Abstract
Metal nanoparticle formation using laser ablation of targets in water by picosecond pulses is a well-developed process. Here we demonstrate how the salt and sugar being dissolved in water differently affect the spectral characteristics of the absorbance of the suspensions produced during ablation of silver and gold targets. We demonstrate the disappearance of the surface plasmon resonances of Ag and Au nanoparticles in the salt-containing solutions. The presence of salt does not allow for maintaining the synthesis of nanoparticles during ablation of metals contrary to the sugar-containing solution. Our nonlinear optical studies show that the saturable absorption observed in the nanoparticles-containing suspensions produced during laser ablation of silver and gold in distilled water and sugar-containing water entirely disappears in the case of the suspension produced during the ablation of these metals in salt-containing solution.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The unusual optical properties of metal nanoparticles (NP) are linked to the confinement of the quasi-free electrons within these species [1]. The resonant collective oscillation of these quasi-free electrons leads to the appearance of surface plasmon resonances (SPRs). The appearance of these specific features in the absorption spectra of the suspensions containing NP is a direct confirmation of the presence of those multi-atomic structures. Some of the metal NP possess those properties in the visible range (Ag, Au, Cu, etc.).
One of the well-developed techniques for the synthesis of NP is the laser ablation of metals in the liquid environment [2,3,4]. The simplicity of NP formation by this method allowed a synthesis of the NP of almost all metals of the periodic table, which cannot be accomplished by the chemical and other methods of formation of these multi-atomic structures. The analysis of the absorbance of these NP-containing liquid suspensions allowed the determination of the conditions for the formation of the non-spherical structures [5]. These NP found applications in various areas of science, particularly, in optical switchers for generation of the short laser pulses [6]. It is important to analyze different conditions of laser-induced formation of NP and determine the influence of various parameters of this process like the fluence, pulse duration, and wavelength of the lasers used for this process. The important characteristic, that can play a crucial role in NP formation and maintenance, is a surrounding liquid.
The most of reports on those topics describe the application of distilled water as a surrounding medium during the laser ablation of metals. The application of other liquids, though rarely reported, did not show significant advantages compared to the water. The application of organic liquids suffers some disadvantages, which did not lead to the formation of stable and small nanostructures. Meanwhile, water itself is a very strong polar medium, which can maintain the best conditions for each stage of NP formation (heating of target, assembly of NP, their cool down, maintenance of NP's properties, etc.). In this connection, the application of some additional components like surfactants being dissolved before laser ablation can either preserve and improve the properties of NP or entirely stop their synthesis and maintenance in the suspensions.
The formation of metal NP by laser ablation in the presence of an ionic surfactant that serves as a medium to control the size of NP was reported in Ref. [7]. Silver NP were also synthesized at a small concentration of salt (NaCl) solution in water during ablation by 5 ns pulses [8]. It was demonstrated that the presence of the chloride ions in the liquid medium during laser ablation can reduce the average size of Ag NP at a 5 mM concentration of NaCl solution to 26 nm.
In this paper, we analyze the influence of two materials (sugar and salt), which being dissolved in distilled water either maintain the conditions of NP formation during laser ablation of metals by picosecond pulses or interrupt the synthesis and maintenance of those species in the suspensions. We demonstrate the disappearance of the SPRs of Ag and Au in the salt-containing solutions and show that the presence of NaCl does not allow for the maintenance of the synthesized NP during the ablation of used metals (gold and silver) contrary to the sugar-containing solution. Finally, we show that the saturable absorption of picosecond pulses in the NP-containing suspensions produced during laser ablation of metals in water and sugar-contained water entirely disappears in the case of the suspension produced during ablation of Au and Ag in the salt-containing solution at high concentration of NaCl. These studies provide advanced insights including the quantum mechanical effects and interparticle interactions during laser-induced ablation of metals in liquids at variable conditions of the liquid medium surrounding the target.
2 Spectral and morphological studies of laser-ablated silver and gold
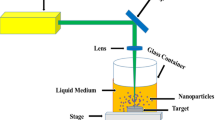
Laser ablation of metals in liquids was performed using the 30 ps, 1064 nm pulses at 50 Hz repetition rate. They were focused using the 100 mm focal length spherical lens on the surfaces of Ag and Au targets. The diameter of the laser beam on the surfaces was 0.3 mm. The targets were placed in the cells. The irradiation of targets was performed from the top of the 20 × 20 × 20 mm3 cells filled with distilled water, water with dissolved sugar (water + sugar), and water with dissolved salt (water + salt). To prepare the latter solutions, 5 g of sugar or 6.8 g of salt were dissolved in 25 mL of water and then diluted with water at the ratio of 1:10. The corresponding molar concentrations of those solutions were 80 mM and 400 mM. The cells were moved along the focal plane during ablation using the programmable X–Y translating stage to provide a fresh position of target surfaces for interaction with a laser beam. During ablation, the plasma spectra were registered using the fiber spectrometer. The ablation of targets lasted for 30 min. The obtained suspensions were rinsed and their absorbance was measured using the fiber spectrometer.
