Abstract
Legumes can host rhizobia and mycorrhizal fungi, and this triple symbiosis might be exploited to improve saline soil fertility. Therefore, a greater understanding of the interaction of rhizobia and arbuscular mycorrhizal fungus during legume growth in saline soil is required. We investigated the efficiency of salt tolerance conferred by rhizobia in mycorrhizal Sesbania cannabina. Greenhouse experiments were conducted in which S. cannabina plants inoculated with Glomus mosseae BGC NM03D (GM), and two rhizobia strains Agrobacterium pusense YIC4105 (4105) and Neorhizobium huautlense YIC4083 (4083), were exposed to 100 and 200 mM NaCl. Under 200 mM NaCl stress, plants inoculated with 4105, rather than 4083, showed significant increases in shoot and root dry mass compared with non-inoculated plants. Simultaneously, a significant increase over GM-inoculated plants in mycorrhizal colonization and dependency was recorded for 4105 + GM-inoculated plants compared with 4083 + GM-inoculated plants. In addition, under NaCl stress, significant increases in the number and mass of nodules, nitrogenase activity, and leghemoglobin content of nodules occurred in 4105 + GM-inoculated plants compared with 4083 + GM-inoculated plants. Furthermore, the activities of antioxidant enzymes in rhizobia-inoculated plants were significantly higher in the GM + 4105 group than the 4083 + GM group. The malondialdehyde content of plants from the 4105 + GM group was significantly lower than in the 4083 + GM group. Thus, the results revealed a synergistic relationship among the 4105 and GM in alleviating salt stress in S. cannabina. Salt-tolerant rhizobia might improve the salinity tolerance of S. cannabina by enhancing the antioxidant system.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Soil salinity is one of the most widespread threats to agricultural productivity in arid and semiarid areas (Ghazi and Al-Karaki 2006). Currently used techniques, such as improved irrigation practices, changing the soil, and using salt-tolerant plants for farming in salt-affected soils, are either expensive or time consuming (Munns 2002). However, using bioprocesses such as mycorrhizae and root nodules may represent a relatively cost-effective and ecologically friendly method to improve saline soils.
Arbuscular mycorrhizal (AM) fungi exist widely in saline soils, and inoculation with AM fungi improves growth of almost every terrestrial plant under saline stress conditions (Al-Karaki and others 2001; Heikham and others 2009). These fungi have been considered bio-ameliorators in a variety of saline soils (Singh and others 1997; Rao 1998). Rhizobial bacteria can form an exclusive symbiotic relationship with legumes by fixing atmospheric nitrogen to supply the plant with ammonium. They contribute not only to the N sources of leguminous crops, but also to the N content of the soil, and therefore have a key role in environmentally friendly agricultural practices (Puppo and others 2005). Legumes can grow under nitrogen-limiting conditions because of their ability to establish endosymbiosis with rhizobia. Sesbania cannabina is a recognized soil-improving legume, used as green manure to increase the yield of many crops. It is widely adaptable to different adverse climatic conditions, such as soil salinity, drought, and waterlogging. Therefore, rhizobia–S. cannabina plant–AM fungi tripartite symbiosis might represent a good strategy for improving saline soil fertility and helping to reintroduce agriculture to these lands.
Many pieces of evidence indicate that rhizobia and AM fungi improve the salinity tolerance of legumes (Rosa and others 2012; Carmen and Roberto 2009). However, studies on how the salt tolerance of these microbe symbionts affects the host plant are limited. Under long-term saline stress, some AM fungi species survive by ecophysiological adaption (Copeman and others 1996; Weissenhorn and others 1993; Camprubi and Calvet 1996; del Val and others 1999). It is plausible that the AM fungi that are able to survive in saline soils should be considered tolerant species and might be better able to improve the survival and growth of host plants than species from non-saline conditions. However, Tian and others (2004) reported that AM fungi from saline soils do not have a higher capacity to alleviate saline stress in plants than fungi from non-saline soils.
To the best of our knowledge, there have been no reports demonstrating whether the salt tolerance of rhizobia contributes to the survival of plants under salt stress with AM fungi. Any relationship between salt-tolerant rhizobia and AM fungi also remains to be determined. In this study, we inoculated S. cannabina plants with a combination of Glomus mosseae and two species of rhizobia with significant differences in their salt tolerance to explore this issue.
Materials and Methods
Preparation of Inocula
Rhizobia strains were isolated from Sesbania cannabina plants collected from DongYing, ShanDong province (37.76°N, 118.98°E). The isolates were grown on yeast extract mannitol agar (YEMA) medium and incubated at 28°C (Vincent 1970). DNA was extracted from bacterial cultures using the sodium dodecyl sulfate/cetyl trimethylammonium bromide lysis and phenol/chloroform extraction method. The housekeeping gene recA was amplified using the following primers:
-
recA-41F:TTC GGC AAG GGM TCG RTS ATG
-
recA-640R:ACA TSA CRC CGA TCT TCA TGC
After the approximately 600-bp recA amplicons were clustered and compared using MEGA5.05 program, 18 representative genotypes were selected. These isolates were designated as YIC4009, YIC4027, YIC4031, YIC4032, YIC4056, YIC4071, YIC4072, YIC4083, YIC4103, YIC4104, YIC4105, YIC4108, YIC4121, YIC4260, YIC4261, YIC5077, YIC5079, and YIC5082 (Fig. S1).
