Abstract
The effects of exogenous foliar glycine betaine (GB) and abscisic acid (ABA) on papaya responses to water stress were investigated under distinct water regimes. Papaya seedlings (Carica papaya L. cultivar “BH-65”) were pretreated with GB or ABA and subsequently subjected to consecutive periods of drought, rehydration, and a second period of drought conditions. Results indicated that water stress induced ABA, jasmonic acid (JA), and proline accumulation but did not modify malondialdehyde (MDA) concentration. In addition, water deprivation reduced photosynthetic rate, stomatal conductance, relative water content (RWC), leaf fresh weight, and increased leaf abscission. GB applied prior to drought imposition decreased the impact of water stress on ABA, JA, proline accumulation, leaf water status, growth, and photosynthetic performance. However, ABA-pretreated plants did not show alteration of most of these parameters under water stress conditions when compared with non-pretreated plants except a clear induction of JA accumulation. Taken together, the data suggest that GB may modulate ABA, JA, and proline accumulation through the control of stomatal movement and the high availability of compatible solutes, leading to improvement of leaf water status, growth, and photosynthetic machinery function. In contrast, exogenous ABA did not stimulate papaya physiological responses under drought, but interestingly ABA in combination with drought could induce progressive JA synthesis, unlike drought alone, which induces a transitory JA increase and may trigger endogenous ABA accumulation. The data also suggest that irrespective of the pretreatments, papaya did not suffer oxidative damage.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Drought is the major environmental threat to agricultural production and distribution worldwide. Papaya is a tropical fruit characterized by warm temperature and high water requirements and was considered relatively tolerant to water stress through the mechanism of dehydration postponement (Marler and others 1994; Marler and Mickelbart 1998). However, in previous experiments, we reported that papaya respond to drought by decreasing plant growth and leaf gas exchange parameters and by increasing leaf abscission (Mahouachi and others 2006, 2007). In addition, those experiments showed an accumulation of foliar inorganic solutes, which might contribute to osmotic adjustment (Mahouachi and others 2006).
It is actually well established that abscisic acid (ABA) plays an important role in mediating the main responses of plants to environmental stresses such as drought. Thus, ABA induces stomatal closure and reduces water loss via transpiration, which helps plants avoid water stress (Zeevaart and Creelman 1988; Davies and Zhang 1991). It is also accepted that endogenous ABA levels increase in response to water deficit (Zeevaart and Creelman 1988), indicating that activation of de novo ABA biosynthesis is required for the induction and maintenance of stomatal closure during water stress. ABA increased continuously under water stress and returned to control levels immediately after rehydration in several species, for instance, papaya seedlings and citrus fruits (Mahouachi and others 2005, 2007).
Exogenous ABA treatments prior to subjecting plants or tissues to adverse conditions have been reported to improve plant tolerance to osmotic stress (Nayyar and Walia 2003). In citrus, application of ABA and its analog 8′-methylene ABA reduced salt stress injuries such as leaf chloride concentration, ethylene production, and leaf abscission (Arbona and others 2006). It has also been reported that application of exogenous ABA increases drought tolerance of Kentucky bluegrass, improving cell turgor maintenance and reducing damage to cell membranes and photosynthetic systems (Wang and others 2003).
Jasmonic acid (JA) is a naturally occurring growth regulator found in higher plants, and several physiological roles have been described for this compound (or a related compound, methyl jasmonate) during plant development (Zhang and others 2006) and in response to biotic and abiotic stresses (Creelman and Mullet 1995). Therefore, JA and its derivatives have been involved in several stress responses such as plant–pathogen interactions (Peña-Cortés and others 2004), water deficit (Creelman and Mullet 1997) and salt stress (Walia and others 2007). Unlike progressive accumulation of ABA under environmental stress, JA levels increase rapidly and transiently in response to biotic and abiotic stresses such as mechanical stress (Weiler and others 1993), salt stress (Pedranzani and others 2003), and water stress (Mahouachi and others 2007).
On the other hand, one of the mechanisms for improving plant tolerance to drought is the process of osmotic adjustment, which leads to a lower osmotic potential through the net accumulation of osmotically active substances (Blum 1989). However, plant species differ greatly in relation to the types of solutes accumulated and their relative contribution in lowering the osmotic potential (Rhodes and others 2002). At the cellular level, osmotic homeostasis could result from sequestration of toxic substances in the vacuolar compartment, although the nontoxic solutes should be preferentially located in the cytoplasm where they could act as compatible solutes (Gagneul and others 2007) and as scavengers for reactive oxygen species (Smirnoff and Cumbes 1989). Some of these compatible solutes are proline and glycine betaine (GB). Several authors have studied the function of GB as an osmoprotectant in the adaptation to water, salt, and cold stress in many higher plants (Rhodes and Hanson 1993; Kishitani and others 1994). GB accumulation is correlated with tolerance of plants to osmotic stress (Saneoka and others 1995) through protection of cells by maintaining osmotic balance (Rhodes and Hanson 1993) and by stabilizing proteins, enzymes, and membranes (Papageorgiou and Murata 1995).
It has also been reported that plants are able to use foliar-applied GB and to translocate it to almost all plant parts, especially developing organs (Mäkelä and others 1996). Thus, foliar applications may increase the levels of GB in plants that are unable to synthesize this compound. The application of exogenous GB to crops at a range of water stress levels showed positive responses. In glasshouse experiments, increases in the leaf area and in the fresh and dry weights of tobacco leaves were reported by Agboma and others (1997). Increases in the growth rates of turnip rape and pea were found in glasshouse and field experiments (Mäkelä and others 1997). When it was applied to tomato plants under salt stress, fruit yield increased by up to 39% (Mäkelä and others 1998).