Silver and gold plates (5 × 5 × 0.2 mm3 each) were placed on the bottom of the cells filled with the corresponding liquids (pure water, water + sugar, and water + salt). The height of the liquids above the target surfaces was 10 mm. The choice of the laser fluence suitable for target ablation was aimed at achieving the optimal conditions for NP formation. Their appearance was qualitatively confirmed by observation of the coloring of the liquids with yellowish (in the case of Ag target) and reddish (in the case of Au target) colors and manifested by the appearance of the SPRs at ~ 400 nm and ~ 520 nm in absorption curves attributed to the synthesis of the spherical Ag and Au NP, respectively. We maintained the fluencies of heating pulses at the level of ~ 1.4 J cm−2 (in the case of ablation of the gold target) and ~ 1.8 J cm−2 (in the case of ablation of the silver target).
The patterns of the obtained suspensions are shown in the insets to Fig. 1a, b. Samples 1, 2, and 3 correspond to the ablated targets in the distilled water, water + sugar, and water + salt, respectively. One can see that approximately the same reddish color of Au NP-containing suspensions was obtained in two former liquids, while the ablation of gold in water + salt did not result in coloring the surrounding liquid (inset in Fig. 1a). Each shot of laser radiation in water + salt solution caused the appearance of a slightly reddish-colored blast moving out from the target surface, which then disappeared, without showing any change of the color of the surrounding liquid after a few ten thousand laser shots. One can assume that the initial appearance of the Au NP was followed by the interaction with the dissociated ions of Na+ and Cl−, which caused the disintegration of those nanostructures, resulting in the absence of the SPR in the absorption curve. Further analysis of this effect is presented in the Discussion section.
Absorption spectra of the suspensions of ablated (a) Au and (b) Ag targets. The laser beams were focused from the top of cells on the Au and Ag targets. 1—ablation of Au and Ag targets in the distilled water. 2—ablation of Au and Ag targets in the distilled water mixed with the sugar. 3—ablation of Au and Ag targets in the distilled water mixed with the salt. Insets show the images of the cells filled in with the products of ablation corresponding to the 1–3 conditions of ablation. One can see that the ablation of Au and Ag targets in salty water shows only the white color of the suspension
Meanwhile, the ablation of the Au target in distilled water and water + sugar solution allowed observation of the gradually changed color of the liquid surrounding the area of ablation resulting in the increased reddish color of a whole suspension contained in the cell. The appearance of synthesized Au NP was maintained during the whole period of laser ablation (30 min) thus gradually increasing their concentration with each laser shot.
The same feature was observed in the case of ablation of the silver target in similar liquids (inset in Fig. 1b). The yellowish color of suspensions was obtained in the distilled water and water + sugar solutions. Again, like in the case of the gold target, the ablation of Ag in water + salt did not lead to the change of the initial white color of the solution.
These experiments showed that the addition of sugar in the water did not change the process of NP formation during ablation by picosecond pulses of the surfaces of two used targets. In contrast, the presence of salt in the water stopped the process of liquid coloring thus pointing out the cancellation of NP formation in this solution.
To analyze the appearance of SPRs, the absorption spectra of all six samples (three with ablated gold species and three with ablated silver species) were measured. Blue and green curves (#1 and #2 in Fig. 1a) show the absorption spectra of the cells containing the suspensions of gold species produced during ablation in water and water + sugar, respectively. These spectra comprise the SPRs attributed to the presence of the spherical Au NP. The position of SPR of Au NP in the case of water + sugar solution was slightly red-shifted with regard to the SPR observed in the case of ablation in the distilled water, which may indicate some variation of the spherical form of synthesized multi-atomic species. The colors of the suspensions in these two cases were almost similar to each other. The colors and SPRs of Au (central wavelength ~ 520 nm) were maintained during the whole period of observation of those two suspensions (two months). In the meantime, in the case of water + salt solution, a weak SPR was observed immediately after the ablation of the Au target (red curve in Fig. 1a), which then entirely disappeared within a couple of days (yellow curve #3). The suspension produced in the water + salt solution was then almost colorless (see the image of cell #3 in the inset to Fig. 1a).
The absorption curves in the case of ablation of silver target demonstrated a similar tendency. Again, blue and green curves (#1 and #2 of Fig. 1b) showed the absorption spectra of the cells containing the liquids surrounding the Ag target during ablation in water and water + sugar, respectively. Similar to the previous case, the position of SPR of Ag NP in the case of water + sugar solution was slightly red-shifted with regard to the SPR observed in the case of silver ablation in the distilled water (correspondingly, 403 and 397 nm). These two suspensions showed a similar yellow color, which did not degrade for a long period. Meanwhile, the ablation of Ag in water + salt solution did not result in the appearance of SPR (red curve #3 of Fig. b).
These spectral measurements of the suspensions produced during the ablation of Au and Ag targets in the water + salt solution allowed a conclusion about the influence of the ions of Na + and Cl− on the process of NP synthesis and maintenance after the ablation of two targets. In two cases, we observed the sedimentation of large Au and Ag blocks. No sedimentation was seen in the case of Au and Ag NP formation in distilled water and water + sugar solution.