Then, the approximately 1500-bp 16S rDNA genes were amplified from the 18 representative strains using the following primers:
-
16S-27F: AGA GTT TGA TCC TGG CTC AG
-
16S-1492R: AAG GAG GTG ATT CCA GCC
The sequences were edited and assembled using BioEdit 7.0 (http://www.mbio.ncsu.edu/BioEdit/bioedit.html). BLAST searches were performed using the NCBI server (www.ncbi.nlm.nih.gov/blast/Blast.cgi). A phylogenetic tree was constructed from the 16S rRNA gene sequences in comparison with 16SrRNA gene sequences from several standard bacterial strains (Figure S1). Two genospecies, characterized as Agrobacterium pusense YIC4105 (4105) and Neorhizobium huautlense YIC4083 (4083), were selected based on their salt-tolerance traits and capacity to promote S. cannabina growth.
The original inoculum of the AM fungi Glomus mosseae (GM, BGC NM03D) provided by the Institute of Plant Nutrition and Resources (Beijing, China) was propagated in pot culture on Trifolium repens for 8 weeks. The spore intensity was 116 spores per 10 ml inoculum.
Experimental Design and Biological Treatments
The experiment had a 6 × 3 factorial design which was composed of six inoculation treatments and three salinity levels. Six inoculation treatments were non-inoculation control (control), inoculations with AM fungi, and rhizobia individually and in combination (GM, 4083, 4105, GM + 4083, and GM + 4105). Three salinity levels were 0, 100, and 200 mM NaCl. Treatments were completely randomized and replicated three times. The NaCl solution was added to the medium at a rate of 100 ml/7 days, five times in total. Seeds of Sesbania cannabina (Retz.) Pers. (Shandong Academy of Agricultural Sciences, Shandong, China) were sterilized in 10 % hydrogen peroxide for 10 min and rinsed several times with distilled water before use. Ten seeds were germinated at 28 °C for 48 h and sown (thinned to three uniform seedlings after germination) in a single 2-L pot containing autoclaved zonolite in a greenhouse with day/night temperatures of 30/22 °C, 60 ± 2 % relative humidity, and a photoperiod of 14/10 h light/dark. AM inoculums and rhizobia were applied as the seeds were sown. 1/4 Hoagland solution (Hoagland 1950) was supplied regularly and the pots were weighed every week to adjust the water content. After five weeks, plants were harvested for analysis.
Mycorrhizal Colonization and Dependency
The percentage of mycorrhizal colonization in the roots was calculated by the gridline intersection method (Giovannetti and Mosse 1980), after staining with trypan blue (Phillips and Hayman 1970).
According to Plenchette and others (1983), the mycorrhizal dependency was calculated as follows:
where ‘M’ represents the mycorrhizal plants and ‘NM’ the non-mycorrhizal plants.
Nitrogenase Activity and Leghemoglobin Content of Nodules
The excised nodulated roots were placed in 100-ml bottles sealed with rubber plugs. Ten ml of air was taken out and the equivalent amount of acetylene gas was injected into the bottle, which was incubated at 28 °C for 4 h. Gas samples (100 μl) from the bottles were then injected into a gas chromatograph (7890A series GC, Hewlett-Packard, Palo Alto, CA, USA) equipped with a flame ionization detector and an HP-PLOT AL2O3/KCL column (30 m, 0.25 mm, 5 um; Agilent, Santa Clara, CA, USA) was used. A calibration curve was constructed using pure ethylene. After the nitrogenase activity was determined, the nodules of each root were counted and measured for fresh and dry mass.
One gram of fresh nodules was ground with 5 ml of distilled water. The homogenate was centrifuged at 12,000×g for 15 min. Leghemoglobin in the supernatant was determined by the method of Hartree (1957), which is based upon the conversion of hematin to pyridine hemochromogen. A standard curve was prepared using graded concentrations of hemin. Unless stated otherwise, all reagents used in present study were purchased from Sigma-Aldrich (St. Louis, MO, USA).
Superoxide Dismutase Activity
The assay is based on the formation of formazone, which absorbs at 560 nm, by nitroblue tetrazolium and O2 − radical. Superoxide dismutase (SOD; E.C.1.15.1.1) decreases this absorbance by reducing the formation of the O2 − radical (Dhindsa and others 1981). Leaves from S. cannabina frozen in liquid nitrogen were ground with phosphate buffer, centrifuged at 4 °C, and the supernatant was used as the enzyme sample. The reaction mixture contained 14.5 mM methionine, 100 μM nitroblue tetrazolium chloride, 0.1 mM EDTA-Na2, 50 mM phosphate buffer (pH 7.8), 50 mM sodium carbonate, and 50 μl of enzyme. The reaction was started by adding 2.25 μM riboflavin and illuminating. A complete reaction mixture without enzyme served as the control. Switching off the light stopped the reaction. A non-irradiated complete reaction mixture served as the blank. One unit of enzyme activity was taken as the amount of enzyme that reduced the absorbance reading by 50 % in comparison with the control.