A well-known effect of water stress is lipid peroxidation that can lead to disturbance or destabilization of membrane structure (Liljenberg 1992). This oxidation is caused by generation of reactive oxygen species (ROS) during desiccation (Smirnoff 1993). Thus, a change in malondialdehyde (MDA) concentration has been widely accepted as an estimation of oxidative damage to lipid membranes (Hodges and others 1999). Accumulation of MDA under water stress has been reported in several plant species (Reddy and others 2004; Bhatnagar-Mathur and others 2009; Pei and others 2010). In other experimental systems, MDA increase and gradual decrease in antioxidant enzymes (peroxidase, superoxide dismutase, and ascorbate peroxidase) were induced by herbicide (ZJ0273) toxicity stress in oil seed rape seedlings. However, treatments of plants with 5-aminolevulinic acid (1 mg l−1) improved antioxidant activities and reduced peroxidation substances (MDA) (Zhang and others 2008). The influence of silicon treatment on drought-induced oxidative stress and antioxidant defense was investigated in wheat and its effects on antioxidant enzyme activities differ according to the stage of development (Gong and others 2008).
In the present work we investigated the responses of papaya plants subjected to a period of water stress and recovery followed by a second period of drought to elucidate how papaya respond to various periods of drought interrupted by rehydration. The established water regime condition is frequent in lands where rainfall is the unique water supply or when irrigation is available only periodically. In this study we have also characterized the influence of exogenous glycine betaine and abscisic acid applications prior to the imposition of both periods of drought stress on papaya responses.
Materials and Methods
Plant Material and Growth Conditions
Seedlings of Carica papaya L. cultivar “BH-65” were used in this work to assess its responses to different water regimes. This cultivar is characterized by marked dwarfism and suitable agronomic properties, especially when cultivated under greenhouses in the Canary Islands. Four-month-old plants received from the nursery were transplanted and grown in 50-l plastic containers (one plant per container) filled with peat substrate (Leader potting soil, Germany) under glasshouse conditions. Nutrients available in the substrate were N (200 mg l−1), P2O5 (200 mg l−1), and K2O (300 mg l−1). Before transplanting, 20 g/pot of granular fertilizer [Osmocote Pro, NPK fertilizer containing Mg with trace elements: 18(N)–9(P)–10(K)–2Mg–Te] was incorporated into the substrate. Plants were watered three times a week to maintain optimum water requirements. During the experimental period, relative humidity oscillated between 60 and 90% and temperature was controlled between 20 and 30°C throughout the day and the night.
Water Stress Treatments
Plants were subjected to a period of water stress and rehydration, and then a later period of drought. Thus, water stress treatment was imposed by suspending irrigation for 18 days (first drought period). After this period, plants were watered to soil maximum field capacity for 30 days to achieve total plant recovery. Plants were then subjected again to a second period of water stress for 26 days. The durations of drought periods were determined in accordance with the establishment of severe symptoms of drought such as strong diminution of soil moisture, leaf dehydration, and growth detention. The whole experiment was repeated twice under the same conditions and similar results were obtained. Then, data corresponding to one of the experiments were used in this study. In the present experiment, a total of 48 plants were established following a randomized design to carry out the different measurements and samplings. These plants were distributed into four treatments: (a) control, well-irrigated plants; (b) water stress, plants subjected to water deprivation during both periods; (c) water stress + ABA, plants pretreated with ABA and subjected to water stress; and (d) water stress + GB, plants pretreated with GB and subjected to water stress.
Chemical Application
Both ABA and GB (Sigma-Aldrich, St. Louis, MO, USA) were applied by foliar spray. All plant leaves received enough chemical solution to be entirely well wet. Exogenous treatments were performed three times a week for 2 weeks before the beginning of each water stress period. ABA (100 μM) and GB (50 mM) were dissolved in 5% (v/v) aqueous ethanol and water, respectively. A few drops of 0.05% Tween-20 were added to each solution. Control and only water-stressed plants were treated with a solution of distilled water and 0.05% Tween-20.
Growth and Sampling
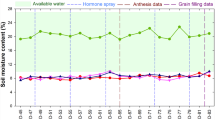
At the beginning and at the end of each period of drought, adult leaf numbers (completely developed leaves) were recorded, and total leaves per plant (except dried leaves, that is, at least half of the leaf acquired yellow color losing the characteristic green color and returned senescent) were collected and their fresh weights were determined. The two first fully expanded leaves counting from the plant apex were harvested at 0, 18 days after water stress 1 (DAWS1), 30 days after rehydration (DARE), and 26 days after water stress 2 (DAWS2) (Fig. 1) for hormonal analysis, MDA, and proline determination. Plant tissues were frozen in liquid nitrogen, lyophilized, ground, and finally used for analysis.
Soil Moisture
Soil moisture was determined during the experimental period by using Trime-FM Time Domain Reflectometry (TDR) equipment (Imko Equipment, Ettlingen, Germany) as described previously (Mahouachi and others 2006). In brief, the instrument was equipped with two rod connector probes 15 cm in length and spaced 5 cm apart. One permanent probe per container was vertically inserted into the substrate to a depth of 15 cm. This equipment determined the percentage of volumetric soil moisture content.
Leaf Water Status
Relative water content (RWC) was determined in papaya leaves throughout the periods of drought and rehydration. Leaves were collected and immediately weighed to determine fresh weight (FW), then transferred to flasks containing 1 l of distilled water where they rehydrated for 8 h at 4°C until full turgor. Afterward, leaves were first surface-dried and then reweighed to obtain turgid weight (TW). Finally, leaves were oven-dried at 72°C for 48 h and reweighed to determine dry weight (DW). This parameter was calculated using the formula: RWC (%) = (FW − DW) ÷ (TW − DW) * 100.