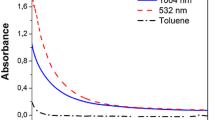
To optimize the conditions of NP formation one has to empirically determine the optimal conditions of ablation of the used targets in the distilled water. The important factor here is the fluence of heating picosecond pulses. Our studies showed that the absence of the ionized species of targets corresponds to the formation of the stable suspensions of Au and Ag NP. To maintain this regime of ablation, we analyzed the emission of the plasmas spreading out from the surfaces of the ablated metals. Figure 2 shows the plasma emissions collected from the (a) Au and (b) Ag targets immersed in the distilled water and ablated by the 1064 nm and 532 nm, 30 ps pulses. The fluencies of 1064 nm heating pulses in that case were maintained at the level of 1.4 J cm−2 (Au target) and 1.8 J cm−2 (Ag target).
Spectra of the (a) Au and (b) Ag plasmas ignited and spread in the distilled water out from the targets during ablation by the 532 and 1064 nm picosecond pulses. The wavelengths of the lines of neural particles (Au I and Ag I) in the case of the ablation by 1064 nm radiation were retrieved from the NIST tables [9]. No lines from singly ionized particles were observed except for some Ag II transitions in the UV range. The fluencies of heating pulses corresponded to the conditions of the optimal formation of NP in a water environment when the SPRs of Au and Ag NPs showed the largest distinction with regard to the other parts of the featureless absorption spectra
The experimental settings for the spectral measurements were as follows. We used the fiber spectrometer (Ocean Optics USB2000) to analyze the emission spectra of the plasmas spread out from the targets’ surfaces. The emission from plasma was collected using the fused silica microlens. The used spectrometer provided up to 0.1 nm resolution with the smallest 5 µm entrance slit. In our case, the 25 µm slit was used, which allowed the 0.8 nm resolution of plasma spectra. This resolution allowed us to distinguish the emission lines of plasma spectra shown in Fig. 2.
The laser plasma was ignited on the surfaces of the silver and gold targets placed inside the glass cells filled with water. The 1064 and 532 nm laser pulses from the picosecond Nd: YAG laser (t = 30 ps) were used for the laser ablation of targets. The fluencies of heating pulses corresponded to the conditions of the optimal formation of NPs in a water environment when the SPRs of Au and Ag showed the largest distinction with regard to other parts of the featureless spectra of absorption (blue curves in Fig. 1). Though the spectra produced using two heating pulses of different wavelengths (532 and 1064 nm) showed almost similar emission lines from the neutral Au and Ag atoms, most studies of NP formation were carried out using the longer wavelength source (1064 nm).
These spectra showed the dominance of the transitions attributed to the excited neural particles (Au I and Ag I), with the presence of the weak lines of Ag II in the UV range (240–260 nm), alongside the stronger transitions of Ag I in the whole UV range. The numbers above the spectral lines correspond to the wavelengths of emissions of two atoms retrieved from the NIST Tables [9]. The lines 479.3 nm, 481.1 nm, and 523.0 from the datasheet of NIST Tables correspond to the transitions of the neutral atoms (Au I). The first two lines in our case were observed as a single line marked as “480” (Fig. 2a). The 479.3 nm line being 5 times stronger than the 481.1 nm line dominated in this spectral range. Moreover, most probably the latter line was not strongly excited during the ablation by laser pulses at the used experimental conditions. Notice that we did not see many transitions attributed to the neutral gold. Only the strongest of the recorded transitions of neutral gold are marked in Fig. 2a. Regarding the 523.0 nm line, it was clearly distinguished in the registered spectrum and marked as “523” on the two curves of Fig. 2a corresponding to the excitation by the 1064 and 532 nm laser pulses. Thus, not all transitions from the neutral gold shown in the NIST Table [9] were observed in present studies due to the specific features of the excitation of the target. It is referred in those tables that some of the transitions were retrieved using other methods of excitation.
Regarding the strong lines of silver presented in Ref. [9] (531.2 nm, 520.9 nm, and 546.5 nm), two former lines belong to the transitions of the neutral Ag and dominate in the green range of the excited emission spectrum. In our case, they marked as “521” and “547” on the bottom curve of Fig. 2b obtained using the 1064 nm excitation. The only strong line in the visible range attributed to the singly charged silver (Ag II) is 531.2 nm marked in NIST Tables, which was not observed in our experiments (bottom curve in Fig. 2b), along with numerous other transitions from the singly-ionized silver. The strong line in the upper curve of Fig. 2b marked as "532" originates from the scattering of the 532 nm emission of the used laser. Overall, the silver plasma produced on the target surface at the used fluencies of 1064 and 532 nm pulses showed only spectral lines attributed to the neutral Ag (Ag I). Even the lines in the UV range (328.0 nm and 338.3 nm) belong to the neutral silver [9]. Very strong transitions of Ag II in the UV shown in the NIST Table were either not observed or showed very low intensity.
