Abstract
Dual emission NaLaMgWO6:Mn4+, Tb3+ phosphor has been synthesized via high temperature solid state reaction method. The phosphor shows both red Mn4+ emission and green Tb3+ emission and its emission color can change from red to green by adjusting the ratio of Mn4+/Tb3+ doping concentration. Moreover, the luminescence intensity ratio of Tb3+/Mn4+ exhibits a monotonic relationship with both temperature and excitation wavelength within certain range, meaning its possible application in optical temperature sensing and wavelength detection.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Photoluminescent materials are widely applied in lasers, lightings, displays, biological molecular detection and imaging and so on [1,2,3,4,5]. Dual emission luminescent materials with suitable luminescent centers have potential application in WLEDs and sensor technologies. For example, Zhang et al. [6] designed a dual emission double-perovskite La2MgTiO6:Pr3+,Dy3+ phosphor for thermometer with a high relative sensitivity of 2.357%K−1; Li et al. [7] reported that NaLa(MoO4)2:Ce3+,Tb3+ dual emission phosphors can be effectively excited by near ultraviolet to achieve different emission colors from indigo to yellow-greenish, which is suitable for use in white LEDs; The properties of the La2ZnTiO6:Mn4+,Cr3+ dual emission phosphor developed by Ou et al. [8] suggested that it can be applied in wavelength detection and indoor cultivation of LEDs.
Mn4+ as a luminescent center has been widely studied for its bright red emission and broad excitation band [9,10,11,12]. The doped Mn4+ ion acting as luminescent activator usually prefers to occupy the octahedral position in the matrix [13,14,15,16,17]. Mn4+ is very sensitive to the matrix environment in which it is located. As well as influencing emission band position, the local environment contributes to thermal quenching speed and luminescence intensity. Tb3+ is a widely used luminescent center in phosphors due to its 5D4 → 7F5 transition, which produces sharp green emission peaks near 545 nm [18,19,20]. The emission peaks of Tb3+ (green) and Mn4+ (red) are well separated, while their excitation spectra overlap. Thus, with suitable wavelength excitation, the luminescence of Tb3+ and Mn4+ can be simultaneously obtained. Changing the concentration of Tb3+ and Mn4+ can produce color-tunable luminescence, which can be applied to WLEDs. Moreover, the luminescence intensity ratio (green/red) can also be used in temperature measurement and light wavelength detection since the emission intensity of Tb3+ and Mn4+ depends on temperature and excitation wavelength in different ways.
Tungstate as a matrix of luminescent materials has been widely studied for its low cost, stable chemical properties, suitable local environment for luminescence center [21,22,23,24]. Especially, the double perovskite tungstate NaLaMgWO6 with the layered Na+/La3+ cation ordering and rock salt Mg2+/W6+ cation ordering is an ideal matrix for both rare earth ions and transition metal ions [25]. The La3+ site in the matrix can be replaced by Tb3+ and other trivalent rare earth ions, and the [WO6] octahedrons can provide octahedral sites suitable for Mn4+ ions. The Mn4+ doped NaLaMgWO6 red phosphors and the Tb3+ doped NaLaMgWO6 phosphors have been widely reported [26,27,28,29]. As far as we know, however, there are no reports so far about NaLaMgWO6:Tb3+, Mn4+ co-doped dual-emitting phosphor.
In this work, a series of NaLaMgWO6:Tb3+, Mn4+ phosphors have been synthesized. The Tb3+/Mn4+ doping concentration, temperature and excitation wavelength dependent luminescence properties have been studied, which suggests that NaLaMgWO6:Tb3+, Mn4+ phosphor is a promising high-performance multifunctional material.
2 Experimental details
The NaLaMgWO6:xTb3+, yMn4+ (x = 0–25%, y = 0–1.5%) phosphors were obtained from one-step high-temperature Solid-state method. The chemical raw materials were NaCO3 (A.R.), La2O3 (99.99%), WO3 (A.R.), 4MgCO3⋅Mg(OH)2⋅5H2O (A.R.), MnCO3 (A.R.) and Tb4O7 (99.99%). The electronic balance was utilized to weigh all the raw materials in stoichiometric ratios, which were subsequently ground in an agate mortar for a duration of 25 min. After that, the materials were calcined for eight hours at 1400 °C in a muffle furnace. Finally, the sample is ground into a powder and then characterized.