Photosynthesis Parameters
Net photosynthetic rate (A) and stomatal conductance (gs) were measured in papaya leaves during the experimental period. A portable photosynthesis system (LCpro, ADC BioScientific Ltd., Hoddesdon, UK) with a leaf chamber window area of 6.25 cm2 was used for these measurements. Measurements were carried out on the third fully expanded leaves counting from the plant apex under an airflow rate of 200 μmol s−1, environmental humidity, and environmental CO2. Light was provided by a red/blue LED array and was set at 1,200 μmol m−2 s−1 of photon flux density (PFD). Measurements were performed in the morning (9:00 to 11:00 a.m.), and the temperature within the leaf chamber was 27.0 ± 0.5°C and leaf-to-air vapor pressure deficit was 1.8 ± 0.3 kPa.
ABA and JA Analysis
Plant hormones were analyzed by liquid chromatography coupled to tandem mass spectrometry following the procedure described in Durgbanshi and others (2005) and Mahouachi and others (2007). In brief, 50 μl of a mixture of internal standards containing 100 ng of [2H6] ABA and 100 ng of [2H6] JA was added to 0.05 g of lyophilized and triturated plant material. The tissue was homogenized in 5 mL of ultrapure water. Extracts were then centrifuged at 5,000×g for 10 min to pellet debris. The pH of the supernatant was adjusted to 2.8 with 15% CH3COOH and partitioned twice against an equal volume of diethyl ether. After discarding the aqueous phase, the organic fraction was evaporated in vacuum at room temperature and the solid residue was resuspended in 1 ml of a water/methanol (90:10 v:v) solution which was filtered through a 0.22-μm cellulose acetate filter. A 20-μl aliquot of this solution was then directly injected into an Alliance 2690 HPLC system (Waters, Milford, MA, USA) coupled to a tandem mass spectrometer (geometry quadrupole-hexapole-quadrupole, Quatro LC, Micromass, Manchester, UK) through an orthogonal Z-spray electrospray interface. Concentrations of each plant hormone were determined using calibration curves performed with known amounts of pure standard samples.
Proline Content
Leaf dry weight (0.05 g) was extracted in 5 ml of 3% sulfosalicylic acid (Panreac, Barcelona, Spain) with the assistance of a homogenizer (Ultra-Turrax, IKA-Werke, Staufen, Germany). Homogenates were centrifuged at 5,000×g for 20 min at 4°C, and proline content was determined following the procedures described by Bates and others (1973). In brief, 1 ml of the supernatant was added to 2 ml of a mixture of glacial acetic acid and ninhydrin reagent (Panreac) in a 1:1 (v:v) ratio. The mixture was reacted in a water bath at 100°C for 1 h and continuously cooled in an ice bath for 15 min. Absorbance was read in the organic phase at 520 nm. A standard curve was performed with proline (Sigma-Aldrich).
Malondialdehyde Production
Malondialdehyde (MDA) concentration was determined following the procedure of Hodges and others (1999). Briefly, dry leaf tissue (0.05 g) was homogenized in 80% of ethanol using a tissue homogenizer (Ultra-Turrax). Extracts were centrifuged at 5,000×g for 30 min and different aliquots of the supernatant were mixed with either 20% trichloroacetic acid or a mixture of 20% trichloroacetic acid and 0.5% thiobarbituric acid. Both mixtures were incubated in a water bath at 90°C for 1 h. Afterward, samples were cooled in an ice bath for 15 min. Absorbance of the supernatant was read at 440, 534, and 600 nm against a blank.
Statistical Analyses and Reproducibility of the Determinations
Plants were distributed in three blocks with 12 plants per treatment in each block. For hormone, MDA, and proline analysis, three plants per block and treatment were collected at each date of sampling. Also, three plants per block and treatment were used for growth measurements and photosynthetic parameters. Mean values were compared using the least significant difference (LSD) test (p ≤ 0.05). Statistical analyses were performed by using Systat 10 (SPSS Inc., Chicago, IL, USA).
Results
Water Status
To assess plant water status, leaf relative water content (RWC) was determined using the third completely developed leaf counting from the plant apex during the periods of water stress and rehydration (Fig. 2). At the onset of drought experiments, leaf RWC in hydrated plants varied between 89 and 93% in control and pretreated plants. Drought stress reduced leaf RWC about 10% in comparison with controls 18 days after irrigation was stopped. At this date, ABA-pretreated water-stressed plants exhibited similar RWC as only-water-stressed plants; however, GB-pretreated water-stressed plants showed similar leaf water content as controls. Rewatering of plants for 30 days returned their water status to control levels. The application of a second cycle of drought (26 days) reduced RWC about 6% with respect to controls; however, no significant changes were produced in relation to controls when these plants were previously treated with ABA or GB.
Relative water content [RWC (%)] in papaya leaves in control and dehydrated plants subjected or not to pretreatments with ABA or GB. Data are means ± SE, and each value was determined from three different plants with three replicates per treatment (n = 9). For each date, dissimilar letters above columns differ significantly by the LSD test at p ≤ 0.05. DAT days after treatments, DAWS1 days after the first period of water stress, DAWS2 days after the second period of water stress, DARE days after rehydration, WS water stress, CT plants irrigated regularly, WS plants subjected to water stress for 18 days, rehydrated for 30 days, and finally subjected again to water stress for 26 days, ABA + WS plants pretreated with ABA and subjected to WS, GB + WS plants pretreated with GB and subjected to WS
Soil moisture was also measured in irrigated and nonirrigated pots and showed low values at the end of each drought period (Fig. 3). TDR equipment registered values of 26 and 15% in hydrated and nonhydrated substrates, respectively, at 18 DAWS1. Similar values in control and 13.5% in dehydrated substrates were determined at 26 DAWS2.
