Abstract
The molecular mechanism involved in cell wall dynamics has not been well clarified, although it is quite important for organ growth. We characterized a rice mutant, root growth inhibiting (rt), which is defective in root elongation. The rt mutant showed a severe defect in cell elongation at the root-elongating zone with additional collapse of epidermal and cortex cells at the root tip caused by the defect in the smooth exfoliation of root cap cells. Consistent with these phenotypes, expression of the RT gene, which encodes a member of the membrane-anchored endo-1,4-β-d-glucanase, was specifically localized in the root-elongating zone and at the junction between epidermal and root cap cells. The enzymatic analysis of root extracts from the wild-type and rt mutant indicated that RT hydrolyzes noncrystalline amorphous cellulose. The cellulose content was slightly increased but the crystallinity of cellulose was decreased in the rt root. In addition, the hemicellulose composition was different between wild-type and rt roots. The total extensibility was significantly lower in the rt root explants. Based on these results, we concluded that RT is involved in the disassembly of the cell wall for cell elongation in roots as well as for root cap exfoliation from the epidermal cell layer by hydrolyzing the noncrystalline amorphous cellulose fibers of cellulose microfibrils resulting in loosening of the hemicellulose and cellulose interaction.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Plant cells are imprisoned within their rigid cell walls; therefore, the morphogenesis of a developing plant depends on orderly cell divisions followed by strictly oriented cell expansion. Plant cell expansion is believed to require a selective loosening of the wall, thereby permitting movement or slippage of cellulose microfibrils in response to the mechanical forces generated by cell turgor (Cosgrove 1997). The growing cell wall consists of a scaffold of cellulose microfibrils embedded in a matrix of crosslinking polysaccharides such as xyloglucans and arabinoxylans. The relative abundance of glucan crosslinkages between cellulose microfibrils could contribute to the strength of the cell wall, and hydrolysis or transglycosylation of matrix polysaccharides enables the cell wall to enlarge by weakening the matrix that holds cellulose microfibrils. To date, four molecular mechanisms have been proposed to explain the loosening of the cell wall. Candidate wall-loosening agents include expansin, xyloglucan endotransglycolase/hydrolase, endo-1,4-β-d-glucanase (EGase), and hydroxyl radicals (Cosgrove 2005).
EGases are enzymes that depolymerize polysaccharides containing 1,4-β-d-glucan linkages and are produced by bacteria, fungi, nematodes, insects, and plants (Beguin 1990). Most microbial EGases have a cellulose-binding domain, which enables them to degrade crystalline cellulose, whereas plant EGases generally lack this domain and do not appear capable of degrading crystalline cellulose (Levy and others 2002). Most plant EGases have an endoplasmic reticulum import signal peptide and are secreted to the periplasm where they modify the cell wall (del Campillo 1999), whereas plant membrane-anchored EGases are type II integral membrane proteins predicted to be integrated in the plasma membrane and to act at the plasma membrane–cell wall interface (Brummell and others 1997; Nicol and others 1998). Because membrane-anchored EGases are expected to be associated with the plasma membrane, they probably do not have access to the majority of the cell wall, and so they probably do not function as cell wall-loosening enzymes (Cosgrove 2001; Mφlhφj and others 2001b). Another role for membrane-anchored EGases in cellulose biosynthesis has become apparent more recently. Mutations in the Arabidopsis (Arabidopsis thaliana L.) KORRIGAN (KOR) gene disrupted the correct assembly of the cellulose–hemicellulose network and caused cellulose deficiency without detectable changes in xyloglucans (Nicol and others 1998; His and others 2001; Lane and others 2001; Sato and others 2001). In addition, the strong allele of kor caused the formation of aberrant cell plates, incomplete cell walls, and multinucleated cells, indicating that KOR also plays a critical role during cytokinesis (Zuo and others 2000).
In rice (Oryza sativa L.), there are at least three genes encoding membrane-anchored EGases, and one of them, OsGLU1, has a similar function to Arabidopsis KOR in cellulose biosynthesis (Zhou and others 2006). Here we report that another rice membrane-anchored EGase encoded by the ROOT GROWTH INHIBITING (RT) gene has a different function from Arabidopsis KOR and rice OsGLU1. The rt mutant was generated by ethylene imine treatment and characterized as a mutant defective in root elongation (Futsuhara and Kitano 1985). Based on the mutant phenotype and the expression pattern of the RT gene in rice plants, we concluded that RT is involved in the disassembly of the cell wall for cell elongation in roots as well as for root cap exfoliation from the epidermal cell layer by hydrolyzing the noncrystalline amorphous cellulose fibers of cellulose microfibrils.