The phase purity of the NaLaMgWO6:10 mol% Tb3+,0.6 mol% Mn4+ phosphor was determined by using X-ray diffraction (XRD) data acquired on a Bruker D8 Focus Diffractometer (Instrument model: PIGAKV Ultima IV) with Cu K radiation. With the help of a monochromator (Zolix Instrument, Omni-λ320i) outfitted with a photomultiplier tube (PMTH-S1-CR928), Data Acquisition System and a xenon lamp (LSP-X150), the photoluminescence excitation (PLE) and emission (PL) spectra were obtained. A steady-state/transient fluorescence spectrometer (Edinburgh Instruments, FLS980) was used to measure the PL spectra changing with temperature of NaLaMgWO6:10%Tb3+, 0.3%Mn4+ phosphor.
3 Results and discussion
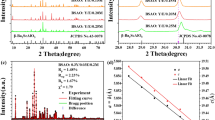
Figure 1a depicts XRD patterns of the NaLaMgWO6:10 mol% Tb3+,0.6 mol% Mn4+ sample, confirming that all the diffraction peaks correspond to standard card (JCPDS # 37–0243). Since no other impurity phases are observed, Tb3+ and Mn4+ activators are successfully incorporated into the NaLaMgWO6 matrix. As revealed in Fig. 1b, the crystal structure of NaLaMgWO6 consists of anomalous polyhedrons with layered ordering of Na/La(the peaks (001)/(111)) and octahedrons with rock salt ordering of Mg/W(the peaks (101)/(121)) [30]. The crystal of NaLaMgWO6 is the monoclinic double-perovskite with space group C2/m [31]. Mn4+ can occupy the [WO6] octahedron because its radius (R = 0.53 Å, Z = 6) is similar to that of W6+ (R = 0. 62 Å, Z = 6), resulting in the 2Eg → 4A2g transition and emitting red light. Because of the same ionic radius and valence state between Tb3+ (R = 0.923 Å, Z = 8) and La3+(R = 0.1032 Å, Z = 8) ions, Tb3+ ions tend to stay in the [LaO8] anomalous polyhedrons.
Figure 2a depict the PL and PLE spectra of the NaLaMgWO6: Mn4+ sample. The NaLaMgWO6: Mn4+ PLE spectra is constituted of two broad bands at 347 nm and 466 nm (monitored at 700 nm).The broad bands can be attributed to the strong electron–phonon interaction [32]. The two excitation bands are well-fitted by three Gaussian peaks at 290 nm, 377 nm and 487 nm, corresponding to the transitions of Mn4+-O2− charge-transfer band(CTB), 4A2 → 4T1 and 4A2 → 4T2(dotted lines in the figure), respectively[27]. Under 487 nm excitation, the PL spectra in Fig. 2a show a broad band with a center at 700 nm, which is ascribed to the spin-forbidden 2Eg → 4A2g transition of Mn4+. Mn4+ ion is located in the [WO6] octahedron and is susceptible to the crystal field environment. Figure 2b depicts the Tanabe-Sugano energy level diagram of Mn4+ with d3 electron configuration. When the value of the crystal field strength Dq/B is large (Dq/B > 2.2), Mn4+ is located in a strong crystal field environment, and the emission originates in the 2Eg → 4A2g transition. On the contrary, Mn4+ is located in a weak crystal field environment, and the electrons returning from the 4T2g state to the 4A2g state will give the Mn4+ emission. The following formula can be used to compute Dq/B [33,34,35].
where \(Dq\) is the crystal field strength, \(B\), \(C\) is the Racah parameters. It can be obtained from Fig. 2a that the values of \(E({\,}^4A_2 - {\,}^4T_1 )\), \(E({\,}^4A_2 - {\,}^4T_2 )\) and \(E({\,}^2E_g - {\,}^4A_{2g} )\) are 26,525.2 cm−1, 20,533.9 cm−1 and 14,285.7 cm−1, respectively. According to calculations, \(Dq\), \(B\) and \(C\) are 2053, 557 and 3332 cm−1, respectively. Mn4+ is found to be located in a strong crystal field environment because Dq/B is 3.69. In addition, the illustration in Fig. 2a depict that the optimal concentration of Mn4+ is 0.6%mol.