Soil moisture (%) in irrigated and nonirrigated pot soils of papaya plants. Data are means ± SE, and each value was determined by three TDR probes with three replicates per treatment (n = 9) (one probe per pot). For each date, dissimilar letters above columns differ significantly by the LSD test at p ≤ 0.05. DAT days after treatments, DAWS1 days after the first period of water stress, DAWS2 days after the second period of water stress, DARE days after rehydration, WS water stress, CT plant pots irrigated regularly, WS plant pots subjected to water stress for 18 days, rehydrated for 30 days, and finally subjected again to water stress for 26 days
Leaf Growth
To evaluate how water restriction could affect plant growth, total leaf fresh weight (Table 1) and adult functional leaf number (Table 2) were quantified at the beginning and at the end of each cycle of drought. Total leaf fresh weight per plant oscillated between 41 and 50 g at the onset of the experiment. Drought applied continuously for 18 days significantly reduced leaf fresh weight at the end of the first period of water deprivation. Application of drought alone and drought plus ABA reduced leaf fresh weight accumulation about 77 and 70%, respectively, in comparison to controls (Table 1). Nevertheless, leaf fresh weight decrease was less severe in plants pretreated with GB (57% with respect to control). Restoration of water supply over 30 days recovered leaf growth and accumulation of fresh weight was similar among control and all drought-treated plants. Elimination of watering through a second phase of drought reduced leaf fresh weight again in plants pretreated or not with ABA or GB about 65–70% compared to controls (Table 1). On the other hand, water removal for 18 days increased leaf abscission about 65 and 56% in water-stressed and ABA-pretreated water-stressed plants, respectively, compared to controls (Table 2). The exogenous application of GB reduced the impact of drought on leaf abscission, and then only 39% of leaves were abscised compared to controls. Hydration of the plants after this period of drought stimulated the emergence and growth of new leaves, and 30 days thereafter leaf persistence was statistically similar between stressed and nonstressed plants. The application of a new cycle of dehydration diminished leaf numbers in water-stressed plants and in plants pretreated with ABA or GB by 56–60% compared to controls. In this second cycle of drought, GB did not alter leaf abscission in comparison with the treatments of water stress alone or in combination with ABA (Table 2).
Photosynthesis and Stomatal Conductance
Photosynthetic rate (A) varied between 9.6 and 10.4 μmol m−2 s−1 in control plants throughout the experimental period (Fig. 4). The imposition of the first period of drought induced a significant reduction of net CO2 assimilation in water-stressed plants (72%) and in ABA-pretreated and dehydrated plants (69%); however, this reduction was less severe in GB-pretreated water stressed plants (48%) in comparison to controls. Rehydration completely recovered leaf assimilation of CO2, and similar values of A were observed in control and in previously dehydrated or pretreated plants 30 days after rewatering. The application of a second period of water stress reduced again the values of A in dehydrated plants in comparison to controls. The photosynthetic rate in GB-pretreated plants was slightly higher than in ABA-pretreated or only-dehydrated plants.
Photosynthetic rate (A) in papaya leaves in control and dehydrated plants subjected or not to pretreatments with ABA or GB. Data are means ± SE, and each value was determined from three different plants with three replicates per treatment (n = 9). For each date, dissimilar letters above columns differ significantly by the LSD test at p ≤ 0.05. DAT days after treatments, DAWS1 days after the first period of water stress, DAWS2 days after the second period of water stress, DARE days after rehydration, WS water stress, CT plants irrigated regularly, WS plants subjected to water stress for 18 days, rehydrated for 30 days, and finally subjected again to water stress for 26 days, ABA + WS plants pretreated with ABA and subjected to WS, GB + WS plants pretreated with GB and subjected to WS
In addition, stomatal conductance (gs) showed a similar pattern of change as photosynthetic rate. The first water stress period significantly reduced gs values about 73 and 71% in dehydrated and in ABA-pretreated dehydrated plants, respectively, in comparison to controls (Fig. 5). GB-pretreated dehydrated plants suffered the effect of drought (55% decrease with respect to control) to a lesser extent. Rehydration recovered gs of all treated plants to control levels. The second phase of drought induced again a significant reduction of gs values, about 81 and 78% in water-stressed and in ABA-pretreated water-stressed plants, respectively, in comparison to control. GB-pretreated water-stressed plants showed lower reductions of gs than water-stressed ABA-pretreated or not plants. This decrease was 65% in comparison to controls.
Stomatal conductance (gs) in papaya leaves in control and dehydrated plants subjected or not to pretreatments with ABA or GB. Data are means ± SE, and each value was determined from three different plants with three replicates per treatment (n = 9). For each date, dissimilar letters above columns differ significantly by the LSD test at p ≤ 0.05. DAT days after treatments, DAWS1 days after the first period of water stress, DAWS2 days after the second period of water stress, DARE days after rehydration, WS water stress, CT plants irrigated regularly, WS plants subjected to water stress for 18 days, rehydrated for 30 days, and finally subjected again to water stress for 26 days; ABA + WS plants pretreated with ABA and subjected to WS, GB + WS plants pretreated with GB and subjected to WS
Proline Accumulation
Proline concentration was determined in papaya leaves from irrigated and dehydrated plants (Fig. 6). At day 0, no proline changes were observed between control and ABA- or GB-pretreated plants and values varied between 22 and 24 μmol g−1 DW. When subjected to progressive water stress over 18 days, proline concentration significantly increased in water-stressed, ABA-pretreated dehydrated, and GB-pretreated dehydrated leaves by 53, 41, and 28%, respectively, compared to controls. Proline accumulation in GB-pretreated leaves was significantly lower than in only-water-stressed plants (Fig. 6). Rewatering of plants for 30 days returned proline to control levels. Application of a second cycle of water stress again increased proline concentrations in only-water-stressed and in ABA- or GB-pretreated water-stressed plants. At this date, proline accumulation was lower in GB-pretreated (40%) and also in ABA-pretreated (45%) than in only-water-stressed leaves (59%) with respect to controls (Fig. 6).