The suspensions prepared in distilled water were analyzed using the SEM (Fig. 3). The mean sizes of the Au and Ag NP synthesized in distilled water were 90 and 55 nm, respectively. Almost no NP were observed in the case of ablation of Au and Ag targets in the water + salt suspension, or they scarcely appeared in the SEM images. In that case, we mostly observed the large irregular agglomerates of NP.
The absorption spectra of prepared suspensions were used to determine the presence or absence of NP in those suspensions. The presence of NP was inferred from the appearance of the strong absorption bands associated with SPR. It was shown in Ref. [3] that the SPR of spherical silver NP induces a strong absorption centered in the range between 400 and 480 nm depending on the preparation technique but not on the sizes of NP. Notice that the optimization in the sizes of NP can significantly enhance the optical limiting effect during the propagation of ultrashort laser pulses through the NP suspension. Particularly, the optical limiting of silver NP with different sizes and shapes was investigated and compared to the optical limiting performance of conventional carbon black suspension [10]. It was found that the optical limiting effect is a strong particle size-dependent process, and the best performance is achieved with the smaller particles. Meanwhile, the peak of absorption identified with the SPR remained almost the same for different used NP.
A unique property of Ag NP is that their SPR can be tuned from 390 to 530 nm or farther by changing the particle morphology (particularly, triangles instead of spheres) and the local refractive index near the particle surface. Even larger shifts of resonance peak towards the IR region can be achieved by producing silver NP with rod or plate shapes using chemical methods. The example of a significant variation of the absorption spectrum of Ag NP suspension in the case of the spherical and triangular shapes of the studied structures was analyzed in Ref. [11]. It was shown that the peak of SPR was significantly red-shifted in the case of the latter NP (~ 600 nm) compared to the spherical NP. Another interesting peculiarity of laser ablation-prepared silver NP is as follows. The modification of Ag NP size distribution by laser irradiation of bulk target, as well as nanoparticles, in water was reported in Ref. [12] and the change of nanoparticles’ characteristics caused by variable laser fluence and duration of irradiation was observed. A considerable narrowing (by a factor of three) of the SPR bandwidth was achieved, which is evidence of a narrowing of the particle size distribution.
Further, in Ref. [13], the analysis of the position of SPR for different sizes of Ag NP was analyzed. It was shown that the spherical NP of a broad range of mean sizes (8–45 nm) demonstrate almost the same positions of SPR. The SPR location for the most often used chemical technique of Ag NP preparation is centered in the vicinity of 415–425 nm, whereas the absorption spectra of such structures prepared by laser ablation show that this resonance shifts toward a short-wavelength range (between 400 and 410 nm) due to the influence of the synthesized small-sized (< 10 nm) NP. Another experimental analysis of the relation between the position of SPR of NPs, particularly gold NPs, and their mean sizes was reported in Ref. [14]. Those studies have shown the insignificant red shift of SPR of Au NP (from 521 to 537 nm) for the 13 nm and 64 nm nanoparticles.
In the present work, we aimed to distinguish the influence of the sugar and salt suspensions on the absorption spectra of the synthesized silver and gold NPs during laser ablation of targets in the liquid environment. Below we present the analysis of the size distribution and mean sizes of synthesized nanoparticles. The SEM images (Fig. 3) were registered at a low resolution to show the general pattern of the gold and silver NPs in a large area. For the analysis of the mean sizes and size distribution, the SEM images at higher resolution and the ImageJ software were used. The histograms (Fig. 4) of the counts of the number of NP with fixed diameters were retrieved after 3 days from the ablation of targets. During this period, the large NP and microparticles were expelled from the suspensions due to sedimentation, and the histograms depict the presence of the size distributions between ~ 50 and ~ 130 nm in the case of gold NP and ~ 35 and ~ 80 nm in the case of silver NP. The values of 90 and 55 nm were determined as the mean sizes of the studied Au and Ag NP, while the size distribution was approximately symmetrical with regard to the mean sizes.
3 Nonlinear optical properties of the nanoparticle suspensions
Below we present the nonlinear optical studies of the suspensions obtained during the ablation of Ag and Au targets in water, water + sugar, and water + salt. The effective nonlinear optical response of laser-produced metal NPs has been observed in numerous studies (for example [15, 16]). The appearance of SPR in the spectral measurements of the absorption of the suspensions containing various NP can serve as a qualitative indicator of the possibility of observation of the nonlinear optical effects like Kerr-induced nonlinearities, various types of nonlinear absorption, third-harmonic generation, etc. The optimization of NP’s morphology (i.e., their shape and size) can lead to the improvement of light confinement in the nanoscale and stronger nonlinear modulation of the local electric field. And vice versa, a disappearance or decrease of SPR can cause a diminishing of the nonlinear optical response of the medium.