Materials and Methods
Plant Materials
Seeds of wild-type rice (Oryza sativa L. cv. Fujiminori) and the rt mutant were sterilized in 1% NaClO for 1 h and sown on an 0.8% agar medium without nutrients. Seedlings were grown in a growth chamber at 30°C under continuous light for 6 days. The seminal roots of both wild-type and rt mutant seedlings grew vertically in the agar medium. The length of the seminal roots of the seedlings was manually measured every day for 6 days. Seedlings were then transplanted to plastic pots (19.5 cm in height, 15.8 cm in diameter) filled with soil without nutrients and grown for 2 months in a greenhouse at 28°C under ambient light conditions.
Histological Analysis
The seminal root segments excised from 5-day-old seedlings were fixed in FAA (formalin:glacial acetic acid:70% ethanol = 1:1:18). These segments were dehydrated in a graded acetone series, embedded in Paraplast Plus (Oxford Labware, St. Louis, MO, USA), and sectioned at 8 μm. Microtome sections were stained with toluidine blue. For scanning electron microscopy (SEM), fixed root segments were observed using a S-3000H scanning electron microscope (Hitachi, Tokyo, Japan).
Molecular Cloning and Sequence Analysis
To map RT, linkage analysis was performed using an F 2 population of approximately 2,000 derived from the cross between rt (japonica variety) and Kasalath (indica variety). A BLAST search was performed against the rice DNA databases of the Rice Genome Research Program (http://rgp.dna.affrc.go.jp/), the Beijing Genomics Institute (http://btn.genomics.org.cn/rice), DDBJ (http://www.ddbj.nig.ac.jp/index-e.html), GenBank (http://www.ncbi.nlm.nih.gov/BLAST/), Gramene (http://www.gramene.org/), and KOME (http://cdna01.dna.affrc.go.jp/cDNA/). The predicted protein sequences were initially clustered using ClustalW (Thompson and others 1994). TreeView was used to generate the graphical output (Page 1996). The numbers at the branching points indicate the percentage of times that each branch topology was found during bootstrap analysis (n = 1,000).
Expression Analysis
RT-PCR was performed with DNase-treated total RNAs separately prepared from various organs of rice by using the Advantage RT-for-PCR Kit (Clontech, Palo Alto, CA, USA). The primer sequences are 5′-CTTCATCCTCATCGGCCTCCCCGTCATC-3′ and 5′-CCCGCCCTTGACGTCCGTCAGATCGGAG-3′ for RT, 5′-CGGCCTCTTCATCGGCTTCGTCATGATG-3′ and 5′-GCTCTTCCGGACGGTGCTGTCGGAGAGG-3′ for AK070408, 5′-TCTTCGCCGGGCTCGTCGCCGGCAT-3′ and 5′-GGCTGCGCCCGACTGCCGGATCCGAGAG-3′ for AK102748, and 5′-CGCCAGTTTGGTCGCTCTCGATTTCG-3′ and 5′-TCAGGAGCTCCGTGCTCTTCTGGTAC-3′ for Histone H3. These primers specifically amplified the target gene sequences.
Plasmid Constructs and Plant Transformation
For complementation of the rt mutation, the wild-type genomic sequence from −2581 to +1969 (taking the translation initiation site as +1) was amplified by PCR and cloned into pBI121. For the RT promoter GUS construct, the wild-type genomic sequence from −2581 to +518 was amplified by PCR and introduced in front of the GUS reporter gene of pBI-Hm to produce a fusion with the GUS reporter gene. The resulting fusion construct was introduced into Agrobacterium tumefaciens strain EHA101 by electroporation. Agrobacterium-mediated transformation of rice was performed as described previously (Hiei and others 1994). Transgenic plants were selected on media containing 50 mg l−1 hygromycin. More than 20 transformants were analyzed for each construct.
Analysis of Cell Wall Polysaccharides
Root segments were washed with distilled water and ground in a mortar with purified quartz powder. Homogenates were centrifuged for 10 min at 1,000×g, and the pellets were washed three times each with distilled water, acetone, a mixture of methanol:chloroform (1:1), ethanol, and distilled water. Cell wall pellets were then kept in 200 mg/ml Pronase (Kaken Chemicals, Tokyo, Japan) in phosphate buffer (pH 7.0). A pectic fraction was obtained by duplicate extraction with 0.5% ammonium oxalate solution at 95°C for 1 h each. The insoluble pellet was further extracted with 4 and 24% KOH solution to obtain hemicellulose fractions. The residual cell wall precipitates were washed with water and with dilute acetic acid solution. The cellulose fraction was obtained by dissolving these pellets in 72% H2SO4. The amounts of sugars in these fractions were determined by the phenol-sulfuric acid method using galacturonic acid as a standard for the pectic fraction and glucose for the hemicellulose and the cellulose fractions.