a Excitation and emission spectra of NaLaMgWO6: Mn4+ phosphor (λem = 700 nm and λex = 487 nm) and b Tanabe-Sugano energy level diagram of Mn4+ with d3 electron configuration c Excitation and emission spectra of NaLaMgWO6: Tb3+ phosphor (λem = 545 nm and λex = 487 nm). d the linear fitting relationship between lg(x) and lg(I/x). The insets in (a) and (b) show the relationship between single-doped Mn4+ and Tb3 concentrations and emission intensity, respectively
Figure 2c depicts the PLE spectra of the NaLaMgWO6:Tb3+ phosphor monitoring at 545 nm. The excitation band within 250–380 nm is originated from CTB of W6+ → O2− and 4f8 → 4f75d1 transition. The most intense excitation peak occurs at 487 nm, which corresponds to the 7F6 → 5D4 transition. The PL spectra under 487 nm excitation have three emission peaks at 545 nm, 594 nm and 623 nm, assigning to 5D4 → 7F5, 7F4, 7F3 transitions, respectively [36]. The intensity of Tb3+ emission increases as Tb3+ content increases. When the content of Tb3+ exceeds 10%, the average distance between Tb3+ ions become small, the energy transfer between Tb3+ become convenient and the excitation energy is easy to reach quenching centers. Therefore, the luminescence efficiency of the system is reduced and the luminescence intensity of Tb3+ is weakened. The critical distance (\(R_c\)) can be used to identify the type of energy transfer between Tb3+ ions. When \(R_c\) is smaller than 5 Å, exchange interaction predominates in the energy transfer between Tb3+ ion. On the contrary, the electric multipole interaction rather than the exchange interaction is responsible for the energy transfer. The following formula can be used to compute \(R_c\) [37].
where, \(V\) is the crystal cell volume, \(x_c\) is the critical concentration of Tb3+, and N is the amount of sites available for Tb3+ ions in NaLaMgWO6 unit cell. For NaLaMgWO6: Tb3+ phosphor, \(N = 4\), \(V = 477.83\)\({\text{\AA}}^3\) and \(x_c = 0.1\). \(R_c\) is determined to be 13.17 Å, which is more than 5 Å. Electric multipole interaction thus dominates the energy transfer mechanism between Tb3+ ion in the NaLaMgWO6 matrix. According to Dexter theory it is possible to further determine which mechanism might be [38, 39].
where \(I\) represents the luminous intensity, \(\mu\) and \(\varpi\) are constants, and \(x\) represents the Tb3+ concentration. The values of \(\theta\) = 6, 8 and 10 correspond to dipole–dipole, dipole-quadrupole and quadrupole–quadrupole interactions, respectively [40]. Figure 2d fits the slope (\(- \frac{\theta }{3}\)) to -1.53, so \(\theta\) is 4.59(close to 6). Therefore, the mechanism of energy transfer between Tb3+ ion in NaLaMgWO6 matrix is the dipole–dipole interaction.
