Abstract
FIR temperature measurement based on thermally coupled levels of rare earth ions has been widely studied, but its sensitivity is limited by the thermal coupling levels’ spacing. In this letter, a transition from non-thermally coupled levels of Ho3+ to thermally coupled levels is reported for the first time. It can effectively improve the sensitivity of temperature measurement based on thermal coupling levels. The Ho3+/Yb3+ co-doped Y2O3 millimeter crystals have been prepared by laser annealing. The fluorescence properties of laser-annealed Ho3+/Yb3+ co-doped Y2O3 millimeter crystals were investigated as a function of temperature. It was found that the I667nm/I549nm show non-thermal coupling below 453K, but changes to thermal coupling above 453K. The maximum relative sensitivity of I667nm/I549nm in thermal coupling is greater than the relative sensitivity of any known thermal coupling energy levels of Ho3+. It provides a new way to improve the sensitivity of thermally coupled FIR temperature measurement.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Rare-earth (RE) ions are suitable to be up-conversion (UC) luminescent materials, as they have stable chemical properties, rich energy levels in the ultraviolet–visible-near infrared spectral region, and a long lifetime of the excited state. It can be applied in fields such as optical storage [1, 2], information processing [3], temperature measurement [4, 5], biological fluorescence labeling [6, 7] etc.
Among various RE ions, the Ho3+ ion has become one of the most effective trivalent RE ions to achieve visible UC luminescence owing to its favorable energy level structure [10]. Yb3+ ions with larger absorption cross sections in the 980nm were codoped with Ho3+ ions as sensitizers [8, 9]. Yttrium oxide is an ideal UC host substrate with excellent properties such as stable physicochemical properties and low phonon energy (~ 550 cm−1) [10,11,12,13]. In addition, the Y3+ ion has a similar radius to the RE ion, which allows for effective and easy co-doping with the RE ion [14].
The fluorescence intensity ratio (FIR) thermometry of RE shows a distinct advantage over other radiation thermometry methods due to the discrete fluorescence emission bands at the two thermally coupled levels [15]. Currently, there are many synthetic materials with doped RE ions are used for FIR temperature measurement, including NaLuF4: Yb3+-Er3+-Ho3+ [16], Eu3+:YVO4 [17], Sm3+ doped TiO2 nanoparticles [18] et al. In general, the two thermally coupled energy levels meet the Boltzmann distribution need the energy level spacing between 200 and 2000 cm−1. The relative sensitivity of thermal-coupled levels is proportional to the gap between the levels [19, 20]. Therefore, there is a limit in the relative sensitivity limit of FIR temperature measurement technology based on thermal coupling.
In this letter, Y2O3:Ho3+/Yb3+ millimeter crystals were obtained by the laser annealed method, and the fluorescence and temperature characteristics of Y2O3:Ho3+/Yb3+ millimeter crystals were studied. It was found that I667nm/I549nm showed non-thermally coupled at temperatures below 463 K and converted to thermally coupled at temperatures higher 463 K. A relative sensitivity higher than any known Ho3+ thermally coupled levels was achieved by the transition from non-thermally to thermally coupled levels.
2 Experimental
The sample used Aladdin’s analytical pure reagent (AR) yttrium nitrate hexahydrate (Y(NO3)3·6H2O, 99.99%), Ho(NO3)3·5H2O,99.99%), ytterbium nitrate pentahydrate (Yb(NO3)3·5H2O,99.99%). Fluorescent precursors with different doping concentrations were prepared by sol–gel combustion. The composition of the compound used was the following,
These oxides were dissolved in deionized water, and a small amount of citric acid was added as organic fuel. The mixture was then heated and stirred on a magnetic stirrer to remove excess water, nitrates, and other volatile organic impurities. With continuous heat, the gel burns automatically, forming a gray-black precursor. The combustion reaction equation is:
The precursor was divided into two parts. One part was annealed with a furnace for 2h at 1073K to obtain white phosphor. The other part was annealed with a 980 nm laser. The laser power was 18 W, the spot diameter was about 10 mm, and the heating time was about 120 s. The gray-black precursor will turn to white powder with the laser annealing. The white powder can be further annealed with a 980 nm laser to form millimeter crystals. The laser power is 18 W, the spot diameter is about 1 mm, and the heating time is about 30 s. (Fluorescent powder obtained by furnace annealing-FPFA; Fluorescent powder obtained by laser annealing-FPLA, Fluorescent millimeter crystals obtained by laser annealing-MCLA).
The structures of the samples in powder form were identified by X-ray diffraction (XRD). The experiment was performed for a 2θ value from 10° to 80°. MCLA was ground to powder with a metal mortar to measure XRD.