X-Ray Diffraction
Roots of two seedlings were sampled and dried completely at 40°C. The cellulose sample was pressed into a cylindrical pellet, 12 mm in diameter, 0.1 mm in thickness, and approximately 20 mg in mass. The X-ray diffraction pattern of the pellet was recorded with a RINT 2000 instrument (Rigaku Co. Ltd., Tokyo, Japan) in the reflection mode using Ni-filtered Cu–K radiation (40 kV, 40 mA) at a scanning rate of 0.6 deg min−1 in 2θ. We sampled the height of the main equatorial reflection of cellulose and compared that of a region with no diffraction peaks present to produce a crystallinity index by Segal’s method (Segal and others 1959).
Enzymatic Activity Measurement
Five grams of roots were homogenized in 15 ml of 10 mM Hepes buffer (pH 7.0) containing 10% sucrose and 0.02% NaN3 using a polytron homogenizer for 30 s, then passed through the 50-mm-mesh nylon cloth. After centrifuging for 60 min at 80,000×g at 5°C, the pellet was washed once with homogenate buffer and resuspended in 2 ml of the same buffer. Enzymatic activity was assayed by measuring the reduction in viscosity of a solution of 0.5% CM-Cellulose 4 M (Megazyme, Wicklow, Ireland) in 50 mM sodium phosphate buffer (pH 6.0) containing 0.04% NaN3 using an Ostwald type KPG viscometer (No. 1; Sansyo, Tokyo, Japan), which was placed in a water bath maintained at 35°C. All assays were run in the presence of 0.17% Tritron X-100.
Determination of Mechanical Properties of Seminal Root
A creep test was carried out as previously described (Tanimoto and others 2000). The seminal root samples were immediately immersed in methanol at 65°C for 10 min and transferred to fresh methanol at 4°C. Root segments were rehydrated with 10 mM MES buffer, pH 6.0, at 0°C for 15 min. The root was secured between two clamps of a Rheoner creep meter (Yamaden RE-33005, Tokyo), one at a position 1 mm from the apex and the other at 4 mm. The creep extension was carried out in the MES buffer at room temperature. A force of 15 g mm−2 was applied to the root by driving the lower clamp down at the maximum speed at 0.5 mm s−1. The extension process was recorded by a computer at 0.5 s intervals for 10 min.
Results
Characterization of the rt Mutant
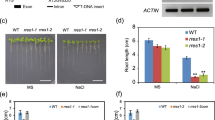
Two-month-old rice plants formed well-developed root systems with various components such as seminal, crown (adventitious), and lateral roots (Fig. 1a), whereas the rt mutant developed all these root components with a severe defect in elongation (Fig. 1b). Such defects in root elongation were observed just after germination (Fig. 1c), although the radicle in the embryo and the crown root primordia in the stem were formed normally in the rt mutant (data not shown). These observations indicate that the RT gene is involved only in root elongation and not in root initiation.
Phenotype of rt mutant. a Two-month-old wild-type. b Two-month-old rt mutant root. Scale bars = 5 cm. c Seminal root growth of wild-type (circle) and rt mutant (square). Each data point is the average of ten plants. d–f Scanning electron microscopy of wild-type (d, e) and rt mutant (f) root. EP epidermis, RC root cap. Scale bars = 500 μm (d, f), 200 μm (e). g, h Light microscopy of longitudinal sections of wild-type (g) and rt mutant (h) root tip regions. Blue, red, and yellow indicate root cap, epidermal, and outer cortex cells, respectively. Scale bars = 200 μm. i, j Light microscopy of longitudinal sections of wild-type (i) and rt mutant (j) root cell elongation zone. CC central cylinder, CO cortex cell. Scale bars = 100 μm
The surface structure of the root tip area was severely disorganized in the rt mutant. In the wild-type plant, the root cap laps the root tip (Fig. 1d) and is exfoliated from the epidermal cell layer starting from the upper side of the root tip (Fig. 1e). Consequently, the root surface above the root tip (where cells are dividing and elongating) is maintained smooth with well-arranged epidermal cells (Fig. 1e, g). In contrast, the root surface of the rt mutant is rough and its outer cells above the root tip are severely damaged (Fig. 1f, h).
Histological observation also revealed defects in the root development of the rt mutant. The basic radial pattern of the root, which consists of epidermis, cortex, and central cylinder, observed in wild-type (Fig. 1g) was established in the rt mutant, but the epidermal and the outermost cortical cell layers were stretched wide and the row of cells was disorganized in the rt mutant (Fig. 1h). Broken root cap cells persistently attached to the epidermal cells suggesting that the collapse of surface cells above the root tip may be due to the defect in smooth exfoliation of root cap cells from the epidermal cell layer.
Another defect in the rt mutant was observed in the root-elongating region. The organized cell files of the cortical and central cylinder cells were maintained; however, longitudinal elongation of these cells was apparently inhibited in the rt mutant (compare Fig. 1i, j). Given adequate supplies of water and fertilizer, the aboveground portion of the rt mutant grew normally and did not differ significantly from wild-type morphologically, even at harvest (data not shown). Based on these results, we concluded that the RT gene functions in root cap exfoliation and root cell elongation but not in root differentiation, root cell division, and shoot development.