Catalase Activity
The catalase activity (CAT; E.C. 1.11.1.6) assay is based on a decrease in the absorbance of H2O2 at 240 nm over a time period, according to Aebi (1984). The reaction mixture consisted of 50 mM (pH 7.0) phosphate buffer, 12.5 mM hydrogen peroxide, and 50 μl of enzyme. Adding H2O2 started the reaction. Enzyme activity was computed by calculating the amount of H2O2 decomposed in 1 min. A standard curve was prepared using graded concentrations of H2O2. The enzyme activity was represented as the H2O2 reduced per min per mg protein.
Peroxidase Activity
The peroxidase activity (POX; E.C. 1.11.1.7) assay is based on the increase in optical density caused by the oxidation of guaiacol to tetraguaiacol (Castillo and others 1984). The reaction mixture contained 8 mM guaiacol, 5 mM H2O2, 100 mM phosphate buffer (pH 6.4), and 150 μl of enzyme extract. Absorbance from the formation of tetraguaiacol was recorded at 470 nm; the extinction coefficient ε was 26.6 mM−1 cm−1. The enzyme activity was represented as μmol tetraguaiacol formed per min per g fresh weight (FW).
Ascorbate Peroxidase Activity
The ascorbate peroxidase (APOX; E.C. 1.11.1.11) activity assay is based on the decrease in absorbance of ascorbic acid at 290 nm resulting from its oxidation (Nakano and Asada 1981). The enzyme sample was prepared with 1 mM ascorbic acid in addition to the extraction buffer. The reaction mixture contained 50 mM phosphate buffer (pH 7.0), 0.5 mM ascorbic acid, 0.1 mM EDTA-Na2, 0.2 mM H2O2, and 100 μl of enzyme. The reaction was started with the addition of 0.2 ml H2O2. The decrease in absorbance at 290 nm for 30 s was measured. A standard curve was prepared using graded concentrations of ascorbic acid. The enzyme activity was represented as the concentration of ascorbic acid oxidized per min per mg protein.
Glutathione Reductase Activity
The glutathione reductase (GR; E.C. 1.11.1.9) activity assay is based on the formation of a colored complex that absorbs at 412 nm by the reduction of glutathione with 5, 5-dithiobis-2-nitrobenzoic acid (DTNB) (Smith and others 1988). The reaction mixture contained 50 mM phosphate buffer (pH 7.5) and 0.1 mM EDTA, 0.5 mM DTNB, 0.5 mM NADPH, 5 mM GSSG (oxidized glutathione), and 100 μl of enzyme. The reaction was started by adding 2 mmol GSSG. The activity was expressed as total absorbance at 412 nm (Abs. 412) per mg protein per min.
Determination of Malondialdehyde Content
Lipid peroxidation was determined by measuring malondialdehyde (MDA) formation, using the thiobarbituric acid reaction described by Sudhakar and others (2001). The colored complex absorbs at 532 nm. Leaves from Sesbania cannabina were homogenized in 0.1 % trichloroacetic acid. The homogenate was centrifuged at 13,000×g for 20 min. The supernatant was used to estimate the MDA content. Thiobarbituric acid (TBA) in 20 % TCA was added to the supernatant. The mixture was heated at 95 °C for 20 min and then cooled in an ice bath. After centrifugation at 10,000×g for 10 min, the absorbance of the supernatant was recorded. Non-specific absorption values recorded at 600 nm were subtracted from the values recorded at 532 nm. The MDA content was calculated according to its extinction coefficient, ε (155 mM−1 cm−1).
Statistical Analyses
Data were compiled using Microsoft Excel (Redmond, WA, USA). Values are represented as means ± SDs of three replicates for each treatment. Student’s t test, one-way ANOVA, and Duncan’s multiple range test were used to identify significant differences, using SPSS ver. 13.0 (SPSS Inc., Chicago, IL, USA).
Results
Effects on Plant Growth
Two rhizobia, 4105 and 4083, with significant differences in salt tolerance, were isolated and selected for inoculation (Fig. 1). These two strains had similar capacities to promote S. cannabina growth: the increases in shoot and root dry mass of 4105- and 4083-inoculated plants were not significantly in 0 mM NaCl (Table 1).
As shown in Table 1, the inhibitory effect of salinity on shoot and root dry mass accumulation of S. cannabina increased as the NaCl concentration increased. NaCl at 100 and 200 mM induced a 25.3 and 51.8 % decline in dry matter accumulation of S. cannabina plants, respectively, compared with plants grown in 0 mM NaCl. In the single inoculation group, 4105 and GM, but not 4083, induced a clear improvement in dry mass accumulation of S. cannabina plants under 200 mM NaCl treatment, compared with non-inoculated plants. In the dual inoculation group, 4105 + GM was more efficient than 4083 + GM in increasing plant growth under salinity stress. Under 100 and 200 mM NaCl, the increase of 4105 + GM plants over non-inoculated plants was 2.54 and 2.38 times, whereas the increases of 4083 + GM plants were 1.93 and 1.71 times, respectively, (Table 1).