Earlier, the degenerate four-wave mixing, Z-scan, and pump-probe techniques were applied to demonstrate the strong third-order optical nonlinearities of metal NP. Third-order nonlinear susceptibility of these species shows a strong spectral dependence due to the resonant behavior of the conduction electrons of NP, particularly Ag NP, thus allowing the growth of different nonlinear optical processes in the vicinity of their SPR (~ 400 nm). This resonance can be tuned during a decrease of Ag NP sizes below 15 nm due to the quantum-size effect. The same can be said about the Au NP.
The standard Z-scan procedure of the nonlinear absorption studies of suspensions [17] was used in the present studies. The open-aperture scheme (inset in Fig. 5a) was used when the aperture (A) in the Z-scan scheme was entirely open. The 532 nm, 30 ps pulses from the laser source (LC) were focused using the 400 mm focal length spherical lens (L) in the cell (C) containing different prepared suspensions. The conditions of experiments were the same for all six measurements of the normalized transmittance of suspensions of three groups of Ag-ablation-induced suspensions and three groups of Au-ablation-induced suspensions. The thickness of the cell was 2 mm. The cell moved through the focal plane of the focusing lens using the translation stage (TS). The propagated radiation was collected by a large-area-photodiode (R).
Open-aperture Z-scans of (a) Au- and (b) Ag-ablated suspensions using the 532 nm, 30 ps probe pulses. The filled brown circles correspond to the ablation in distilled water. The filled red circles correspond to the ablation in water + sugar solution. The blue empty circles correspond to the ablation in the water + salt solution. The inset in (a) shows the Z-scan scheme. LS laser source, L lens, C cell with studied suspension, TS translating stage, A aperture, R registrar
The open-aperture Z-scans of distilled water, water + sugar solution, and water + salt solution did not show the nonlinear optical response of those liquids at the used intensity of the probe 532 nm pulses (2 × 1010 W cm−2). The nonlinear optical response was observed only in the case of the suspensions obtained during the ablation of the targets in distilled water and water + sugar solution. Figure 5a shows three open-aperture Z-scans of the studied suspensions produced during ablation of the gold target. Two suspensions demonstrated strong and almost similar saturable absorption (brown and red-filled circles) when the transmittance of samples increased during the approach of the cell towards the focal plane (z = 0). Meanwhile, the suspension produced during the ablation of the gold target in the water + salt solution demonstrated very weak saturable absorption (blue empty circles).
These studies showed that the nonlinear optical response of the two above suspensions is attributed to the presence of the NP in the suspension. The insignificant nonlinear optical response in the suspension of the ablated Au in water + salt solution points out the significantly smaller number of NP in this suspension. A similar conclusion can be retrieved in the case of Ag NPs-contained suspensions (Fig. 5b) when the ablation of silver in water + salt solution resulted in the entire disappearance of the saturable absorption. The saturable absorption in Au NP suspension was larger compared with the same process in Ag NP suspension. The difference in the nonlinear optical response of these two groups of NP was attributed to the closeness of the wavelength of probe pulses (532 nm) to the SPR of Au NP, which can cause the resonance-induced growth of the saturable absorption.
The closed-aperture Z-scans did not show the nonlinear optical refraction of the studied suspensions at the same used intensities of the probe pulses. The same can be said about the application of 1064 nm, 30 ps pulses for the analysis of the nonlinear optical properties of the samples under investigation at similar intensities of the probe emission.
4 Discussion
Sodium chloride (NaCl) represents a 1:1 ratio of sodium and chloride ions. Only highly polar solvents like water can dissolve NaCl since the attraction between the Na+ and Cl− ions in the solid is very strong. This solution has very different properties with regard to the distilled water. In solution, NaCl disintegrates into two ions (Na+ and Cl−). They become surrounded by polar water molecules thus creating a metal aquocomplex [Na(H2O)8]+ and the strongly solvated chloride ions surrounded by six molecules of water. The combination of those complexes with NP introduces new coupling terms and affects the electromagnetic response of the system. Our studies show that this combination may lead to a significant decrease in the saturable absorption of both Au NPs and Ag NP. The mechanism of decreasing the electromagnetic response of NPs can be a shielding of the local field of those particles leading to the suppression of SPR and the corresponding decrease of the nonlinear optical response. A similar effect was considered theoretically in the case of the polar molecules surrounding the metal nanostructures [18]. One can note that this effect can play a role in the case when NPs are presented in the suspension. They can survive the aggregation at the small concentration of NaCl in water and then can be surrounded by the abovementioned aquocomplexes thus decreasing the nonlinear optical response of the new NPs-contained complexes. At high concentrations of NaCl, the NPs sediment after aggregation in the presence of a large number of surrounding auqocomplexes. In that case, the absence of NPs in such suspension leads to the cancellation of the nonlinear optical processes.
Another species used in present studies is sugar, which is the generic name for sweet-tasting, soluble carbohydrates, many of which are used in food. Sugar refers to monosaccharides, disaccharides, or oligosaccharides. Monosaccharides are also called "simple sugars", the most important being glucose. Most monosaccharides have a formula that conforms to CnH2nOn with n between 3 and 7. Particularly, glucose has the molecular formula C6H12O6. No changes in the nonlinear response of used NP were observed during the ablation of metals in the environment comprising the aqueous solution of sugar, which shows that, at these conditions, the complexes of molecules surrounding metal NP do not shield the local field and suppress the SPR.