The RT Gene Encodes a Membrane-anchored EGase
The RT locus was cloned using the map-based chromosome walking procedure. The RT locus was mapped on the long arm of chromosome 4, tightly linked to a molecular marker C335 (Fig. 2a). According to the gene prediction database (RICEGAAS; http://ricegaas.dna.affrc.go.jp), this region includes one open reading frame (ORF), which encodes a protein of 623 amino acids with a predicted molecular mass of 71.1 kDa and a pI of 9.42 (accession No. AB682995). Sequence comparison suggested that RT is a member of the membrane-anchored E-type EGase, also known as cellulase. By comparing the nucleotide sequences of this gene region between wild-type and the rt mutant, we found a single nucleotide substitution at Lys291 (AAG to TAG), which generates a premature stop codon. Because this mutation results in the loss of the C-terminal half of endo-1,4-β-D-glucanase domain, including two of the four amino acid residues essential for catalytic activity identified in celD which are also conserved in KOR and RT (Nicol and others 1998), we consider this rt mutant to be a null allele. Complementation analysis by introduction of a 7.9 kb genomic DNA fragment containing the entire candidate gene confirmed that the rt phenotype is caused by a loss-of-function mutation in this predicted RT gene (data not shown).
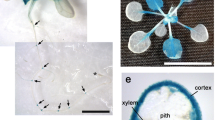
Molecular characterization of RT gene. a High-resolution linkage and physical map of the RT locus. The vertical bars represent molecular markers and the numbers of recombinant plants are indicated above the linkage map. The RT locus was tightly linked to a molecular marker C335. b Phylogenetic relationships of plant EGases. At Arabidopsis thaliana, Bn Brassica napus, Hv Hordeum vulgare, Le Lycopersicon esculentum, Ta Triticum aestivum. c Hydropathic profile of RT-deduced protein. d Expression of RT and two rice paralogs in various organs of wild-type rice. Histone H3 was used as a control. e–g Localization of GUS activity under the control of the RT promoter. Root (e), root tip region (f), and transverse sections through the cell elongating zone (g). Scale bar = 2 mm (e), 150 μm (f); 350 μm (g)
As the cDNA sequence corresponding to the RT gene was not found in any available DNA databases, we isolated and sequenced its cDNA clone and confirmed that the predicted ORF is correct (data not shown). A BLAST search using the whole amino acid sequence of RT revealed that RT showed the highest homology with tomato (Lycopersicon esculentum Mill.) mRNA clone, 132247F (62% amino acid identity) and was related to two rice cDNA clones, AK102748 and AK070408 (60 and 58% amino acid identities, respectively). Phylogenetic analysis grouped RT with membrane-anchored EGases from various plant species and further divided this group into small subgroups (Fig. 2b). The two rice paralogs, AK102748 and AK070408, were clustered in the largest subgroup that included nine members from both dicot and monocot species, and they showed higher similarity with Arabidopsis KOR (AtKOR1; 74 and 70% amino acid identities, respectively). However, RT was distantly related to this subgroup and to tomato 132247F and Arabidopsis KOR2 (56% amino acid identity).
In contrast to the secreted EGases, membrane-anchored EGases do not have a characteristic endoplasmic reticulum import signal sequence but instead contain a transmembrane domain (TMD; Brummell and others 1997; Nicol and others 1998). The predicted TMD of RT contained a stretch of highly hydrophobic amino acids (Fig. 2c), although it showed lower amino acid identity with other membrane-anchored EGases (17–43%). The remainder of the RT possessed six potential N-glycosylation sites (N-X-S/T), which have a protective or stabilizing function for EGases (Brummell and others 1997; Mφlhφj and others 2001b). The C terminus of RT is rich in proline, a characteristic of the linker regions connecting different domains in microbial cellulases (Beguin 1990). The putative polarized targeting motifs (Zuo and others 2000) and the characteristics of EGases belonging to the glycoside hydrolysis family 9 characterized by an inverting hydrolysis mechanism (Brummell and others 1997) were conserved in RT (data not shown). The cell attachment signature (R–G–D), which is observed in proteins that function in cell adhesion, was found in AtKOR2 (Mφlhφj and others 2001a) and tomato 132247F but not in RT. These structural characteristics suggest that RT encodes a putative membrane-anchored EGase, although we cannot exclude the possibility that RT has different functions from other membrane-anchored EGases.
Expression of the RT Gene
Semiquantitative reverse-transcription PCR analysis revealed that RT was expressed in root, whereas the transcripts of the two rice paralogs AK102748 and AK070408 were broadly detected in all the organs we tested (Fig. 2d). We also examined the RT expression pattern indicated by GUS activity under the control of the RT promoter. GUS staining was observed in the root (Fig. 2e) but not in any aboveground tissues or organs (data not shown). In the root, staining was observed in the elongation zone where cells actively elongate along the root axis (Fig. 2e), and at the junction between epidermal and root cap cells (Fig. 2f). In the elongation zone, the staining was preferentially localized in outer tissues such as the epidermis and outside cells of the cortex but not in vascular tissues (Fig. 2g). The localized expression of the RT gene in root corresponds well to the mutant phenotype of the rt mutant, that is, inhibition of cell elongation at the elongation zone and a defect in root cap exfoliation from the epidermal cell layer.