Notably, GM promoted stronger increases in root dry mass compared with shoot dry mass. This effect also seemed to occur in dual-inoculated plants (Table 1).
Mycorrhizal Colonization and Dependency
The results of mycorrhizal colonization showed that different levels of NaCl did not affect the colonization ability of GM significantly. The mycorrhizal infection of S. cannabina roots observed under different saline concentrations was not significantly different (Fig. 2a). Moreover, the colonization rate was even higher when the mycorrhizal plants were inoculated with 4105 compared with 4083 under 100 and 200 mM NaCl (Fig. 2a).
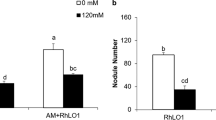
Glomus mosseae mycorrhizal colonization (%) and mycorrhizal dependency (%) of Sesbania cannabina plants inoculated with rhizobium under NaCl stress. a Percentage of Glomus mosseae colonization of the roots of Sesbania cannabina plants inoculated with Rhizobium pusense YIC4105 and Rhizobium huautlense YIC4083. b Mycorrhizal dependency (%) of Sesbania cannabina plants inoculated with Rhizobium pusense YIC4105 and Rhizobium huautlense YIC4083. Bars with different letters among the inoculations within an NaCl level are significantly different, and with those different numbers among salinity levels within the same inoculation are significantly different (one-way ANOVA, Duncan’s multiple range test, P < 0.05)
The mycorrhizal dependency was calculated in mycorrhizal plants with or without rhizobial inoculation. It is clear that the maximum mycorrhizal dependency of dry mass was obtained at the higher NaCl level of 200 mM. However, the mycorrhizal dependency of 4083-inoculated plants was not significantly different from GM-inoculated plants, while the mycorrhizal dependency of 4105-inoculated plants was 39 % higher than mycorrhizal plants under NaCl stress of 200 mM (Fig. 2b). The results above indicated that 4105 had greater capacity than 4083 to promote S. cannabina growth under salt stress in a single inoculation and in a double inoculation with GM.
Effect of Arbuscular Mycorrhizal Fungi on Nodulation and Nitrogen Fixation
To study the effect of AM fungi on rhizobia in dual-inoculated S. cannabina, nodulation and nitrogen fixation were investigated.
Data presented in Table 2 revealed that the number and mass of nodules significantly decreased under treatment with 200 mM NaCl in rhizobia-inoculated and dual-inoculated plants. Interestingly, dual inoculation of 4105 and GM increased the average number of nodules by 11.33 compared with the 4105 plants, whereas dual inoculation with 4083 and GM increased the average number of nodules by 5 compared with the 4083 plants. Total nodule weight per plant followed a similar pattern to the number of nodules.
Exposing the plants to NaCl stress of 100 and 200 mM resulted in a sharp reduction in the leghemoglobin content in rhizobia-inoculated plants. However, significantly less damage to the leghemoglobin protein was recorded in 4105 + GM-inoculated plants under stress compared with 4105 + GM- and 4105-inoculated plants. Nodule activity, according to the ethylene transformation from acetylene, corresponded to the reduction in nodule dry mass and leghemoglobin content under salt stress (Table 2). The efficiency of S. cannabina–4105 symbiosis increased significantly and the symbiotic performance was better in the 4105-inoculated than the 4083-inoculated plants under salt stress (Table 2).
Antioxidant Enzyme Activities
Salt stress could lead to the excessive production of reactive oxygen species (ROS), such as superoxide O2 − radicals and hydrogen peroxide H2O2, resulting in nodule senescence. Antioxidant enzymes, such as SOD, CAT, and POX, play key roles in the detoxification of ROS (Moran and others 2003). ROS-generating and activated antioxidant systems have been found not only at biotic/abiotic stress sites but also in distal leaf, acting as systemic signal (Freeman 2003; Martha and Clarence 1999). To exclude the ROS interference in AM fungal tissues, shoots instead of roots were used for measurements of antioxidant enzymes activities. The results showed that SOD activity (Fig. 3a) was elevated in response to moderate (100 mM) and heavy (200 mM) salt stress in 4105 + GM-inoculated plants. NaCl levels of 100 and 200 mM increased the SOD activity by 1.52 and 3.99 times, respectively, in 4105 + GM-inoculated plants compared with the corresponding plants at 0 mM. Higher SOD activity (1.93 times) was also observed in 4105 plants at 100 mM, but not at 200 mM NaCl, compared with non-stressed plants (Fig. 3a). The SOD activity of 4105 + GM plants was significantly higher than that in 4083 + GM plants. Increased levels of CAT and POX (Fig. 3b, c) activities were also observed in 4105 + GM-inoculated plants compared with non-inoculated plants subjected to moderate to heavy salt stress. The presence of 4105 and GM in the plant root increased CAT activity by 1.35 and 3.24 times at 100 and 200 mM, respectively, relative to non-stressed controls. The POX activity of stressed 4105 + GM-inoculated plants was 1.23 and 2.07 times higher than the corresponding non-stressed controls, at 100 and 200 mM, respectively. The dual inoculations of 4083 and GM also increased CAT and POX activity under saline stress, but not to as high a level as 4105 + GM-inoculation. SOD and POX activities of 4105 + GM plants were significantly higher than those in 4083 + GM plants (Fig. 3b, c).
