating and characterizing ACC-deaminase containing plant growth promoting rhizobacteria. J Plant Physiol 118:10–18
Pu X, Lv X, Tan T, Fu F, Qin G, Lin H (2015) Roles of mitochondrial energy dissipation systems in plant development and acclimation to stress. Ann Bot 116:583–600
Ramesh A, Sharma SK, Sharma MP, Yadav N, Joshi OP (2014) Plant growth-promoting traits in Enterobacter cloacae subsp. Dissolvens MDSR9 isolated from soybean rhizosphere and its impact on growth and nutrition of soybean and wheat upon inoculation. Agric Res 31:53–66
Rizhsky L, Liang H, Mittler R (2002) The combined effect of drought stress and heat shock on gene expression in tobacco. Plant Physiol 130:1143–1151
Sandhya V, Ali SZ, Grover M, Reddy G, Venkateswarlu B (2009) Alleviation of drought stress effects in sunflower seedlings by exopolysaccharides producing Pseudomonas putida strain P45. Biol Fert Soils 46:17–26
Sandhya V, Ali SZ, Grover M, Reddy G, Venkateswarlu B (2010) Effect of plant growth promoting Pseudomonas spp. on compatible solutes, antioxidant status and plant growth of maize under drought stress. Plant Growth Regul 62:21–30
Sarikhani MR, Ebrahimi M, Oustan S, Aliasgharzad N, Madani O (2016) Isolation of potassium releasing bacteria from soil and assessment of its ability in potassium nutrition of tomato. 2nd international conference on integrated environmental management for sustainable development pp 235–251
Scheibe R (2004) Malate valves to balance cellular energy supply. Physiol Plant 120:21–26
Shao HB, Chu LY, Abdul Jaleel C, Manivannan P, Panneerselvam R, Shao MA (2009) Understanding water deficit stress-induced changes in the basic metabolism of higher plants-biotechnologically and sustainably improving agriculture and the ecoenvironment in arid regions of the globe. Crit Rev Biotechnol 29:131–151
Shirani Rad AH, Abbasian A, Aminpanah H (2013) Evaluation of rapeseed (Brassica napus L.) cultivars for resistance against water deficit stress. Bulg J Agric Sci 19:266–273
Soussi M, Ocana A, Lluch C (1998) Effects of salt stress on growth, photosynthesis and nitrogen fixation in chick-pea (Cicer arietinum L.). J Exp Bot 49:1329–1337
Stephen J, Shabanamol S, Rishad KS, Jisha MS (2015) Growth enhancement of rice (Oryza sativa) by phosphate solubilizing Gluconacetobacter sp. (MTCC 8368) and Burkholderia sp. (MTCC 8369) under greenhouse conditions. Biotech 5:831–837
Suh MC, Yi SY, Lee S, Sim W, Pai HS, Choi D (2001) Pathogen-induced expression of plant ATP:citrate lyase. FEBS Lett 488:211–212
Umeda M, Hara C, Matsubayashi Y, Li HH, Liu Q, Tadokoro F, Aotsuka S, Uchimiya H (1994) Expressed sequence tags from cultured cells of rice (Oryza sativa L.) under stressed conditions: analysis of genes engaged in ATP-generating pathways. Plant Mol Biol 25:469–478
Usadel B, Nagel A, Thimm O, Redestig H, Blaesing OE, Palacios-Rofas N, Selbig J, Hannemann J, Piques MC, Steinhauser D, Scheible WR, Gibon Y, Morcuende R, Weicht D, Meyer S, Stitt M (2005) Extension of the visualization tool MapMan to allow statistical analysis of arrays, display of corresponding genes, and comparison with known responses. Plant Physiol 138:1195–1204
Usadel B, Obayashi T, Mutwil M, Giorgi FM, Bassel GW, Tanimoto M, Chow A, Steinhauser D, Persson S, Provart NJ (2009) Co-expression tools for plant biology: opportunities for hypothesis generation and caveats. Plant Cell Environ 32:1633–1651
Vacheron J, Desbrosses G, Bouffaud ML, Touraine B, Moënne-Loccoz Y, Muller D, Legendre L, Wisniewski-Dyé F, Prigent-Combaret C (2013) Plant growth-promoting rhizobacteria and root system functioning. Front Plant Sci 17:356