The rt Mutant Has an Altered Cell Wall Polysaccharide Composition
To examine the relationship of cell wall components to the RT activity and the phenotype of the rt mutant, cell wall polysaccharide content was compared between wild-type and the rt mutant roots. Interestingly, cellulose content was significantly increased in the rt mutant root approximately 1.25 times that of the wild-type root, whereas the mutant roots contained smaller amounts of pectin than wild-type roots (Table 1). In addition, the composition of hemicellulose was quite different between wild-type and mutant roots; the 4% KOH-soluble or KOH-insoluble fraction was significantly decreased (67%) and increased (195%) in the mutant root relative to each fraction of the wild-type root, respectively. Because the 4% KOH-soluble or KOH-insoluble fraction contains hemicellulose combined with cellulose microfibrils, increased insoluble and decreased soluble hemicellulose in the rt mutant root indicates that hemicellulose in the rt mutant root tightly binds to the surface of cellulose microfibrils.
We further determined the crystallinity of cellulose in wild-type and mutant roots by X-ray diffraction. The diffraction curves for both wild-type and mutant cellulose samples prepared from roots fit the typical curve of cellulose I (data not shown). In contrast, the curve for the cellulose fraction isolated from the mutant root reflected a significantly lower level of crystallinity (0.172 ± 0.016, wild-type was 0.352 ± 0.012, P < 0.01), indicating a high proportion of noncrystalline amorphous cellulose than did that for the wild-type fraction. Consistent with this result, the EGase activity of the membrane fraction for noncrystalline amorphous cellulose was significantly decreased in the rt mutant root to 44% (1.41 ± 0.27 unit μg−1 protein) of that of the wild-type (3.24 ± 0.47 unit μg−1 protein, P < 0.01).
The above results strongly suggest that the RT gene is involved in cell elongation and cell–cell separation in roots through the digestion of noncrystalline amorphous cellulose and the release of hemicellulose tightly bound to cellulose microfibrils. Therefore, in the rt mutant, defects in hydrolysis of amorphous cellulose maintain the strong cellulose–hemicellulose network resulting in decreased plastic extensibility of the cell wall. This idea was supported by the result that the total extensibility at the breaking point was significantly lower in the rt mutant root explants (1.33 ± 0.06 mm in rt and 1.81 ± 0.11 mm in wild-type, P < 0.01).
Transmission electron microscopic observations of the cell wall between the root cap and epidermal cells also support this idea. In wild-type, the cell wall between the root cap and epidermal cells was thin and staining materials were observed at the root tip (CW in Fig. 3a), whereas the cell wall became thick and staining materials were not found at the upper region where abruption of the root cap from the epidermal cell layer occurs (CW in Fig. 3b). In contrast, a thin cell wall with staining materials between the root cap and epidermal cells was maintained in rt even at the upper region where root cap cells persistently attached to the epidermal cells (CWs in Fig. 3c, d).
Discussion
Phenotypic observations indicate that the defect in elongation of the rt root is caused by inhibition of longitudinal cell elongation in the cell-elongating zone (Fig. 1j) and the collapse of epidermal and cortex cells at the root tip region (Fig. 1f, h). Consistent with these phenotypes, the RT gene expression is specifically localized in the root-elongating zone (Fig. 2e) and at the junction between the epidermal and root cap cells (Fig. 2f). In addition, RT was preferentially expressed in outer tissues such as the epidermis and outside cells of the cortex in the cell-elongating zone. Numerous studies have shown that the cell wall of peripheral cells of stems and coleoptiles constrain the extension of the internal tissues and thus limit elongation of these organs (Dale 1988; Kutschera 1992; Savaldi-Goldstein and others 2007). Thus, the preferential expression of RT in peripheral cells of the cell-elongating zone strongly supports the idea that the RT protein is involved in root elongation through regulation of cell wall dynamics.