Figure 3a depicts the normalized excitation spectra of the NaLaMgWO6:10%Tb3+,0.6%Mn4+ sample, in which the monitored wavelength is 545 nm and 700 nm corresponding to the emission peak of Tb3+ and Mn4+ ions, respectively. Figure 3a shows that light within the range 272–400 nm and 468–508 nm can simultaneously excite Tb3+ and Mn4+ ion, with 487 nm being the optimal excitation wavelength. The emission spectra for the NaLaMgWO6:x%Tb3+,0.6%Mn4+ and NaLaMgWO6:10%Tb3+, y%Mn4+ samples under 487 nm excitation are depicted in Fig. 3b and c, respectively. The PL spectra of NaLaMgWO6:Tb3+, Mn4+ phosphors are mainly composed of the 5D4 → 7F5 transition(545 nm) of Tb3+ ions and Mn4+2Eg → 4A2g transition(700 nm). The inset of Fig. 3b depicts the variation of emission intensities of Tb3+ and Mn4+ with Tb3+ concentration under 487 nm excitation. Along the increasing content of Tb3+, the emission intensity of Mn4+ diminishes, suggesting that Tb3+ can quench the emission of Mn4+. Simultaneously, the Tb3+ ion concentration quenching is responsible for the subsequent decline in the intensity of Tb3+ emission at high Tb3+ concentration. Similarly, as depicted in Fig. 3c, when the concentration of Tb3+ is fixed and the concentration of Mn4+ increases (0.1%-1.5%mol), Tb3+ emission gradually weakens, and Mn4+ emission increases and then decreases because of concentration quenching. This means that Mn4+ can also quench the emission of Tb3+. Therefore, it is further proved that the interaction between Tb3+ and Mn4+ in NaLaMgWO6 matrix may be a mutual quenching process [41]. Importantly, since Tb3+ dominates green emission and Mn4+ dominates red emission, a red-yellow-green tunable emission color can be achieved by altering the content ratio of Mn4+ and Tb3+ in the sample. Under 487 nm excitation, Fig. 3d displays the normalized relative emission intensity of Tb3+ and Mn4+ ions in NaLaMgWO6:x%Tb3+,0.6% Mn4+ phosphors. The red emission of Mn4+ predominates when Tb3+ concentration is very low, as shown more clearly in Fig. 3d. The Mn4+ emission weakens gradually as Tb3+ concentration increases, whereas Tb3+ emission is intensified, resulting in yellow emission from the phosphor. When Tb3+ concentration increases to 25%, the green emission of Tb3+ dominates and Mn4+ emission is hardly observed. Figure 3e displays the CIE chromaticity coordinates diagram of NaLaMgWO6:x%Tb3+,0.6%Mn4+ (x = 1%-25%) samples (\(\lambda_{ex} = 487nm\)). A tunable red-yellow-green emission is displayed in these samples with CIE chromaticity coordinates from A(0.7333,0.2667) to G(0.3606,0.6327), indicating the potential application in WLEDs.
a Normalized excitation spectra of the NaLaMgWO6:10%Tb3+,0.6%Mn4+; Emission spectra of b NaLaMgWO6:x%Tb3+,0.6%Mn4+ and c NaLaMgWO6:10%Tb3+,y%Mn4+ phosphors under 487 nm excitation; d The relative normalized emission intensity of Mn4+ and Tb3+ ions in NaLaMgWO6:x%Tb3+,0.6%Mn4+ phosphors;e CIE chromaticity coordinates for NaLaMgWO6:x%Tb3+,0.6%Mn4+ phosphors under 487 nm excitation
It is well known that rare earth ions (Tb3+) are less sensitive to temperature due to the weak electron-lattice interaction because of the 4f level structure shielded by external 5s25p6 shells. However, transition metal ions (Mn4+) with 3dn electron configurations exhibit a strong electron–phonon coupling and are more sensitive to temperature, so the Mn4+, Tb3+ co-doped phosphor can be used as temperature probes. The PL spectra changing with temperature of NaLaMgWO6: 10%Tb3+,0.3%Mn4+ is investigated to explore its temperature sensing properties. Figure 4a displays the PL spectra changing with temperature of the NaLaMgWO6:10%Tb3+,0.3%Mn4+ normalized at 545 nm under 487 nm excitation, and Fig. 4b displays the integrated intensity histogram for the Tb3+:5D4 → 7F5 and Mn4+:2E → 4A2 transitions. In 330–500 K range, Tb3+ and Mn4+ both exhibit temperature increasing dependent emission intensity weakening, but the emission reduction of Tb3+ is slower than that of Mn4+. Figure 4c demonstrates the configurational coordinate diagram of Mn4+ and Tb3+. In Fig. 4c, A is the bottom of the 2E state, and B is the intersection of the 4A2 state and the 2E state parabola. The thermal quench activation energy (∆E) between A and B represents the energy barrier for electron to overcome to realize the nonradiative decay [42]. As the temperature rises, the electron–phonon coupling provides enough thermal quenching activation energy to allow a large number of electrons to travel from A to B and then relax to the ground state via a non-radiative process [43]. This is the reason for the luminescence thermal quenching of Mn4+ ion. However, Tb3+ has a completely different thermal quenching process than Mn4+ because the excited and ground states of Tb3+ do not intersect. As depicted in Fig. 4c,the large energy gap (~ 14,000 cm−1) between the 5D4 and the next state 7F0 of Tb3+ sufficiently reduces the possibility of depopulation of the 5D4 through the multi-phonon relaxation process [44].