Previously, the application of different additions to the distilled water was examined to determine the conditions lowering the sizes of synthesizing NP during either laser ablation or chemical reduction. Well-controlled sizes were obtained in the case of application of the chemical reduction. However, this method has a disadvantage in several steps of purification. Meanwhile, the laser ablation of a bulk metal immersed in a liquid allows rapid vaporization and condensation of the target material in a single step. The important parameter here is a liquid surrounding the metal target [19,20,21]. The optimization of other parameters of the process like laser fluence, pulse duration, and laser wavelength also plays a crucial role in improving the morphology of NPs.
Contrary to the studies of Bae et al. [8], we demonstrated the entire disappearance of SPR and absence of NPs in the case of ablation of Au and Ag targets in NaCl suspension. Additionally, this suspension did not allow the observation of saturable absorption. In the case of the ablation of Au, some indications of SPR were detected immediately after ablation. However, the absorption in the region of 520 nm fully disappeared after two days (curve 3 in Fig. 1a). A time-dependent decrease in SPR was also reported in [8]. They attributed a rapid decrease in the absorbance for Ag NP suspensions containing NaCl to the aggregation effect promoted by Cl− ions, which can modify the surface of the Ag NP generated by laser ablation.
A difference between our results showing the disappearance of NP, SPR, and saturable absorption during ablation of metals in NaCl-contained liquid and the results of Bae et al. [8] can be, to some extent, attributed to the difference in the durations of the 532 nm pulses used during laser ablation (30 ps and 5 ns in our and their cases, respectively). In the case of short pulses, the formation of NP can be suffered due to the involvement of Cl− ions at a much larger scale compared with the case of 5 ns pulses. However, the most probable reason for the distinction of our results from those of Ref. [8] is a difference in the used concentrations of NaCl. In our case, the used molar concentration of NaCl (400 mM) was 80 times larger than the one used in the refereed paper (5 mM). Notice that even this relatively small molar concentration of NaCl (5 mM) led, in their case, to the suppression of the SPR peak of Ag by a factor close to 2. Correspondingly, one can expect that, at an 80 times stronger concentration of NaCl, the SPR in their case will also be strongly suppressed.
Another proof for the decisive role of Na+ and Cl− ions in the disappearance of the SPR and the corresponding diminishing of the NP concentration in such suspensions can be supported by the analysis of the addition of pure water + salt solution to the already synthesized suspension of NP in the distilled water. Below we show the modification of the absorption spectra of NP at these conditions.
Figure 6a shows the absorption spectrum of Au NP suspension in distilled water (blue curve), which was similar to the one shown in Fig. 1a (curve 1). Once we added a similar volume of water + sugar solution to the Au NP-containing suspension the amplitude of the SPR peak in the resulting absorption curve predictably decreased due to lesser concentration of NP in the new suspension (green curve). Thus the addition of water + sugar solution does not change the nanostructure of the already-prepared multi-particle species. However, once we added the same volume of water + salt solution to the Au NP-containing suspension a significant modification of the absorption spectrum was observed (red curve). The SPR almost disappeared and broadened. After one day, the broadened area in the range of 500–700 nm disappeared, and the absorption curve did not show any sign of the presence of SPR. Thus the maintenance of Au NP was strongly affected by the addition of salt solution. In that case, we observed the sedimentation of the NP aggregates, similar to the case observed during the ablation of Au in the water + salt solution.
Similar experiments using the Ag NPs suspension (Fig. 6b) showed almost the same modifications of the SPR in the case of the addition of the water + sugar and water + salt solutions. Thus the entire disappearance of the SPR after the addition of the water + salt solution confirms a decisive role of the sodium and chlorine ions in the modification of the absorption spectra of NP suspensions. The most probable scenario could be the sedimentation of NPs under the action of those ions leading to the aggregation and formation of large, a few μm sized, agglomerates of silver and gold nanostructures. We observed such a sedimentation of large blocks of NPs in the case of the mixtures of Ag NP and Au NP suspensions and NaCl solutions (Fig. 7).
The resonance peaks in Fig. 1 correspond to the SPRs of Ag and Au spherical NP. Notice that previous studies of these NP have shown similar maximums (400 and 525 nm, respectively) for the spherical particles of different mean sizes [22,23,24,25]. The deviation from these maximums for the gold and silver NP suspensions in most cases was explained by the modification of the shape of NP being changed from spherical to elliptical or triangle shape. Particularly, this modification can be attributed to the aggregation of particles during the aging of the suspensions. Another reason for the modification of the absorption spectrum (see the red curve in Fig. 6a showing the broadening and red shifting of the absorption spectrum of Au NP suspension after adding the salt solution) is the artificial triggering of the gold particles toward the aggregation (Fig. 7b). The same, to some extent, can be said about the silver particles, when some amount of salty water was added to the NP suspension (Fig. 7a). So, the resonance peaks in Fig. 1 have no direct relation with the NP sizes but rather related with the shape of synthesized NP.