The RT gene encodes a membrane-anchored EGase, with highest similarity to tomato 132247F and Arabidopsis KOR2. Libertini and others (2004) classified plant EGases into three subfamilies, α, β, and γ, by phylogenetic analysis. According to that, α- and β-EGases are predicted to be secreted proteins and believed to be involved in a number of physiological roles such as fruit ripening (Lashbrook and others 1994), anther dehiscence (del Campillo and Lewis 1992), vascular tissue differentiation (Milioni and others 2001, 2002), abscission of plant organs (Tucker and others 1988; del Campillo and Bennett 1996; Gonzalez-Bosch and others 1997), and sloughing of root cap cells from the root tip (del Campillo and others 2004). In contrast, γ-EGases are predicted to contain an N-terminal membrane anchor and therefore to function on the membrane (Brummell and others 1997; Nicol and others 1998). In Arabidopsis, there are at least 22 genes encoding secreted EGases and only three encoding membrane-anchored EGases: KOR (Nicol and others 1998) and KOR2 and KOR3 (Mφlhφj and others 2001a). There is evidence to support a role for KOR in the synthesis and/or assembly of cellulose in plants: all kor alleles invariably show a cellulose deficiency without detectable change in xyloglucans (His and others 2001; Lane and others 2001; Sato and others 2001); recombinant CEL16, an oilseed rape protein orthologous to KOR and expressed in Pichia pastoris, has a hydrolyzing activity of amorphous cellulose but does not hydrolyze crystalline cellulose and xyloglucan (Mφlhφj and others 2001b); and KOR and its homolog in cotton are actively expressed during secondary wall synthesis in Arabidopsis and in cotton fibers, which contain very little xyloglucans (Turner and Somerville 1997; Peng and others 2002). Recently, Takahashi and others (2009) clearly showed that cellulose crystallinity was decreased in transgenic Arabidopsis overexpressing the KOR homolog in hybrid aspen (Populus tremula L. × P. tremuloides Michx.), PttCel9A1, whereas it was increased in the irx2 mutant carrying a point mutation in KOR. Based on these observations, they suggested that the activities of both KOR and PttCel9A1 facilitate cellulose biosynthesis in a way that increases the amount of noncrystalline cellulose. In addition, KOR2 is expressed in developing root hairs and the proximal parts of leaves, floral organs, and trichomes, and KOR3 is expressed in trichome support cells and in the bundle sheath cells (Mφlhφj and others 2001a). Based on these observations, Mφlhφj and others (2001a) suggested that KOR2 and KOR3 genes are involved in assembly and reinforcement of cell walls at these tension points, similar to KOR.
In this study, we showed a slight but significant increase of cellulose content in the rt mutant roots. In contrast, as mentioned previously, all kor alleles invariably showed a cellulose deficiency and pectin accumulation without detectable changes in xyloglucans (hemicellulose) (His and others 2001; Lane and others 2001; Sato and others 2001). When tobacco or tomato suspension cells were treated with 2,6-dichlorobenzonitrile, a specific inhibitor of cellulose synthesis, a marked reduction in the amount of cellulose and accumulation of a large amount of pectin occurred in the cell wall (Shedletzky and others 1990; Nakagawa and Sakurai 1998). An increased amount of pectin was also observed in the kor mutant, which was predicted to compensate for the weakness attributable to the reduced number of cellulose microfibrils (Sato and others 2001). However, the rt mutant root produced almost the same amount of pectin as the wild-type root. An increase in cellulose content and no change in pectin content strongly suggest that RT has a different function from KOR. This idea is also supported by the recent report that mutation of OsGLU1, another membrane-anchored EGase in rice, resulted in a decrease in cellulose content but an increase in pectin content (Zhou and others 2006). Thus, OsGLU1 is predicted to have the same function as KOR, that is, cellulose synthesis and cell wall assembly in rice. That the defect in the epidermal and root cap cell separation observed in the rt mutant cannot be explained by the synthesis or assembly of cellulose, and this phenotype was not found in the glu mutant, the loss-of-function mutant of OsGLU1, strongly supports our conclusion that RT has a different function from KOR.
What function does the RT protein have in cell wall dynamics? The most probable function of RT is loosening the hemicellulose [probably β-1,3:1,4-glucan, because it is present exclusively in grasses, while dicot plants are rich in xyloglucan (Carpita 1996)] and cellulose interaction by hydrolyzing the noncrystalline amorphous cellulose fibers of cellulose microfibrils. A previous report that poplar cellulase attacked the paracrystalline sites of cellulose microfibrils to loosen xyloglucan interaction and this irreversible wall modification promotes the enlargement of transgenic Arabidopsis cells (Park and others 2003) supports this idea. Furthermore, we found that the content of hemicellulose in the 4% KOH-soluble fraction was decreased and that the insoluble fraction was increased in the rt root. In contrast, Park and others (2003) reported that the overexpression of a poplar secreted EGase in transgenic Arabidopsis increased and decreased the hemicellulose contents in the 4% KOH-soluble and KOH-insoluble fractions, respectively, and resulted in promotion of the expansion growth of leaves. These results indicate that decreased and increased hemicellulose contents in the 4% KOH-soluble and KOH-insoluble fractions, respectively, in the rt root can explain the defects in root elongation in the rt mutant. As total extensibility was decreased in the rt root, our present analysis suggested that one of the membrane-anchored EGases has a similar function to that of secreted EGases in cell wall loosening. In addition, sloughing of root cap cells from the root tip in Arabidopsis was regulated by secreted EGase (del Campillo and others 2004). Based on these results and our findings, we hypothesize that RT hydrolyzes the noncrystalline amorphous cellulose fibers of cellulose microfibrils to loosen the hemicellulose and cellulose interaction, and this activity of RT expressed in cells at the root elongation zone and at the root tips is necessary for the disassembly of the cell wall for cell elongation and for root cap exfoliation from the epidermal cell layer, respectively. The molecular mechanism behind why a membrane-anchored EGase can function to hydrolyze the amorphous cellulose fibers like secreted EGases is unknown. Further studies are necessary to reveal the multiple functions of membrane-anchored EGases in cell wall dynamics.