The Struck and Fonger theory [45] is used to analyze the temperature dependence of Tb3+ and Mn4+ luminescence intensity to further evaluate the temperature sensing capability of sample. In terms of Tb3+ and Mn4+, temperature relates to emission intensity as follows [46]:
where \(I\) is the luminous intensity at the initial temperature and \(I_0\) is the luminous intensity at different temperatures; \(A\) represents a pre-exponential constant, \(E\) represents the thermal quenching activate energy, \(k_B\) is the Boltzmann constant.
Then according to Eq. (7), the luminescence intensity ratio(\(LIR\)) between Tb3+ and Mn4+ can be deduced and expressed by the following formula[47]:
where \(u\), \(v\), and \({\Delta }E\) are parameters. \(I_{Tb}\) is the integrated emission intensity of Tb3+ ions ranging from 532 to 555 nm, while \(I_{Mn}\) is the integrated emission intensity of Mn4+ ions ranging from 650 to 800 nm. Figure 5a illustrates the relationship between temperature and measured \(LIR\) of NaLaMgWO6: 10%Tb3+,0.3%Mn4+ sample. The experimental data can be adequately fitted by Eq. (8). \(LIR\) decreases monotonically with increasing temperature because Mn4+ emission thermally quenches more quickly than Tb3+ emission.
The absolute temperature sensitivity(\(S_A\)) and the relative temperature sensitivity(\(S_R\)) can be further deduced by the following equations[48]:
Figure 5b show the relationship between \(S_A\), \(S_R\) and temperature for sample of NaLaMgWO6: 10%Tb3+,0.3%Mn4+. It is observed that the values of the \(S_A\) and \(S_R\) in the temperature range 330-500 K are both increasing. The maximum \(S_A\) and \(S_R\) values for the NaLaMgWO6:10%Tb3+, 0.3%Mn4+ phosphor are obtained at 500 K, which are 0.011 K−1 and 2.35% K−1 respectively. Finally, temperature repeatability is assessed by subjecting 5 heating–cooling cycles on the phosphor and calculating the intensity ratio at 330 and 498 K (Fig. 5c). Temperature repeatability(\(R\)) can be calculated as follows[32]:
where \(\left\langle {LIR(T)} \right\rangle\) is the average of the luminous intensity ratio in all cycles, and \(LIR_j (T)\) is the luminescence intensity ratio in the j cycle. It can be calculated from Eq. (11) that the temperature repeatability rate of NaLaMgWO6:10%Tb3+, 0.3%Mn4+ phosphor is up to 98%.
Properties of some temperature sensing materials combined with Tb3+ or Mn4+ are shown in Table 1. The comparison of SR and temperature measurement range shows that the performance of NaLaMgWO6:Mn4+, Tb3+ is better than that of most optical temperature measuring materials reported previously. In addition, the monitoring peaks of Tb3+ (≈545 nm) and Mn4+ (≈700 nm) are well separated, indicating that NaLaMgWO6:Mn4+, Tb3+ temperature measuring materials have good signal recognition ability. Therefore, the high relative sensitivity, wide temperature measurement range and excellent signal resolution of NaLaMgWO6:Mn4+, Tb3+ dual emission make this material possible to be an excellent temperature measurement material without any additional luminescence intensity calibration.
In addition, the excitation intensity of Tb3+ exhibits a consistent decrease as the wavelength increases, whereas the excitation intensity of Mn4+ displays a consistent increase within the wavelength range of 293-348 nm, as depicted in Fig. 3(a). Therefore, as the excitation wavelength progressively increases from 294 to 348 nm, the Tb3+ emission diminishes, while Mn4+ emission intensifies. As depicted in Fig. 6a, the PL spectra of the NaLaMgWO6:10%Tb3+,0.6%Mn4+ phosphor excited at different wavelengths (293-348 nm) are measured. The luminescence intensity ratio of Mn4+ to Tb3+ in NaLaMgWO6:10%Tb3+,0.6%Mn4+ phosphor may exhibit monotonic wavelength-dependent change in 293-348 nm region. The calculation of the integrated \(LIR\) of Mn4+ to Tb3+ at various excitation wavelengths is depicted in Fig. 6b. \(LIR\) increases monotonically as the excitation wavelength increasing from 293 to 348 nm. The wavelength measurement sensitivity S can be defined as follows:
Here \(\lambda_{ex}\) is the excitation wavelength[8, 10]. Figure 6b illustrates the relationship between \(S\) and \(\lambda_{ex}\). A maximum \(S\) of 6.67%nm−1 is obtained at 340 nm. The results indicate that NaLaMgWO6:Tb3+, Mn4+ phosphor can be used in wavelength detection when temperature is maintained, which provides a possible method for designing new compact photodetectors or spectrometers based on this phosphor in the future.