Proline concentration in papaya leaves in control and dehydrated plants subjected or not to pretreatments with ABA or GB. Data are means ± SE, and each value was determined from three different plants with three replicates per treatment (n = 9). For each date, dissimilar letters above columns differ significantly by the LSD test at p ≤ 0.05. DAT days after treatments, DAWS1 days after the first period of water stress, DAWS2 days after the second period of water stress, DARE days after rehydration, WS water stress, CT plants irrigated regularly, WS plants subjected to water stress for 18 days, rehydrated for 30 days, and finally subjected again to water stress for 26 days, ABA + WS plants pretreated with ABA and subjected to WS, GB + WS plants pretreated with GB and subjected to WS
MDA Concentration
MDA was determined in papaya leaves to estimate the putative oxidative damage to lipid membranes induced by drought in papaya leaves (Fig. 7). MDA concentrations were high in control plants and varied between 195 and 247 mmol g−1 DW. The different treatments of water stress and pretreatments with ABA or GB slightly reduced or maintained the concentration of MDA similar to that of controls at the end of the two periods of drought and after rehydration.
Malondialdehyde (MDA) concentration in papaya leaves in control and dehydrated plants subjected or not to pretreatments with ABA or GB. Data are means ± SE, and each value was determined from three different plants with three replicates per treatment (n = 9). For each date, dissimilar letters above columns differ significantly by the LSD test at p ≤ 0.05. DAT days after treatments, DAWS1 days after the first period of water stress, DAWS2 days after the second period of water stress, DARE days after rehydration, WS water stress, CT plants irrigated regularly, WS plants subjected to water stress for 18 days, rehydrated for 30 days, and finally subjected again to water stress for 26 days, ABA + WS plants pretreated with ABA and subjected to WS, GB + WS plants pretreated with GB and subjected to WS
Hormonal Changes
To elucidate the hormonal changes in papaya after various episodes of water stress interrupted by rehydration, foliar ABA and JA were analyzed. Leaf ABA concentrations varied between 25 and 41 ng g−1 DW in control plants during the experimental period (Fig. 8). GB pretreatment did not modify foliar ABA concentration; however, application of ABA significantly increased endogenous ABA accumulation (63% with respect to control) at day 0. The suppression of watering for 18 days significantly increased ABA concentrations in water-stressed and in ABA-pretreated water-stressed plants about 79% in comparison to controls. However, GB and drought induced a significant increase of ABA concentration of about 57% with respect to controls. Rehydration in contrast returned ABA in dehydrated and/or pretreated plants to control levels. The application of a second cycle of drought increased again ABA accumulation in leaves of stressed plants pretreated or not. In comparison to controls, such increases were 74, 80, and 61% in non-pretreated, ABA-pretreated, and GB-pretreated water-stressed plants, respectively.
Abscisic acid (ABA) concentration in papaya leaves in control and dehydrated plants subjected or not to pretreatments with ABA or GB. Data are means ± SE, and each value was determined from three different plants with three replicates per treatment (n = 9). For each date, dissimilar letters above columns differ significantly by the LSD test at p ≤ 0.05. DAT days after treatments, DAWS1 days after the first period of water stress, DAWS2 days after the second period of water stress, DARE days after rehydration, WS water stress, CT plants irrigated regularly, WS plants subjected to water stress for 18 days, rehydrated for 30 days, and finally subjected again to water stress for 26 days, ABA + WS plants pretreated with ABA and subjected to WS, GB + WS plants pretreated with GB and subjected to WS
Leaf JA was also analyzed in irrigated and nonirrigated plants subjected or not to pretreatments of ABA or GB (Fig. 9). At the onset of the experiment (day 0), JA concentrations were quite similar in control and in ABA-pretreated plants; however, GB-pretreated leaves showed significantly less accumulation of JA compared to control and ABA-pretreated leaves. JA levels were not modified in water-stressed and in GB-pretreated water-stressed leaves, but they increased significantly in ABA-pretreated water-stressed leaves (40% with respect to control, 18 DAWS1). Rehydration restored JA concentrations to around control levels. The application of a second cycle of drought increased JA concentrations in water-stressed and in ABA-pretreated water-stressed leaves about 57 and 44%, respectively. GB treatment did not alter foliar JA concentration.