References
Beguin P (1990) Molecular biology of cellulose degradation. Annu Rev Microbiol 44:219–248
Brummell DA, Catala C, Lashbrook CC, Bennett AB (1997) A membrane-anchored E-type endo-1,4-β-glucanase is localized on Golgi and plasma membranes of higher plants. Proc Natl Acad Sci USA 94:4794–4799
Carpita NC (1996) Structure and biogenesis of the cell walls of grasses. Annu Rev Plant Physiol Plant Mol Biol 47:445–476
Cosgrove DJ (1997) Relaxation in a high-stress environment: the molecular bases of extensible cell walls and cell enlargement. Plant Cell 9:1031–1041
Cosgrove DJ (2001) Wall structure and wall loosening. A look backwards and forwards. Plant Physiol 125:131–134
Cosgrove DJ (2005) Growth of the plant cell wall. Nat Rev Mol Cell Biol 6:850–861
Dale JE (1988) The control of leaf expansion. Ann Rev Plant Physiol Plant Mol Biol 39:267–295
del Campillo (1999) Multiple endo-1,4-β-d-glucanase (cellulase) genes in Arabidopsis. Curr Top Dev Biol 46:39–61
del Campillo E, Bennett AB (1996) Pedicel breakstrength and cellulase gene expression during tomato flower abscission. Plant Physiol 111:813–820
del Campillo E, Lewis LN (1992) Occurrence of 9.5 cellulase and other hydrolases in flower reproductive organs undergoing major cell wall disruption. Plant Physiol 99:1015–1020
del Campillo E, Abdel-Aziz A, Crawford D, Patterson SE (2004) Root cap specific expression of an endo-β-1,4-d-glucanase (cellulase): a new marker to study root development in Arabidopsis. Plant Mol Biol 56:309–323
Futsuhara Y, Kitano H (1985) Inheritance of a root-growth inhibiting mutant in rice. Rice Genet Newslett 2:70–71
Gonzalez-Bosch C, del Campillo E, Bennett AB (1997) Immunodetection and characterization of tomato endo-β-1,4-glucanase cel1 protein in flower abscission zones. Plant Physiol 114:1541–1546
Hiei Y, Ohta S, Komari T, Kumashiro T (1994) Efficient transformation of rice (Oryza sativa L.) mediated by Agrobacterium and sequence analysis of boundaries of the T-DNA. Plant J 6:271–282
His I, Driouich A, Nicol F, Jauneau A, Hofte H (2001) Altered pectin composition in primary cell walls of korrigan, a dwarf mutant of Arabidopsis deficient in a membrane-bound endo-1,4-β-glucanase. Planta 212:348–358
Kutschera U (1992) The role of the epidermis in the control of elongation growth in stems and coleoptiles. Bot Acta 105:246–252
Lane DR, Wiedemeier A, Peng L, Hofte H, Vernhettes S, Desprez T, Hocart CH, Birch RJ, Baskin TI, Burn JE, Arioli T, Betzner AS, Williamson RE (2001) Temperature-sensitive alleles of RSW2 link the KORRIGAN endo-1,4-β-glucanase to cellulose synthesis and cytokinesis in Arabidopsis. Plant Physiol 126:278–288
Lashbrook CC, Gonzalez-Bosch C, Bennett AB (1994) Two divergent endo-β-1,4-glucanase genes exhibit overlapping expression in ripening fruit and abscising flowers. Plant Cell 6:1485–1493
Levy I, Shani Z, Shoseyov O (2002) Modification of polysaccharides and plant cell wall by endo-1,4-β-glucanase and cellulose-binding domains. Biomol Eng 19:17–30
Libertini E, Li Y, McQueen-Mason SJ (2004) Phylogenetic analysis of the plant endo-β-1,4-glucanase gene family. J Mol Evol 58:506–515
Milioni D, Sado PE, Stacey NJ, Domingo C, Roberts K, McCann MC (2001) Differential expression of cell-wall-related genes during the formation of tracheary elements in the Zinnia mesophyll cell system. Plant Mol Biol 47:221–238
Milioni D, Sado PE, Stacey NJ, Roberts K, McCann MC (2002) Early gene expression associated with the commitment and differentiation of a plant tracheary element is revealed by cDNA-amplified fragment length polymorphism analysis. Plant Cell 14:2813–2824
Mφlhφj M, Jorgensen B, Ulvskov P, Borkhardt B (2001a) Two Arabidopsis thaliana genes, KOR2 and KOR3, which encode membrane-anchored endo-1,4-β-d-glucanases, are differentially expressed in developing leaf trichomes and their support cells. Plant Mol Biol 46:263–275