4 Conclusion
In summary, NaLaMgWO6:Mn4+, Tb3+ phosphors have been synthesized by one-step high temperature solid method. Red emission of Mn4+ and green emission of Tb3+ can be observed by 487 nm excitation. At room temperature, the red-yellow-green emission color transition can be attained by altering the doping concentration ratio of Tb3+ and Mn4+. Owing to their distinct thermal quenching behaviors, the LIR of Mn4+ and Tb3+ in NaLaMgWO6:Mn4+, Tb3+ phosphors are monotonically related to temperature in 330–500 K, and the SR is up to 2.35% K−1 at 500 K, showing excellent temperature sensing performance. At a constant temperature, the LIR of Mn4+ to Tb3+ also increases monotonically as the excitation wavelength increases from 293 to 348 nm, indicating that it can serve as a wavelength detector within 293-348 nm. The results demonstrate the possible wide range of applications for NaLaMgWO6:Mn4+, Tb3+ phosphor, including illumination, wavelength detection and optical temperature measurement.
Data availability
The corresponding author will supply the relevant data in response to reasonable requests.
References
B. Dong, B. Cao, Y. He, Z. Liu, Z. Li, Z. Feng, Temperature sensing and in vivo imaging by molybdenum sensitized visible upconversion luminescence of rare-earth oxides. Adv. Mater. 24, 1987–1993 (2012)
C. Bouzigues, T. Gacoin, A. Alexandrou, Biological applications of rare-earth based nanoparticles. ACS Nano 5, 8488–8505 (2011)
H.Z. Lian, Z.Y. Hou, M.M. Shang, D.L. Geng, Y. Zhang, J. Lin, Rare earth ions doped phosphors for improving efficiencies of solar cells. Energy 57, 270–283 (2013)
T.J.C.R. Ronda, H. Nikol, Rare earth phosphors: fundamentals and applications. J. Alloy. Compd. 275–277, 669–676 (1988)
B. Shao, J. Huo, H. You, Prevailing strategies to tune emission color of lanthanide-activated phosphors for WLED applications. Adv. Opt. Mater. (2019). https://doi.org/10.1002/adom.201900319
H. Zhang, Y. Liang, H. Yang, S. Liu, H. Li, Y. Gong, Y. Chen, G. Li, Highly sensitive dual-mode optical thermometry in double-perovskite oxides via Pr3+/Dy3+ energy transfer. Inorg. Chem. 59, 14337–14346 (2020)
G. Li, S. Lan, L. Li, M. Li, W. Bao, H. Zou, X. Xu, S. Gan, Tunable luminescence properties of NaLa(MoO4)2:Ce3+, Tb3+ phosphors for near UV-excited white light-emitting-diodes. J. Alloy. Compd. 513, 145–149 (2012)
J. Ou, X. Yang, S. Xiao, Luminescence performance of Cr3+ doped and Cr3+, Mn4+ co-doped La2ZnTiO6 phosphors. Mater. Res. Bull. (2020). https://doi.org/10.1016/j.materresbull.2019.110764
Y. Chen, J. He, X. Zhang, M. Rong, Z. Xia, J. Wang, Z.Q. Liu, Dual-mode optical thermometry design in Lu3Al5O12:Ce3+/Mn4+ phosphor. Inorg. Chem. 59, 1383–1392 (2020)
T. Chen, X. Yang, W. Xia, W. Li, S. Xiao, Deep-red emission of Mn4+ and Cr3+ in (Li1−xAx)2MgTiO4 (A=Na and K) phosphor: potential application as W-LED and compact spectrometer. Ceram. Int. 43, 6949–6954 (2017)