Effect of AM inoculation on the antioxidant enzymes activity of Sesbania cannabina plants inoculated with rhizobium and Glomus mosseae under salt stress. a Effect of AM inoculation on the superoxide dismutase (SOD) activity (units mg−1 protein min−1) of Sesbania cannabina plants. b Effect of AM inoculation on the catalase (CAT) activity (nmol H2O2 reduced mg−1 prot. min−1) of Sesbania cannabina plants. c Effect of AM inoculation on the peroxidase (POX) activity (nmol tetraguaiacol formed min−1 g−1FW) of Sesbania cannabina plants. d Effect of AM inoculation on the ascorbate peroxidase (APOX) activity (l mol asc. acid oxidized min−1 mg−1 protein) of Sesbania cannabina plants. e Effect of AM inoculation on the glutathione reductase (GR) activity (absorbance at 412 nm mg−1 protein min−1) of Sesbania cannabina plants. Bars with different letters among inoculations within NaCl levels are significantly different (one-way ANOVA, Duncan’s multiple range test, P < 0.05)
Glutathione ascorbate peroxidase (APOX) and reductase (GR), which are enzymes involved in the ascorbate–glutathione pathway that is responsible for the removal of H2O2, were also investigated. No significant increase in APOX activity was recorded in single inoculation of rhizobia or in dual inoculations of rhizobia and arbuscular mycorrhizal fungi (Fig. 3d). An increase in GR activity was recorded only in 4083 and 4105 + GM-inoculated plants under heavy salt stress (Fig. 3e). However, the difference between the APOX and GR activities in 4105 + GM and 4083 + GM-inoculated plants was not significant.
Lipid Peroxidation
Salt stress-induced accumulation of ROS leads to oxidation damage of cell components, such as peroxidation of polyunsaturated fatty acids in membranes via free radical reactions. The results shown in Fig. 4 revealed that only the MDA content of 4105 + GM-inoculated plants was not significantly increased compared with corresponding plants under 0 mM NaCl treatment. This was consistent with the data from the antioxidant enzyme assays.
Discussion
In stressed edaphic environments, many plants establish a symbiosis with soil microorganisms, including AM fungi or rhizobium in legumes. Both symbionts improve plant growth under severe environmental conditions (Mohamed and others 2014). In fact, it is common that dual symbiosis of rhizobium and AM fungi enhances the growth and yield of many legumes (Zahran 1999; Kazunori and others 2013). In the present study, rhizobial bacteria 4083 promoted the growth of S. cannabina under moderate salt stress, whereas 4105 showed a clear improvement under moderate and severe salt stress. The AM fungus GM had a similar growth-promoting effect to 4105 (Table 1). The plant growth response to dual symbiosis is influenced by microbial strains, as well as by the compatibility of the interactions among them (Azcón and others 1991). In AM fungi and rhizobium dual-inoculated plants, 4105 + GM was more efficient than 4083 + GM in increasing plant growth under salinity stress. In addition, the dry mass of dual-inoculated plants was higher than in the corresponding rhizobial-inoculated plants (Table 1).
There is ample evidence of a positive effect of dual inoculation with different rhizobium strains and AM fungi on legume growth (Aryal and others 2003; Mortimer and others 2008). Here, we examined whether this positive effect also occurred under saline stress. Interestingly, the colonization rate was even higher when the mycorrhizal plants inoculated with 4105 were treated with 200 mM NaCl (Fig. 2a). The mycorrhizal dependency of 4105-inoculated plants was 39 % higher than mycorrhizal plants under 200 mM NaCl. However, the mycorrhizal dependency of 4083-inoculated plants was not significantly different from GM-inoculated plants (Fig. 2b).