Wang Y, Xu L, Tang M, Jiang H, Chen W, Zhang W, Wang R, Liu L (2016) Functional and integrative analysis of the proteomic profile of radish root under Pb exposure. Front Plant Sci 7:1871
Xu C, Sibicky T, Huang B (2010) Protein profile analysis of salt responsive proteins in leaves and roots in two cultivars of creeping bent grass differing in salinity tolerance. Plant Cell Rep 29:595–615
Zahir ZA, Munir A, Asghar HN, Arshad M, Shaharoona B (2008) Effectiveness of rhizobacteria containing ACC-deaminase for growth promotion of peas (Pisum sativum) under drought conditions. J Microbiol Biotechnol 18:958–963
Zhou S, Sauve R, Fish T, Thannhauser T (2009) Proteome changes induced by aluminium stress in tomato roots. J Exp Bot 60:1849–1857
Zhu KX, Zhou HM, Qian HF (2006) Antioxidant and free radical scavenging activities of Wheat Germ Protein Hydrolysates (WGPH) prepared with Alcalase. Proc Biochem 4:1296–1302
Acknowledgements
The authors thank Mr. T. Gasemzade from University of Tabriz for useful assistance. The authors also thank Ms. X. Wang from the National Institute of Crop Science for valuable discussions. BKO was partially supported by a scholarship from University of Tabriz.
Author information
Authors and Affiliations
Contributions
KOB, BA, SMR, and SK initiated and designed the project. KOB, BA, SMR, and SK carried out the experiments. Bacterial experiment was performed by SMR and proteomic analysis was performed by KOB and SK. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Additional information
Accession Code The mass spectrometry proteomics data have been deposited with the ProteomeXchange Consortium (http://proteomecentral.Proteomexchange.org) via the PRIDE partner repository (Vizcaino and others 2013) under the dataset identifiers PXD005707 and PXD005784.
Electronic supplementary material
Below is the link to the electronic supplementary material.
344_2017_9754_MOESM1_ESM.pptx
Fig. S1. Experimental design for proteomic, morphological, and enzymatic analyses of canola root cultivars under osmotic stress (PPTX 39 KB)
344_2017_9754_MOESM2_ESM.docx
Table S1. List of changed proteins in non-inoculated drought-sensitive Sarigol roots treated without or with osmotic stress (DOCX 70 KB)
344_2017_9754_MOESM3_ESM.docx
Table S2. List of changed proteins in inoculated drought-sensitive Sarigol roots treated without or with osmotic stress (DOCX 51 KB)
344_2017_9754_MOESM4_ESM.docx
Table S3. List of changed proteins in non-inoculated drought-tolerant Hyola308 roots treated without or with osmotic stress (DOCX 20 KB)
344_2017_9754_MOESM5_ESM.docx
Table S4. List of changed proteins in inoculated drought-tolerant Hyola308 roots treated without or with osmotic stress (DOCX 43 KB)
344_2017_9754_MOESM6_ESM.docx
Table S5. List of changed proteins in inoculated drought-sensitive Sarigol leaves treated without or with osmotic stress (DOCX 19 KB)
344_2017_9754_MOESM7_ESM.docx
Table S6. List of changed proteins in inoculated drought-tolerant Hyola308 leaves treated without or with osmotic stress (DOCX 37 KB)
Rights and permissions
About this article
Cite this article
Oskuei, B.K., Bandehagh, A., Sarikhani, M.R. et al. Protein Profiles Underlying the Effect of Plant Growth-Promoting Rhizobacteria on Canola under Osmotic Stress. J Plant Growth Regul 37, 560–574 (2018). https://doi.org/10.1007/s00344-017-9754-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00344-017-9754-y