B. Yan, Y. Wei, W. Wang, M. Fu, G. Li, Red-tunable LuAG garnet phosphors via Eu3+→Mn4+ energy transfer for optical thermometry sensor application, Inorganic Chemistry. Frontiers 8, 746–757 (2021)
T. Kurunthatil Kuttiat, M. Abraham, A.K. Kunti, N. Amador-Mendez, M. Tchernycheva, S. Das, Enriching the deep-red emission in (Mg, Ba)3M2GeO8: Mn4+ (M = Al, Ga) compositions for light-emitting diodes. ACS Appl. Mater. Interfaces 15, 7083–7101 (2023)
B. Wang, H. Lin, F. Huang, J. Xu, H. Chen, Z. Lin, Y. Wang, Non-rare-earth BaMgAl10–2xO17:xMn4+, xMg2+: a narrow-band red phosphor for use as a high-power warm w-LED. Chem. Mater. 28, 3515–3524 (2016)
M. Kim, W.B. Park, J.-W. Lee, J. Lee, C.H. Kim, S.P. Singh, K.-S. Sohn, Rb3SiF7:Mn4+ and Rb2CsSiF7:Mn4+ red-emitting phosphors with a faster decay rate. Chem. Mater. 30, 6936–6944 (2018)
S. Liang, M. Shang, H. Lian, K. Li, Y. Zhang, J. Lin, Deep red MGe4O9:Mn4+(M = Sr, Ba) phosphors: structure, luminescence properties and application in warm white light emitting diodes. Journal of Materials Chemistry C 4, 6409–6416 (2016)
Y. Jin, M.H. Fang, M. Grinberg, S. Mahlik, T. Lesniewski, M.G. Brik, G.Y. Luo, J.G. Lin, R.S. Liu, Narrow red emission band fluoride phosphor KNaSiF6:Mn4+ for warm white light-emitting diodes. ACS Appl. Mater. Interfaces 8, 11194–11203 (2016)
F. Ding, Y. Zhou, Y. He, Y. Liang, P. Luo, W. Zhou, J. Zhang, L. Yu, Z. Qiu, S. Lian, Broadband UV-excitation and red/far-red emission materials for plant growth: tunable spectrum conversion in Eu3+, Mn4+ Co-doped LaAl0.7Ga0.3O3 phosphors. Inorganic Chem. 62, 3141–3152 (2023)
X. Wu, Y. Jiao, O. Hai, Q. Ren, F. Lin, H. Li, Photoluminescence, energy transfer, color tunable properties of Sr3La(BO3)3:Ce, Tb phosphors. J. Alloy. Compd. 730, 521–527 (2018)
L. Qiu, P. Wang, X. Wei, F. Chi, Y. Chen, M. Yin, Investigation of a phosphor mixture of LiAl5O8: Cr3+ and LuPO4: Tb3+ as a dual-mode temperature sensor with high sensitivity. J. Alloys Compd. 879, 160461 (2021)
P. Kumar, S. Singh, I. Gupta, A. Dalal, V. Kumar, D. Singh, Preparation, structural and photometric properties of single-phased Gd3Al5O12:Tb3+ green-emitting phosphors for solid state lighting purpose. Mater. Sci. Eng. B 288, 116189 (2023)
V. Petrov, M. Cinta Pujol, X. Mateos, Ò. Silvestre, S. Rivier, M. Aguiló, R.M. Solé, J. Liu, U. Griebner, F. Díaz, Growth and properties of KLu(WO4)2, and novel ytterbium and thulium lasers based on this monoclinic crystalline host. Laser Photon. Rev. 1, 179–212 (2007)
R.V. Yadav, R.S. Yadav, A. Bahadur, A.K. Singh, S.B. Rai, Enhanced quantum cutting via Li+ doping from a Bi3+/Yb3+-Codoped gadolinium tungstate phosphor. Inorg. Chem. 55, 10928–10935 (2016)
F. Lei, B. Yan, Morphology-controlled synthesis, physical characterization, and photoluminescence of novel self-assembled pomponlike white light phosphor: Eu3+-Doped sodium gadolinium tungstate. J. Phys. Chem. C 113, 1074–1082 (2009)