Unlike rhizobia, legume-rhizobium symbiosis and nodule formation on legumes are more sensitive to salt (Zahran 1991). Salt stress not only inhibits the initial steps of nodule formation, but also affects nitrogen fixation of legumes (Delgado and others 1994; Nair and others 1993; Salwa and others 2005). The reduction of N2-fixing activity by salt stress is usually attributed to a reduction in respiration of the nodules (Walsh 1995) and a reduction in leghemoglobin production by nodules (Delgado and others 1994). Agrobacterium pusense YIC4105 and Neorhizobium huautlense YIC4083 used in this work could tolerate 4 and 2 % NaCl in tryptone-yeast media, respectively. However, the number, mass of nodules, leghemoglobin content, and nitrogenase activity were all significantly suppressed by 200 mM NaCl treatments in rhizobia-inoculated plants. In addition to intrinsic protective systems of plants against saline stress, arbuscular mycorrhizal symbiosis can alleviate saline-induced nodule senescence in legume plants (Porcel and others 2003). The present study showed that dual inoculation of 4105 + GM increased the number and weight of nodules to a greater extent than in 4083 + GM-inoculated plants. In addition, significantly lower damage to the leghemoglobin content and N2-fixing efficiency was recorded in 4105 + GM-inoculated plants under stress compared with 4083 + GM as well as 4105-inoculated plants (Table 2). Notably, although the leghemoglobin content and nitrogenase activity of all rhizobia-inoculated plants declined under saline stress, the nodule number and mass did not; the nodule number of 4083-inoculated plants and the fresh weight of 4105-inoculated plants even increased significantly under 100 mM NaCl (Table 2). The data revealed that the rhizobium actively infected the roots and developed nodules; however, saline stress prevented the nodules from enlarging or activating symbiosis. Similar results have been observed in previous studies: Anthraper and DuBios (2003) reported that although salt-stressed plants produced more nodules than the controls, most of the nodules were small or inactive. In addition, an increase in the average nodule weight with increasing salinity level was observed in chickpeas (Garg and Singla 2004) and faba beans (Cordovilla and others 1999).
As a consequence of salinity stress, the accumulation of ROS can cause oxidative damage to membrane lipids, proteins, and nucleic acids, eventually leading to programed cell death (Gomez and others 1999; Hernandez and Almansa 2002). Increased antioxidative enzyme activities could be involved in the beneficial effects of mycorrhizal colonization on the performance of plants grown under semiarid conditions (Alguacil and others 2003). Our results revealed that SOD activity increased significantly in 4105 and 4105 + GM plants compared with 4083 and 4083 + GM plants under salt stress. This induction of SOD activity was consistent with changes in hydrogen peroxide-scavenging enzymes, CAT and POD (Fig. 3). The increased SOD activity induced by O2 − radicals results in increased H2O2 levels, and this is accompanied by an increased enzymatic capacity to decompose H2O2 (Hernandez and Almansa 2002). Although the difference between the APOX and GR activities in 4105 + GM and 4083 + GM-inoculated plants was not significant (Fig. 3d, e), the MDA content of 4105 + GM-inoculated plants was significantly lower than that in 4083 + GM-inoculated plants (Fig. 4).
In summary, the present study revealed that the salt tolerance of rhizobia contributes to the host plant under salt stress, together with AM fungi. In higher salt-tolerant rhizobia-inoculated plants, antioxidative enzyme activities, such as SOD, POX, CAT, and GR, were induced to protect the plants from the oxidative effects of the ROS. The higher salt-tolerant strain obviously had greater protective effects, and exhibited a synergistic interaction with AM fungi to improve the salinity tolerance of S. cannabina. The results suggested that screening salt-tolerant rhizobia that have synergistic interactions with AM fungi in triple symbiosis could be a useful strategy to enhance the tolerance of legumes to saline stress and, therefore, improve the fitness of plants. The development of sustainable biofertilizer technology is desirable for maximizing environmentally friendly crop production in saline soils (Singh and others 2011).
References
Aebi H (1984) Catalase in vitro. In: Packer L (ed) Methods in enzymology. Academic Press, Orlando, pp 121–126
Alguacil MM, Hernandez JA, Caravaca F, Portillo B, Roldan A (2003) Antioxidant enzyme activities in shoots from three mycorrhizal shrub species afforested in a degraded semi-arid soil. Physiol Plant 118:562–570. doi:10.1034/j.1399-3054.2003.00149.x