A 980 nm diode laser was used to irradiate the sample. The steady-state photoluminescence curves were measured by a microspectrometer (QE Pro, Ocean Optics). The temperature range of the thermostat is 40–400 °C, and the temperature control accuracy is 0.1 °C.
3 Results and discussions
3.1 X-ray diffraction
The XRD patterns of FPFA, FPLA, and MCLA in the range of 10 ~ 80° are shown in Fig. 1. The diffraction peaks of FPFA, FPLA, and MCLA are consistent with the standard PDF (No. 88–1040) card. There are no other impurity diffraction peaks and secondary phases in the XRD patterns, which indicates that Ho3+ and Yb3+ are successfully doped in the Y2O3 host lattice. MCLA appears as a millimeter crystal, as shown in the inset. The XRD data are analyzed, and the results are shown in Table 1. The full width at half maximum (FWHM) of the diffraction peaks of the FPLA became sharper compared to FPFA. It indicates laser annealing results in larger grain size and better crystallization than furnace annealing. The crystallite size of FPFA and FPLA are calculated using Scherrer's formula [21].
where K is the Scherrer constant, D is the average thickness of the grain perpendicular to the crystal plane, B is the half-peak height width of the diffraction peak of the measured sample, and θ is the Bragg Angle, γ is the X-ray wavelength of 1.54056Å.
The particle size of FPFA is about 20.2 nm, and FPLA is about 37.6 nm. The particle size obtained by laser annealing is larger than furnace annealing. It may be due to the higher temperature caused by laser annealing heat the lattice grows [22,23,24].
3.2 UC luminescence characteristics
As shown in Fig. 2, there are three emission bands in the visible region: the green band of 525–575 nm attributed to the 5F4/5S2 → 5I8 transition with its peak at 549 nm; the red band of 635–675 nm attributed to the 5F5 → 5I8 transition with its peak at 667 nm, and the infrared band of 730–780 nm attributed to the 5F4/5S2 → 5I7 transition with its peak at 761 nm [25]. Figure 2a shows the UC spectra of FPFA were essentially the same as those of FPLA at the same excitation power density of 2.486 W/mm2. The phosphor prepared by laser annealing has the same fluorescence intensity as that prepared by muffle furnace annealing, which is consistent with the conclusion obtained by Deng [26]. Figure 2b shows the UC intensity of MCLA about 40 times stronger than FPFA excited by 980 nm laser with the same excitation power density of 2.486 W/mm2. It indicates that laser annealing can obviously enhance the fluorescence intensity.
In order to better understand the UC emission mechanism of MCLA, the relationship between the UC luminescence intensity (I) and laser power (P) was investigated. When the pump power of the laser is small, the UC luminescence process is in the unsaturated phase. The pump power and fluorescence intensity satisfy the following formula [27, 28].
where n is the value of pump photons needed to emit one photon in the UC emission process.
As shown in Fig. 3, the blue emission, green emission, and red emission are three-photon process, two-photon process, and two-photon process, respectively.
According to the above results and other reports [29,30,31], the UC energy-transfer mechanism of MCLA is shown in Fig. 4.
Under the excitation of 980 nm wavelength, Yb3+ transits from the ground state 2F7/2 to 2F5/2 by absorbing an infrared photon. Due to the larger absorption cross-section of Yb3+ and the resonance between Ho3+ (5I8 → 5I6) and Yb3+ (2F5/2 → 2F7/2), the 5I6 intermediary level is populated via energy transfer (ET) from the 2F5/2 state of Yb3+ to Ho3+:
The ion in the 5I6 level can go to the 5S2/5F4 level by another ET from the 2F5/2 state of Yb3+. In the 5S2/5F4 level, the population becomes large and emits a strong green and weak red emission through 5S2/5F4 → 5I8 and 5S2/5F4 → 5I7 transitions at 549 nm and 761 nm, respectively. And this level absorbs the third incident the near-infrared photon through the ESA process to populate 5F3 levels. Ions in these levels decay radiatively to the ground level and give emissions at 493 nm through the 5F3 → 5I8 transitions in blue regions. The ion in the 5I6 level can also decay to the 5I7 level through multiphonon relaxation. Further, the ion in the 5I7 level can be excited to the 5F5 level by ET from the 2F5/2 state of Yb3+. The transition of 5F5 → 5I8 causes red emission at 667nm.
3.3 Temperature dependence of UC luminescence
The UC emission intensity variation of the thermally coupled follows the following relation [32],
where B is a pre-exponential constant, ΔE is the fitting energy difference between the two thermally coupled levels, k is the Boltzmann constant; and T is the absolute temperature.