Jasmonic acid (JA) concentration in papaya leaves in control and dehydrated plants subjected or not to pretreatments with ABA or GB. Data are means ± SE, and each value was determined from three different plants with three replicates per treatment (n = 9). For each date, dissimilar letters above columns differ significantly by the LSD test at p ≤ 0.05. DAT days after treatments, DAWS1 days after the first period of water stress, DAWS2 days after the second period of water stress, DARE days after rehydration, WS water stress, CT plants irrigated regularly, WS plants subjected to water stress for 18 days, rehydrated for 30 days, and finally subjected again to water stress for 26 days, ABA + WS plants pretreated with ABA and subjected to WS, GB + WS plants pretreated with GB and subjected to WS
Discussion
We previously reported that papaya accumulate inorganic solutes in response to water deficit that could help plants to withstand the effects of drought through osmotic adjustment (Mahouachi and others 2006). In the present work, data show that foliar GB, an osmoprotectant organic solute, exhibits a positive effect on papaya water stress responses leading to reduced drought impact. The application of this osmolyte prior to drought imposition increased photosynthetic rate (Fig. 4), leaf relative water content (Fig. 2), leaf persistence (Table 2), and consequently biomass accumulation (Table 1). Therefore, results suggest that GB-pretreated plants acquired the ability to maintain an adequate water status under drought through the control of stomatal movement (Fig. 5) and an improvement in the process of osmotic adjustment resulting from the increase of its availability as a compatible solute. In this process, we propose that GB regulates the movements of stomata by prolonging stomatal opening under high leaf turgor as a consequence of elevated osmoprotective activity, and stomatal closure occurs when a threshold turgor induced by water loss is established. The useful effects of GB application have been reported in several plant species and crops when applied at a range of water stress levels. Thus, increases in leaf area and in fresh and dry weights were reported in tobacco (Agboma and others 1997), increases in growth rates were found in turnip rape and pea (Mäkelä and others 1997), and increases in photosynthesis parameters were found in tomato (Mäkelä and others 1999). In maize, Yang and Lu (2006) showed an increase of growth, net CO2 assimilation rate, and stomatal conductance at low GB concentrations (2–20 mM). When it was applied to tomato plants under salt stress, fruit yield increased by up to 39% (Mäkelä and others 1998). Our data also suggest that the availability of foliar GB could modulate endogenous proline accumulation under water stress, because leaves of stressed plants accumulated less proline in GB-pretreated than in ABA-pretreated or nontreated plants. In this context, GB may act indirectly on stomata movements either by changing the turgor of the epidermis or by guard cells, which would allow plants to save water and avoid at least temporarily water stress effects leading to reduced proline levels.
It is widely accepted that ABA is one of the signals that trigger numerous plant adaptations under drought conditions (Davies and Jones 1991). In previous experiments we reported an increase of ABA concentration in papaya tissues under water deficit and a decrease to control levels immediately after rehydration (Mahouachi and others 2007). In the present work, data indicate that exogenous ABA reinforces the accumulative role of endogenous ABA under drought stress, whereas foliar GB supply moderates this accumulation probably as a result of the alleviation of drought damage on plant tissue through the control of stomatal movement and improvement of RWC. This might suggest that papaya plants activate the mechanism of osmotic adjustment through GB action, and ABA synthesis could be increased when drought damage becomes more severe. ABA has also been shown to be involved in promoting drought tolerance when applied exogenously (Wang and others 2003; Li and others 2004).
Currently, it is well accepted that JA and its derivative methyl jasmonate are involved in several physiological processes during plant development and in response to environmental stresses (Creelman and Mullet 1997). In response to water deficit and osmotic stress, the endogenous levels of jasmonates markedly increase in plants (Lehmann and others 1995; Xin and others 1997). Recently, we reported that drought induced a transient increase of JA and that increase was not related to an accumulative pattern but rather to a sharp increase compatible with a triggering signal upstream of ABA (Mahouachi and others 2007). Here, it is worth emphasizing the effectiveness of exogenous GB on the JA decrease induced in control leaves (0 DAWS1, Fig. 9) in addition to the maintenance of its levels similar to that of controls at the end of both stress periods. These results strengthen the role of JA as an early sensitive signal that detects environmental changes in plant tissues also under control conditions which signifies that JA levels are naturally high and that GB conditioned plants to lower these levels in the absence and also in the presence of water stress. Data also show that ABA-pretreated water-stressed plants accumulated JA after water shortage, suggesting that there is an effect of ABA supply on JA synthesis and accumulation. Actually, the inducing effect of ABA on JA accumulation is not well known; however, the inverse process has been reported in several experimental systems such as rice plants that produce methyl jasmonate under drought which in turn stimulates the production of ABA (Kim and others 2009).
It has been reported that lipid membranes are vulnerable targets for stress-induced cellular damage and that the extent of damage is commonly used as a measure of tolerance to the imposed stress (Zhao and Harris 1992). Our experimental data show that MDA concentrations in water-stressed plants were similar or even lower than in control plants. In addition, GB and ABA pretreatments did not affect MDA content. These results might indicate that papaya did not suffer oxidative damage to lipid membranes under the two periods of drought. Therefore, papaya plants appear to escape oxidative stress through the maintenance of high natural MDA levels, which would help plants to activate the antioxidant machinery leading to the evasion or at least the postponement of dehydration damage. Increased levels of MDA under water stress were reported in numerous species such as wheat (Pei and others 2010), peanut (Bhatnagar-Mathur and others 2009), and mulberry (Reddy and others 2004). Lipid peroxidation increase was also reported in leaves of oilseed rape plants under waterlogging (Leul and Zhou 1999). In that system, the authors suggested that uniconazole improved waterlogged plant performance possibly by improving the antioxidant defense mechanism and delaying lipid peroxidation and membrane deterioration. The involvement of uniconazole in the induction of antioxidant activities and the improvement of root oxidizability and plant vigor was also revealed in winter rape (Zhou and Ye 1996).
Overall, the results presented here suggest that GB mediates induction of water stress responses in papaya plants. Thus, GB applied prior to drought imposition may modulate ABA, JA, and proline accumulation through the control of stomatal movement and the high availability of the compatible solute leading to improvement of leaf water status and growth. In contrast, exogenous ABA did not mitigate papaya responses under drought, but interestingly ABA in combination with drought could induce progressive JA synthesis unlike drought alone, which induces a transitory JA increase and may trigger endogenous ABA accumulation. The data also suggest that papaya did not suffer oxidative damage.