Al-Karaki GN, Hammad R, Rusan M (2001) Response of two tomato cultivars differing in salt tolerance to inoculation with mycorrhizal fungi under salt stress. Mycorrhiza 11:41–47. doi:10.1007/s005720100098
Anthraper A, Dubois JD (2003) The effect of NaCl on growth, N2 fixation (acetylene reduction), and percentage total nitrogen in Leucaena leucocephala (Leguminosae) Var. K-8. Am J Bot 90:683–692. doi:10.3732/ajb.90.5.683
Aryal UK, Xu HL, Fujita M (2003) Rhizobia and AM fungal inoculation improve growth and nutrient uptake of bean plants under organic fertilization. J Sustain Agric 21:29–41. doi:10.1300/J064v21n03_04
Azcón R, Rubio R, Barea JM (1991) Selective interactions between different species of mycorrhizal fungi and Rhizobium meliloti strains, and their effects on growth, N2 fixation (N15) in Medicago sativa at four salinity levels. New Phytol 117:399–404. doi:10.1111/j.1469-8137.1991.tb00003.x
Camprubi A, Calvet C (1996) Isolation and screening of mycorrhizal fungi from Citrus nurseries and orchards and inoculation studies. HortScience 31:366–369
Carmen B, Roberto D (2009) Medicago truncatula improves salt tolerance when nodulated by an indole-3-acetic acid-overproducing Sinorhizobium meliloti strain. J Exp Bot 60:3097–3107. doi:10.1093/jxb/erp140
Castillo FI, Penel I, Greppin H (1984) Peroxidase release induced by ozone in Sedum album leaves. Plant Physiol 74:846–851
Copeman RH, Martin CA, Stutz JC (1996) Tomato growth in response to salinity and mycorrhizal fungi from saline or nonsaline soil. HortScience 31:341–344
Cordovilla MP, Ligero F, Lluch C (1999) Effects of NaCl on growth and nitrogen fixation and assimilation of inoculated and KNO3 fertilized Vicia faba L. and Pisum sativum L. plants. Plant Sci 140:127–136. doi:10.1016/S0168-9452(98)00201-5
Del VC, Barea JM, Azcon-Agular C (1999) Diversity of arbuscular mycorrhizal fungus populations in heavy-metal-contaminated soils. Appl Environ Microbiol 65:718–723
Delgado MJ, Ligero F, Lluch C (1994) Effects of salt stress on growth and nitrogen fixation by pea, faba-bean, common bean and soybean plants. Soil Biol Biochem 26:371–376. doi:10.1016/0038-0717(94)90286-0
Dhindsa RS, Plumb-Dhindsa P, Throne TA (1981) Leaf senescence: correlated with increased levels of membrane permeability and lipid peroxidation and decreased levels of superoxide dismutase and catalase. J Exp Bot 32:93–101
Freeman S (2003) Chapter 37: plant defense systems. Prentice Hall, Englewood Cliffs
Garg N, Singla R (2004) Growth, photosynthesis, nodule nitrogen and carbon fixation in the chickpea cultivars under salt stress. Braz J Plant Physiol 16:137–146. doi:10.1590/S1677-04202004000300003
Ghazi N, Al-Karaki GN (2006) Nursery inoculation of tomato with arbuscular mycorrhizal fungi and subsequent performance under irrigation with saline water. Sci Hortic 109:1–7. doi:10.1016/j.scienta.2006.02.019
Giovannetti M, Mosse B (1980) An evaluation of techniques for measuring vesicular arbuscular mycorrhizal infection in roots. New Phytol 84:489–500. doi:10.1111/j.1469-8137.1980.tb04556.x
Gomez JM, Hernandez JA, Jimenez A, del Rio LA, Sevilla F (1999) Differential response of antioxidative enzymes of chloroplast and mitochondria to long term NaCl stress of pea plants. Free Radical Res 31:S11–S18
Hartree EF (1957) Haematin compounds. In: Paech K, Tracey MV (eds) Modern methods of plant analysis. Springer, New York, pp 197–245
Heikham E, Rupam K, Bhoopander G (2009) Arbuscular mycorrhizal fungi in alleviation of salt stress: a review. Ann Bot 104:1263–1280. doi:10.1093/aob/mcp251
Hernandez JA, Almansa MS (2002) Short-term effects of salt stress on antioxidant systems and leaf water relations of pea leaves. Physiol Plant 115:251–257. doi:10.1034/j.1399-3054.2002.1150211.x
Hoagland A (1950) The water-culture method for growing plants without soil. University of California, College of Agriculture, Agricultural Experiment Station, Berkeley, California
Kazunori S, Natsuko O, Tomomitsu K (2013) Involvement of autoregulation in the interaction between rhizobial nodulation and AM fungal colonization in soybean roots. Biol Fertil Soils 49:1141–1152. doi:10.1007/s00374-013-0804-8
Martha O, Clarence AR (1999) Hydrogen peroxide is generated systemically in plant leaves by wounding and systemin via the octadecanoid pathway. Proc Natl Acad Sci USA 25:6553–6557
Mohamed HA, Abdel-Wahab EE, Nivien AN, David MK, Fatthy MM (2014) Synergistic interaction of Rhizobium leguminosarum bv. Viciae and arbuscular mycorrhizal fungi as a plant growth promoting biofertilizers for faba bean (Vicia faba L.) in alkaline soil. Microbiol Res 169:49–58. doi:10.1016/j.micres.2013.07.007
Moran JF, James EK, Rubio MC, Sarath G, Klucas RV, Becana M (2003) Functional characterization and expression of a cytosolic iron-superoxide dismutase from Cowpea root nodules. Plant Physiol 133:773–782. doi:10.1104/pp.103.023010