Naturally, it shows a linear relationship between ln(FIR) and the inverse temperature (1/T) by taking the logarithm of the above equation:
Meanwhile, the variation of UC emission intensity of the non-thermal coupling was studied by Wang et al. [33, 34], and the relationship between FIR and absolute temperature is expressed as:
where A and C are constants.
The spectrograms at different temperatures are shown in Fig. 5. As the temperature increases, the fluorescence intensity decreases. The reason for this result is precisely that the multiphonon transition rate increases with the increase in temperature because the thermally populated phonon modes in the host lattice can induce stimulated emission of phonons thus reducing the UC efficiency [35,36,37].
Temperature sensing of MCLA was investigated using the FIR technique. The ratio of 549 nm (5F4 → 5I8) and 667 nm (5F5 → 5I8) was selected.
In Fig. 6a, ln(I667nm/I549nm) shows a linear relationship with the absolute temperature T of 323–453 K, which is an obvious non-thermal coupling. In Fig. 6b, as the temperature increases, there is a linear relationship between ln(I667nm/I549nm) and inverse temperature 1/T at 453–673K. According to formula (4), this is a typical thermal coupling. It indicated the I667nm/I549nm transition from non-thermal coupling to thermal coupling as the temperature rises.
The slope in Fig. 6a is −1520.37, and the slope is equal to ΔE/k in formula (4). Therefore, the calculated energy gap ΔE is 1056.63 cm−1. The ΔE is between 200 ~ 2000cm−1, so I667nm/I549nm satisfies the thermal coupling law. It should be noted that the temperature in Fig. 5 represents the ambient temperature, and the actual temperature should also include the temperature caused by the laser. In other words, the energy gap we obtained by formula (4) is lower than the true value.
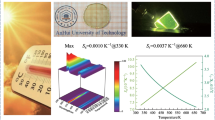
Sensitivity is an important parameter in evaluating the temperature-sensing performance of a thermometer. Sensitivity is usually divided into absolute sensitivity(Sa) and relative sensitivity(Sr). The definitions are as follows,
where ΔE is the energy gap, k is Boltzmann's constant, and T is the temperature.
The maximum value of Sr is 0.0074%K−1 when it is in the thermally coupled energy level in the temperature range of 453–673K in Fig. 7.
A comparison of the relative sensitivities of the Ho3+ ions with other thermally coupled energy level pairs is shown in Table 2. The results show that Y2O3: Ho3+/Yb3+ millimeter crystals under laser annealing can be a good candidate for high-sensitivity optical temperature sensing.
4 Conclusion
In summary, the Y2O3: Ho3+/Yb3+ millimeter crystals were prepared by laser annealing. The fluorescence intensity of the millimeter crystals under laser annealing and the phosphor under furnace annealing were compared under 980 nm laser excitation, and it was found that the fluorescence intensity of the millimeter crystals was enhanced about 40 times compared to the phosphor. The luminescence characteristics of Y2O3: Ho3+/Yb3+ millimeter crystals were measured as the temperature changes from 303 to 673 K. The non-thermal coupling of I667nm/I549nm changes to thermal coupling with the increase in temperature above 453K. The maximum relative sensitivity of thermal coupling of I667nm/I549nm is 0.0074 at 453 K. This work has not only proposed new ideas for obtaining higher fluorescence intensity but also offered more possibilities for temperature sensing.
Data availability
The datasets generated during and/or analyzed during this work are available from the corresponding author upon reasonable request.