References
Agboma PC, Peltonen-Sainio P, Hinkkanen R, Pehu E (1997) Effect of foliar glycinebetaine on yield components of drought-application of stressed tobacco plants. Exp Agric 33:345–352
Arbona V, López-Climent MF, Mahouachi J, Pérez-Clemente RM, Abrams SR, Gómez-Cadenas A (2006) Use of persistent analogues of abscisic acid as palliatives against salt-stress induced damage in citrus plants. J Plant Growth Regul 25:1–9
Bates LS, Waldren RP, Teare ID (1973) Rapid determination of free proline for water-stress studies. Plant Soil 39:205–220
Bhatnagar-Mathur P, Devi MJ, Vadez V, Sharma KK (2009) Differential antioxidative responses in transgenic peanut bear no relationship to their superior transpiration efficiency under drought stress. J Plant Physiol 166:1207–1217
Blum A (1989) Osmotic adjustment and growth of barley genotypes under drought stress. Crop Sci 29:230–233
Creelman RA, Mullet JE (1995) Jasmonic acid distribution and action in plants: regulation during development and response to biotic and abiotic stress. Proc Natl Acad Sci USA 92:4114–4119
Creelman RA, Mullet JE (1997) Biosynthesis and action of jasmonates in plants. Annu Rev Plant Physiol Plant Mol Biol 48:355–381
Davies WJ, Jones HG (1991) Abscisic acid: physiology and biochemistry. BIOS Scientific Publishers, Oxford
Davies WJ, Zhang J (1991) Root signals and the regulation of growth and development of plants in drying soil. Annu Rev Plant Physiol Plant Mol Biol 42:55–76
Durgbanshi A, Arbona V, Pozo O, Miersch O, Sancho JV, Gómez-Cadenas A (2005) Simultaneous determination of multiple phytohormones in plant extracts by liquid chromatography-electrospray tandem mass spectrometry. J Agric Food Chem 53:8437–8442
Gagneul D, Aïnouche A, Duhazé C, Lugan R, Larher FR, Bouchereau A (2007) A reassessment of the function of compatible solutes in the halophytic Plumbaginaceae Limonium latifolium. Plant Physiol 144:1598–1611
Gong HJ, Chen KM, Zhao ZG, Chen GC, Zhou WJ (2008) Effects of silicon on defense of wheat against oxidative stress under drought at different developmental stages. Biol Plant 52:592–596
Hodges D, DeLong J, Forney C, Prange R (1999) Improving the thiobarbituric acid-reactive-substances assay for estimating lipid peroxidation in plant tissues containing anthocyanin and other interfering compounds. Planta 207:604–611
Kim EH, Kim YS, Park SH, Koo YJ, Choi YD, Chung YY, Lee IJ, Kim JK (2009) Methyl Jasmonate reduces grain yield by mediating stress signals to alter spikelet development in rice. Plant Physiol 149:1751–1760
Kishitani S, Watanabe K, Yasuda S, Arakawa K, Takabe T (1994) Accumulation of glycinebetaine during cold acclimation and freezing tolerance in leaves of winter and spring barley plants. Plant Cell Environ 17:89–95
Lehmann J, Atzorn R, Bruckner C, Reinbothe S, Leopold J, Wasternack C, Parthier B (1995) Accumulation of jasmonate, abscisic acid, specific transcripts and proteins in osmotically stressed barley leaf segments. Planta 197:156–162
Leul M, Zhou WJ (1999) Alleviation of waterlogging damage in winter rape by uniconazole applications: effects on enzyme activity, lipid peroxidation, and membrane integrity. J Plant Growth Regul 18:9–14
Li C, Yin C, Liu S (2004) Different responses of two contrasting Populus davidiana populations to exogenous abscisic acid application. Environ Exp Bot 51:237–246
Liljenberg CS (1992) The effects of water deficit stress on plant membrane lipids. Prog Lipid Res 3:335–343
Mahouachi J, Gómez-Cadenas A, Primo-Millo E, Talón M (2005) Antagonistic changes between abscisic acid and gibberellins in citrus fruits subjected to a series of different water conditions. J Plant Growth Regul 24:179–187
Mahouachi J, Socorro AR, Talon M (2006) Responses of papaya seedlings (Carica papaya L.) to water stress and re-hydration: growth, photosynthesis and mineral nutrient imbalance. Plant Soil 281:137–146
Mahouachi J, Arbona V, Gómez-Cadenas A (2007) Hormonal changes in papaya seedlings subjected to progressive water stress and re-watering. Plant Growth Regul 53:43–51
Mäkelä P, Peltonen-Sainio P, Jokinen K, Pehu E, Setälä H, Hinkkanen R, Somersalo S (1996) Uptake and translocation of foliar-applied glycine-betaine in crop plants. Plant Sci 121:221–230
Mäkelä P, Kleemola J, Jokinen K, Mantila J, Pehu E, Peltonen-Sainio P (1997) Growth response of pea and summer turnip rape to foliar application of glycinebetaine. Acta Agric Scand B Soil Plant Sci 47:168–175
Mäkelä P, Jokinen K, Kontturi M, Peltonen-Sainio P, Pehu E, Somersalo S (1998) Foliar application of glycinebetaine—a novel product from sugar beet—as an approach to increase tomato yield. Ind Crops Prod 7:139–148
Mäkelä P, Kontturi M, Pheu E, Somersalo S (1999) Photosynthetic response of drought and salt-stressed tomato and turnip rape plants to foliar-applied glycinebetaine. Physiol Plant 105:45–50