Silver and gold are among the most useful metals suited for NP preparation for optoelectronics and nonlinear optics. NP formation in liquids during laser ablation of these solid-state targets using short laser pulses is a well-developed technique. In this connection, Au NP, and especially Ag NP, are extraordinarily efficient at absorbing and scattering light and, unlike many dyes and pigments, have a color that depends upon the size and shape of the particle. These atoms and clusters of Ag and Au tend to aggregate during the laser pulse or soon afterward leading to the formation of larger clusters. Heterogeneous decomposition, liquid phase ejection and fragmentation, homogeneous nucleation and decomposition, and photomechanical ejection are among those processes that can lead to NP formation [26, 27]. Short (picosecond) laser pulses heat a solid to a higher temperature and pressure than longer (nanosecond) pulses of comparable fluence since the energy is delivered before significant thermal conduction can take place.
Our studies showed that, at high concentrations of NaCl (400 mM), the process of NP formation is reverted. Additionally, during the mixing of the NaCl suspension with the suspensions of synthesized NP those small NPs aggregate leading to the formation of the extremely large Ag and Au microparticles (Fig. 7). The SPRs at these conditions almost entirely disappear from the extinction spectra (Fig. 6). Meanwhile, without NaCl, the synthesized NP remain intact. We did not see any change in the color of our suspensions, which can indicate the change of SPR based on the change of the sizes of silver and gold NP.
5 Conclusions
In conclusion, we have analyzed two materials (sugar and salt), which, being dissolved in the distilled water, can either maintain the conditions of NP formation during laser ablation of metals by picosecond pulses or significantly change the process of the maintenance of the already synthesized species. We have demonstrated the disappearance of the SPRs of Ag and Au during their ablation in the salt-containing solutions. We have also demonstrated that the addition of a salt-contained solution does not allow the maintenance of the synthesized NP obtained during the ablation of used metals (gold and silver), contrary to the sugar-contained solution. Finally, we have shown that the saturable absorption observed in the NP-contained suspensions produced during the laser ablation of metals in distilled water and sugar-containing water entirely disappeared in the case of the suspension produced during the ablation of the same metals in the salt-contained solution. We discussed a difference in the studies of this process using the pulses of different durations (30 ps and 5 ns) and molar concentrations of NaCl (5 and 400 mM).
Data availability
Data underlying the results presented in this paper are not publicly available at this time but may be obtained from the corresponding author upon reasonable request.
References
J.A.A.J. Perenboom, P. Wyder, F. Meier, Electronic properties of small metallic particles. Phys. Rep. 78, 173–292 (1981)
G. Fan, S. Ren, S. Qu, Z. Guo, Q. Wang, Y. Wang, R. Gao, Mechanisms for fabrications and nonlinear optical properties of Pd and Pt nanoparticles by femtosecond laser. Opt. Commun. 295, 219–225 (2013)
R.A. Ganeev, M. Baba, A.I. Ryasnyansky, M. Suzuki, H. Kuroda, Characterization of optical and nonlinear optical properties of silver nanoparticles prepared by laser ablation in various liquids. Opt. Commun. 240, 437–448 (2004)
A. Chehrghani, M.J. Torkamany, Nonlinear optical properties of laser synthesized Pt nanoparticles: saturable and reverse saturable absorption. Laser Phys. 24, 015901 (2014)
G.S. Boltaev, R.A. Ganeev, P.S. Krishnendu, K. Zhang, C. Guo, Nonlinear optical characterization of copper oxide nanoellipsoids. Sci. Rep. 9, 11414 (2019)
R.A. Ganeev, R.I. Tugushev, T. Usmanov, Application of the nonlinear optical properties of platinum nanoparticles for the mode locking of Nd:glass laser. Appl. Phys. B 94, 647–651 (2009)
M. Tajdidzadeh, B. Z. Azmi, W. M. Yunus, Z. A. Talib, A. R. Sadrolhosseini, K. Karimzadeh, S. A. Gene, and M. Dorraj, Synthesis of silver nanoparticles dispersed in various aqueous media using laser ablation, Sci. World J. 324921 (2014).