References
J.C. Zhang, Q.S. Qin, M.H. Yu, Chin. Phys. Lett. 027802, 28 (2011)
X.Z. Wang, Y.S. Wang, Spectrosc. Spect. Anal. 26, 399–402 (2006)
S.P. Horvath, M.F. Reid, J. Wells, J. Lumin. 169, 773–776 (2015)
S.S. Wang, H. Zhou, X.X. Wang, J. Phys. Chem. Solids 98, 28–31 (2016)
V. Bachmann, C. Ronda, A. Meijerink, Chem. Mater. 21, 2077–2084 (2009)
C. Louis, R. Bazzi, C.A. Marquett, Chem. Mater. 17, 1673–1682 (2005)
J. Riegler, T. Nann, Anal. Bioanal. Chem. 379, 913–919 (2004)
R. Bao, L. Yu, L. Ye, Sens. Actuator A Phys. 269, 182–187 (2018)
X. Hou, S. Zhou, T. Jia, Physica B Condens. Matter 406, 3931–3937 (2011)
T. Biljan, A. Gajovic, Z. Meic, J. Alloys Compd. 431, 217–220 (2007)
K. Vineet, Z. Brandon, M. Paul, Dalton Trans. 47, 11158–11165 (2018)
Y.Y. Guo, D.Y. Wang, Y. He, J. Alloys Compd. 741, 1158–1162 (2018)
L. Laversenne, Y. Guyot, C. Goutaudier, Opt. Mater. 16, 1158–1162 (2001)
A. Ubaldini, M. Carnasciali, J. Alloys Compd. 454, 374–378 (2008)
Q. Feng, Z. Hua, C. Wei, Appl. Phys. Lett. 108, 241907 (2016)
R. Marcin, B. Aleksandra, M. Monika, J. Lumin. 201, 104–109 (2018)
P. Lixin, Z. Yuan, Q. Feng, Opt. Lett. 46, 5818–5821 (2021)
M.D. Dramićani, Ž Antić, Nanotechnology 25, 485501 (2014)
R. Yun, J.Q. He, L. Luo, X.H. Liu, Ceram. Int. 47, 16062–16069 (2021)
M. Zacharias, P.C. Kelires, Phys. Rev. B 101, 245122 (2020)
L. Robert, Defect and microstructure analysis by diffraction (Oxford University Press, Oxford, 1999)
A. Bednarkiewicz, D. Wawrzynczyk, Nanotechnology 23, 145705 (2012)
L. Xu, J. Zhang, D. Gao, Nanotechnology 30, 435703 (2019)
A. Kumar, J. Manam, Opt. Mater. 96, 109373 (2019)
E. De, P. Salas, H. Desirena, Appl. Phys. Lett. 87, 241912 (2005)
D. Yawen, Y. Yan, Z. Shuai, Opt. Mater. 135, 113270 (2023)
M. Pollnau, D.R. Gamelin, S.R. Lüthi, Phys. Rev. B 61, 3337 (2000)
A. Polman, Appl. Phys. 82, 1–39 (1997)
A. Roy, H. Dwivedi, Mishra. J. Alloys Compd. 865, 158938 (2021)
L. Songbin, Y. Xinyu, L. Shuifu, J. Am. Ceram. Soc. 100, 3530–3539 (2017)
G. Linna, W. Yuhua, Nanoscale Res. Lett. 7, 636–636 (2012)
W. Chuanlong, J. Yahong, J. Alloys Compd. 894, 162494 (2022)
H. Li, Y. Zhang, L. Shao, Opt. Mater. 7(8), 3003–3010 (2017)
X. Wang, Q. Liu, Opt. Mater. 24(16), 17792–17804 (2016)
A. Kumar, J. Manam, J. Alloys Compd. 829, 154610 (2020)
S.K. Singh, K. Kumar, S.B. Rai, Appl. Phys. B 94, 165–173 (2009)
A.R.C. Márcio, G.S. Maciel, Appl. Phys. Lett. 84, 4753–4755 (2004)
R. Dey, A. Kumari, A.K. Soni, Sens. Actuators B Chem. 210, 581–588 (2015)
P. Singh, K.P. Shahi, A. Rai, Opt. Mater. 58, 432–438 (2016)
H. Bofei, J. Mochen, L. Panpan, J. Am. Chem. Soc. 58, 7939–7946 (2019)
H. Aiguo, Z. Xi, L. Xiaojuan, W. Long, J. Alloys Compd. 803, 450–455 (2019)
Acknowledgements
The work is support by the Science and Technology Research Program of Chongqing Education Commission of (KJQN202201403). The University Innovation Research Group of Shale Gas Optical Fiber Intelligent Sensing Technology (CXQT20027). Co-operative Projects between Undergraduate Universities in Chongqing and Institutes affiliated with Chinese Academy of Sciences (HZ2021014). “New Generation of Information Technology Innovation Project” sponsored by China University Innovation Fund (2021ITA04001).
Author information
Authors and Affiliations
Contributions
Qiu-Yue Ran: Investigation, Validation, Experiment, Paper writing, Paper revision, Software. Yu-Long Lian: Conceptualization, Experiment, Writing-original draft, Software. Yong Tang: Data curation, Visualization, Software, Writing-review & editing. Jian-Hong Hao: Investigation, Resources. Yun-Feng Bai: Conceptualization, Formal analysis, Funding acquisition, Methodology, Writing-review & editing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ran, QY., Lian, YL., Tang, Y. et al. Luminescence and temperature properties of Y2O3: Ho3+/Yb3+ millimeter crystals obtained by laser annealing. Appl. Phys. B 130, 38 (2024). https://doi.org/10.1007/s00340-024-08181-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00340-024-08181-5