Mortimer PE, Pérez-Fernández MA, Valentine AJ (2008) The role of arbuscular mycorrhizal colonization in the carbon and nitrogen economy of the tripartite symbiosis with nodulated Phaseolus vulgaris. Soil Biol Biochem 40:1019–1027. doi:10.1016/j.soilbio.2007.11.014
Munns R (2002) Comparative physiology of salt and water stress. Plant Cell Environ 25:239–250. doi:10.1046/j.0016-8025.2001.00808.x
Nair S, Jha PK, Babu CR (1993) Induced salt tolerant rhizobia, from extremely salt tolerant Rhizobium gene pools, from reduced but effective symbiosis under non-saline growth. Microbios 74:39–51
Nakano Y, Asada K (1981) Hydrogen peroxide is scavenged by ascorbate specific peroxidase in spinach chloroplasts. Plant Cell Physiol 22:867–880
Phillips JM, Hayman DS (1970) Improved procedures for clearing and staining parasitic and vesicular-arbuscular mycorrhizal fungi for rapid assessment of infection. Trans Br Mycol Soc 55:158–160
Plenchette C, Fortin JA, Furlan V (1983) Growth response of several plant species to mycorrhiza in soil of moderate P fertility. I: mycorrhizal dependency under field conditions. Plant Soil 70:191–209. doi:10.1007/BF02374780
Porcel R, Barea JM, Ruiz-Lozano JM (2003) Antioxidant activities in mycorrhizal soybean plants under drought stress and their possible relationship to the process of nodule senescence. New Phytol 157:135–143. doi:10.1046/j.1469-8137.2003.00658.x
Puppo A, Groten K, Bastian F, Carzaniga R, Soussi M, Lucas MM, de Felipe MR, Harrison J, Vanacker H, Foyer CH (2005) Legume nodule senescence: roles for redox and hormone signalling in the orchestration of the natural aging process. New Phytol 165:683–701. doi:10.1111/j.1469-8137.2004.01285.x
Rao DLN (1998) Biological amelioration of salt-affected soils. Microbial interactions in agriculture and forestry, vol 1. Science Publishers, Enfield, pp 21–238
Rosa P, Ricardo A, Juan MR (2012) Salinity stress alleviation using arbuscular mycorrhizal fungi: a review. Agron Sustain Dev 32:181–200. doi:10.1007/s13593-011-0029-x
Salwa J, Moez J, Férid L, Mohamed EA (2005) Changes in ascorbate peroxidase, catalase, guaiacol peroxidase and superoxide dismutase activities in common bean (Phaseolus vulgaris) nodules under salt stress. J Plant Physiol 162:929–936. doi:10.1016/j.jplph.2004.10.005
Singh RP, Choudhary A, Gulati A, Dahiya HC, Jaiwal PK, Sengar RS (1997) Response of plants to salinity in interaction with other abiotic and factors. In: Jaiwal PK, Singh RP, Gulati A (eds) Strategies for improving salt tolerance in higher plants. Science Publishers, Enfield, pp 25–39
Singh JS, Pandey VC, Singh DP (2011) Efficient soil microorganisms: a new dimension for sustainable agriculture and environmental development. Agric Ecosyst Environ 140:339–353
Smith IK, Vierheller TL, Thorne CA (1988) Assay of glutathione reductase in crude tissue homogenates using 5,5′-dithiobis(2-nitrobenzoic acid). Anal Biochem 175:408–413. doi:10.1016/0003-2697(88)90564-7
Sudhakar C, Lakshmi A, Giridarakumar S (2001) Changes in the antioxidant enzymes efficacy in two high yielding genotypes of mulberry (Morus alba L.) under NaCl salinity. Plant Sci 161:613–6199. doi:10.1016/S0168-9452(01)00450-2
Tian CY, Feng G, Li XL, Zhang FS (2004) Different effects of arbuscular mycorrhizal fungal isolates from saline or non-saline soil on salinity tolerance of plants. Appl Soil Ecology 26:143–148. doi:10.1016/j.apsoil.2003.10.010
Vincent JM (1970) A manual for the practical study of root nodule bacteria. Black-well Scientific, Oxford
Walsh KB (1995) Physiology of the legume nodule and its response to stress. Soil Biol Biochem 27:637–655. doi:10.1016/0038-0717(95)98644-4
Weissenhorn I, Leyval C, Berthelin J (1993) Cd-tolerant arbuscular mycorrhizal (AM) fungi from heavy-metal polluted soils. Plant Soil 158:250–256. doi:10.1007/BF00011053
Zahran HH (1991) Conditions for successful Rhizobium-legume symbiosis in saline environments. Biol Fertil Soils 12:73–80. doi:10.1007/BF00369391
Zahran HH (1999) Rhizobium–legume symbiosis and nitrogen fixation under severe conditions and in an arid climate. Microbiol Mol Biol Rev 63:968–989
Acknowledgments
This work was financed by the Strategic Priority Research Program of the Chinese Academy of Sciences (Grant No. XDA11020403), the Key Research Program of the Chinese Academy of Sciences (Grant No. KZZD-EW-14), the National Natural Science Foundation of China (31370108 and 31570063), One Hundred-Talent Plan of Chinese Academy of Sciences (CAS), Yantai Science and Technology Project (2013JH021).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ren, CG., Bai, YJ., Kong, CC. et al. Synergistic Interactions Between Salt-tolerant Rhizobia and Arbuscular Mycorrhizal Fungi on Salinity Tolerance of Sesbania cannabina Plants. J Plant Growth Regul 35, 1098–1107 (2016). https://doi.org/10.1007/s00344-016-9607-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00344-016-9607-0