Mφlhφj M, Ulvskov P, Dal Degan F (2001b) Characterization of a functional soluble form of a Brassica napus membrane-anchored endo-1,4-β-glucanase heterologously expressed in Pichia pastoris. Plant Physiol 127:674–684
Nakagawa N, Sakurai N (1998) Increase in the amount of celA1 protein in tobacco BY-2 cells by a cellulose biosynthesis inhibitor, 2,6-dichlorobenzonitrile. Plant Cell Physiol 39:779–785
Nicol F, His I, Jauneau A, Vernhettes S, Canut H, Hofte H (1998) A plasma membrane-bound putative endo-1,4-β-d-glucanase is required for normal wall assembly and cell elongation in Arabidopsis. EMBO J 17:5563–5576
Page RD (1996) TreeView: an application to display phylogenetic trees on personal computers. Comput Appl Biosci 12:357–358
Park YW, Tominaga R, Sugiyama J, Furuta Y, Tanimoto E, Samejima M, Sakai F, Hayashi T (2003) Enhancement of growth by expression of poplar cellulase in Arabidopsis thaliana. Plant J 33:1099–1106
Peng L, Kawagoe Y, Hogan P, Delmer D (2002) Sitosterol-β-glucoside as primer for cellulose synthesis in plants. Science 295:147–150
Sato S, Kato T, Kakegawa K, Ishii T, Liu YG, Awano T, Takabe K, Nishiyama Y, Kuga S, Sato S, Nakamura Y, Tabata S, Shibata D (2001) Role of the putative membrane-bound endo-1,4-β-glucanase KORRIGAN in cell elongation and cellulose synthesis in Arabidopsis thaliana. Plant Cell Physiol 42:251–263
Savaldi-Goldstein S, Peto C, Chory J (2007) The epidermis both drives and restricts plant shoot growth. Nature 446:199–202
Segal L, Creely JJ, Martin AE, Conrad CM (1959) An empirical method for estimating the degree of crystallinity of native cellulose using the X-ray diffractometer. Text Res J 29:786–794
Shedletzky E, Shmuel M, Delmer DP, Lamport DT (1990) Adaptation and growth of tomato cells on the herbicide 2,6-dichlorobenzonitrile leads to production of unique cell walls virtually lacking a cellulose-xyloglucan network. Plant Physiol 94:980–987
Takahashi J, Rudsander UJ, Hedenström M, Banasiak A, Harholt J, Amelot N, Immerzeel P, Ryden P, Endo S, Ibatullin FM, Brumer H, del Campillo E, Master ER, Scheller HV, Sundberg B, Teeri TT, Mellerowicz EJ (2009) KORRIGAN1 and its aspen homolog PttCel9A1 decrease cellulose crystallinity in Arabidopsis stems. Plant Cell Physiol 50:1099–1115
Tanimoto E, Fujii S, Yamamoto R, Inagana S (2000) Measurement of viscoelastic properties of root cell walls affected by low pH in lateral roots of Pisum sativum L. Plant Soil 226:21–28
Thompson JD, Higgins DG, Gibson TJ (1994) CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res 22:4673–4680
Tucker ML, Sexton R, del Campillo E, Lewis LN (1988) Bean abscission cellulase: characterization of a cDNA clone and regulation of gene expression by ethylene and auxin. Plant Physiol 88:1257–1262
Turner SR, Somerville CR (1997) Collapsed xylem phenotype of Arabidopsis identifies mutants deficient in cellulose deposition in the secondary cell wall. Plant Cell 9:689–701
Zhou HL, He SJ, Cao YR, Chen T, Du BX, Chu CC, Zhang JS, Chen SY (2006) OsGLU1, a putative membrane-bound endo-1,4-β-d-glucanase from rice, affects plant internode elongation. Plant Mol Biol 60:137–151
Zuo J, Niu QW, Nishizawa N, Wu Y, Kost B, Chua NH (2000) KORRIGAN, an arabidopsis endo-1,4-β-glucanase, localizes to the cell plate by polarized targeting and is essential for cytokinesis. Plant Cell 12:1137–1152
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Inukai, Y., Sakamoto, T., Morinaka, Y. et al. ROOT GROWTH INHIBITING, a Rice Endo-1,4-β-d-Glucanase, Regulates Cell Wall Loosening and is Essential for Root Elongation. J Plant Growth Regul 31, 373–381 (2012). https://doi.org/10.1007/s00344-011-9247-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00344-011-9247-3