C.H. Bae, S.H. Nam, S.M. Park, Formation of silver nanoparticles by laser ablation of a silver target in NaCl solution. Appl. Surf. Sci. 197–198, 628–634 (2002)
A. Kramida, Y. Ralchenko, J. Reader, and NIST ASD Team (2022). NIST Atomic Spectra Database (ver. 5.10), [Online]. Available: https://physics.nist.gov/asd [2023, July 10]. National Institute of Standards and Technology, Gaithersburg, MD. https://doi.org/10.18434/T4W30F
O. Muller, S. Dengler, G. Ritt, B. Eberle, Size and shape effects on the nonlinear optical behavior of silver nanoparticles for power limiters. Appl. Opt. 52, 139–145 (2013)
A.I. Zvyagin, A.S. Perepelitsa, M.S. Lavlinskaya, O.V. Ovchinnikov, M.S. Smirnov, R.A. Ganeev, Demonstration of variation of the nonlinear optical absorption of non-spherical silver nanoparticles. Optik 175, 93–98 (2018)
V. Nikolov, R. Nikov, I. Dimitrov, N. Nedyalkov, P. Atanasov, M. Alexandrov, D. Karashanova, Modification of the Ag NPs size-distribution by means of laser light irradiation of their water suspensions. Appl. Surf. Sci. 280, 55–59 (2013)
S.K. Maurya, A. Rout, R.A. Ganeev, C. Guo, Effect of size on the saturable absorption and reverse saturable absorption in silver nanoparticle and ultrafast dynamics at 400 nm. J. Nanomater. 2019, 9686913 (2019)
Y. Fu, R.A. Ganeev, P.S. Krishnendu, C. Zhou, K.S. Rao, C. Guo, Size-dependent off-resonant nonlinear optical properties of gold nanoparticles and demonstration of efficient optical limiting. Opt. Mater. Express 9, 976–991 (2019)
F. Hache, D. Richard, C. Flytzanis, Optical nonlinearities of small metal particles: surface-mediated resonance and quantum size effects. J. Opt. Soc. Am. B 3, 1647–1655 (1986)
M.A. Garcia, Surface plasmons in metallic nanoparticles: fundamentals and applications. J. Phys. D Appl. Phys. 44, 283001 (2011)
M. Sheik-Bahae, A.A. Said, E.W. Van Stryland, High sensitivity, single-beam n(2) measurements. Opt. Lett. 14, 955–957 (1989)
N. Domenikou, I. Thanopulos, V. Yannopapas, E. Paspalakis, Nonlinear optical rectification in a polar molecule-plasmonic nanoparticle structure. Mater. Proc. 4, 8 (2021)
R. Tilakiand, S. Mahdavi, The effect of liquid environment on size and aggregation of gold nanoparticles prepared by pulsed laser ablation. J. Nanopart. Res. 9, 853–860 (2007)
O. Olea-Mejía, H. Pote-Orozco, M.A. Camacho-López, O. Olea-Cardoso, R. López-Castañares, A. Vilchis-Nestor, Silver nanoparticles obtained by laser ablation using different stabilizers. Jpn. J. Appl. Phys. 52, 1–6 (2013)
W.M. Khilkala, G.A. Al-Dahash, S.N.A. Wahid, Preparation and characterization of Cu nanoparticles by laser ablation in NaOH aqueous solution. Int. J. Curr. Eng. Technol. 4, 2577–2579 (2014)
M. Darroudi, M. Ahmad, R. Zamiri, A. Abdullah, N. Ibrahim, A. Sadrolhosseini, Time-dependent preparation of gelatin-stabilized Ag NPs by pulsed Nd:YAG laser. Solid Stat. Sci. 13, 520 (2011)
R. Das, S. Nath, D. Chakdar, G. Gope, R. Bhattacharjee, Synthesis of Ag NPs and their optical properties. J. Exp. Nanosci. 5, 357 (2010)
G.S. Boltaev, R.A. Ganeev, P.S. Krishnendu, S.K. Maurya, P.V. Redkin, K.S. Rao, K. Zhang, C. Guo, Strong third-order optical nonlinearities of Ag nanoparticles synthesized by laser ablation of bulk silver in water and air. Appl. Phys. A 124, 766 (2018)
T. Tsuji, K. Iryo, N. Watanabe, M. Tsuji, Preparation of silver nanoparticles by laser ablation in solution: influence of laser wavelength on particle size. Appl. Surf. Sci. 202, 80–85 (2002)
H.O. Jeschke, M.E. Garsia, K.H. Bennemann, Theory for the ultrafast ablation of graphite films. Phys. Rev. Lett. 87, 015003 (2001)
T.E. Glove, Hydrodynamics of particle formation following femtosecond laser ablation. J. Opt. Soc. Am. B 20, 125–131 (2003)
Acknowledgements
Institute of Solid State Physics, University of Latvia as the Center of Excellence has received funding from the European Union’s Horizon 2020 Framework Programme H2020-WIDESPREAD-01-2016-2017-TeamingPhase2 under grant agreement No. 739508, project CAMART2.
Funding
European Regional Development Fund (1.1.1.5/19/A/003), World Bank Project (REP-04032022–206).
Author information
Authors and Affiliations
Contributions
Conceptualization: RAG; methodology: RAG, VVK; formal analysis and investigation: AZB, KK, VVK, ANKR, AS, AB, AA, AU; writing—original draft preparation: RAG; writing—review and editing: RAG, AS, AA, AB, AU.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicts of interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Bunkas, A.Z., Kalnins, K., Kim, V.V. et al. Influence of NaCl on the morphological, spectral, and nonlinear optical characteristics of laser-produced silver and gold nanoparticles. Appl. Phys. B 130, 21 (2024). https://doi.org/10.1007/s00340-023-08159-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00340-023-08159-9