Marler TE, Mickelbart MV (1998) Drought, leaf gas exchange, and chlorophyll fluorescence of field-grown papaya. J Am Soc Hortic Sci 123:714–718
Marler TE, George AP, Nissen RJ, Andersen PC (1994) Miscellaneous tropical fruits. In: Schaffer B, Andersen PC (eds) Sub-tropical and tropical crops. Handbook of environmental physiology of fruit crops, vol 2. CRC Press, Boca Raton, pp 199–224
Nayyar H, Walia DP (2003) Water stress induced proline accumulation in contrasting wheat genotypes as affected by calcium and abscisic acid. Biol Plant 46:275–279
Papageorgiou GC, Murata N (1995) The unusually strong stabilizing effects of glycinebetaine on the structure and function of the oxygen-evolving photosystem complex. Photosynth Res 44:243–252
Pedranzani H, Racagni G, Alemano S, Miersch O, Ramírez I, Peña-Cortés H, Machado-Domenech E, Abdala G (2003) Salt tolerant tomato plants show increased levels of jasmonic acid. Plant Growth Regul 41:149–158
Pei ZF, Ming DF, Liu D, Wan GL, Geng XX, Gong HJ, Zhou WJ (2010) Silicon improves the tolerance to water-deficit stress induced by polyethylene glycol in wheat (Triticum aestivum L.) seedlings. J Plant Growth Regul 29:106–115
Peña-Cortés H, Barrios P, Dorta F, Polanco V, Sánchez C, Sánchez E, Ramírez I (2004) Involvement of jasmonic acid and derivatives in plant responses to pathogens and insects and in fruit ripening. J Plant Growth Regul 23:246–260
Reddy AR, Chaitanya KV, Jutur PP, Sumithra K (2004) Differential antioxidative responses to water stress among five mulberry (Morus alba L.) cultivars. Environ Exp Bot 52:33–42
Rhodes D, Hanson AD (1993) Quaternary ammonium and tertiary sulfonium compounds in higher plants. Annu Rev Plant Physiol Plant Mol Biol 44:357–384
Rhodes D, Nadolska-Orczyk A, Rich PJ (2002) Salinity, osmolytes and compatible solutes. In: Laüchli A, Lüttge U (eds) Salinity: environment–plant–molecules. Kluwer Academic Publishers, Amsterdam, pp 181–204
Saneoka H, Nagasaka C, Hanh DT, Yang WJ, Premachandra GS, Joly RJ, Rhodes D (1995) Salt tolerance of glycinebetaine-deficient and containing maize lines. Plant Physiol 107:631–638
Smirnoff N (1993) The role of active oxygen in the response of plants to water deficit and desiccation. New Phytol 125:27–58
Smirnoff N, Cumbes QJ (1989) Hydroxyl radical scavenging activity of compatible solutes. Phytochemistry 28:1057–1060
Walia H, Wilson C, Condamine P, Liu X, Ismail AM, Close TJ (2007) Large-scale expression profiling and physiological characterization of jasmonic acid-mediated adaptation of barley to salinity stress. Plant Cell Environ 30:410–421
Wang Z, Huang B, Xu Q (2003) Effects of abscisic acid on drought responses of Kentucky bluegrass. J Am Soc Hortic Sci 128:36–41
Weiler EW, Albrecht T, Groth B, Xia ZQ, Luxem M, Liss H, Andert L, Spengler P (1993) Evidence for the involvement of jasmonates and their octadecanoid precursors in the tendril coiling response of Bryonia dioica. Phytochemistry 32:591–600
Xin ZY, Zhou X, Pilet PE (1997) Level changes of jasmonic, abscisic, and indole-3yl-acetic acids in maize under desiccation stress. J Plant Physiol 151:120–124
Yang X, Lu C (2006) Effects of exogenous glycinebetaine on growth, CO2 assimilation, and photosystem II photochemistry of maize plants. Physiol Plant 127:593–602
Zeevaart JAD, Creelman R (1988) Metabolism and physiology of abscisic acid. Annu Rev Plant Physiol Plant Mol Biol 39:439–473
Zhang ZJ, Zhou WJ, Li HZ, Zhang GQ, Subrahmaniyan K, Yu JQ (2006) Effect of jasmonic acid on in vitro explant growth and microtuberization in potato. Biol Plant 50:453–456
Zhang WF, Zhang F, Raziuddin R, Gong HJ, Yang ZM, Lu L, Ye QF, Zhou WJ (2008) Effects of 5-aminolevulinic acid on oilseed rape seedling growth under herbicide toxicity stress. J Plant Growth Regul 27:159–169
Zhao KF, Harris PJC (1992) The effects of iso-osmotic salt and water stresses on the growth of halophytes and non-halophytes. J Plant Physiol 139:761–763
Zhou W, Ye Q (1996) Physiological and yield effects of uniconazole on winter rape (Brassica napus L.). J Plant Growth Regul 15:69–73
Acknowledgments
This work was supported by the Spanish Ministerio de Ciencia e Innovación and the Instituto Nacional de Investigaciones Agrarias through grant RTA09-159. HPLC-MS equipment used for hormonal analyses was facilitated by the SCIC of the Universitat Jaume I.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mahouachi, J., Argamasilla, R. & Gómez-Cadenas, A. Influence of Exogenous Glycine Betaine and Abscisic Acid on Papaya in Responses to Water-deficit Stress. J Plant Growth Regul 31, 1–10 (2012). https://doi.org/10.1007/s00344-011-9214-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00344-011-9214-z