M. Song, W. Zhao, W. Ran, J. Xue, Y. Liu, J.H. Jeong, Multicolor tunable luminescence and energy transfer mechanism in a novel single-phase KBaGd(WO4)3:Tb3+, Eu3+ phosphor for NUV WLEDs. J. Alloy. Compd. 803, 1063–1074 (2019)
L. Zhang, Z. Lu, P. Han, L. Wang, Q. Zhang, Synthesis and photoluminescence of Eu3+-activated double perovskite NaGdMg(W, Mo)O6– a potential red phosphor for solid state lighting. J. Mater. Chem. C 1, 54–57 (2013)
S. Wang, Y.-J. Han, L. Shi, M.-X. Jia, Y.-L. Tong, N.-N. Bao, Z.-F. Mu, Z.-Y. Mao, Z.-W. Zhang, S.-L. Niu, Charge compensation assisted enhanced photoluminescence derived from Al3+-codoped NaLaMgWO6:Mn4+ phosphors for plant growth lighting applications. J. Lumin. 226, 117438 (2020)
X. Huang, J. Liang, B. Li, L. Sun, J. Lin, High-efficiency and thermally stable far-red-emitting NaLaMgWO6:Mn4+ phosphorsfor indoor plant growth light-emitting diodes. Opt. Lett. 43, 3305–3308 (2018)
G. Li, Y. Wang, Y. Wei, X. Wang, Structure, energy transfer, and luminescence properties of NaLaMgWO6: Tb3+, Eu3+ phosphors for solid-state lighting. J. Mater. Sci. Mater. Electron. 31, 3835–3844 (2020)
J. Hou, X. Yin, F. Huang, W. Jiang, Synthesis and photoluminescence properties of NaLaMgWO6:RE3+ (RE=Eu, Sm, Tb) phosphor for white LED application. Mater. Res. Bull. 47, 1295–1300 (2012)
A.R. Sharits, J.F. Khoury, P.M. Woodward, Evaluating NaREMgWO6 (RE = La, Gd, Y) doubly ordered double perovskites as Eu3+ phosphor HOSTS. Inorg. Chem. 55, 12383–12390 (2016)
F. Xiao, H. Xie, C. Xie, R. Yi, Y. Zeng, H. Yuan, Achievement of broadband NIR emission in double perovskite NaLaMgWO6 via non-equivalent substitution of Cr3+. J. Lumin. 255, 119588 (2023)
Y. Fang, Y. Zhang, Y. Zhang, J. Hu, Achieving high thermal sensitivity from ratiometric CaGdAlO4:Mn4+, Tb3+ thermometers. Dalton Trans. 50, 13447–13458 (2021)
J. Long, C. Ma, Y. Wang, X. Yuan, M. Du, R. Ma, Z. Wen, J. Zhang, Y. Cao, Luminescent performances of Mn4+ ions during the phase evolution from MgTiO3 to Mg2TiO4. Mater. Res. Bull. 85, 234–239 (2017)
J. Xiang, J. Chen, N. Zhang, H. Yao, C. Guo, Far red and near infrared double-wavelength emitting phosphor Gd2ZnTiO6:Mn4+, Yb3+ for plant cultivation LEDs. Dyes Pigm. 154, 257–262 (2018)
Z. Liu, X. Yu, Q. Peng, X. Zhu, J. Xiao, J. Xu, S. Jiang, J. Qiu, X. Xu, NIR mechanoluminescence from Cr3+ activated Y3Al5O12 with intense zero phonon line. Adv. Funct. Mater. (2023). https://doi.org/10.1002/adfm.202214497
D.K. Amarasinghe, F.A. Rabuffetti, Ratiometric thermometry using thermochromic Tb3+:Mn4+:Na4Mg(WO4)3 phosphors. Inorg. Chem. 60, 3165–3171 (2021)
D. Xu, X. Wu, Q. Zhang, W. Li, T. Wang, L. Cao, J. Meng, Fluorescence property of novel near-infrared phosphor Ca2MgWO6:Cr3+. J. Alloy. Compd. 731, 156–161 (2018)
P. Cai, L. Qin, C. Chen, J. Wang, S. Bi, S.I. Kim, Y. Huang, H.J. Seo, Optical thermometry based on vibration sidebands in Y2MgTiO6:Mn4+ double perovskite. Inorg. Chem. 57, 3073–3081 (2018)
C.H. Huang, Y.C. Chiu, Y.T. Yeh, T.S. Chan, T.M. Chen, Eu2+-activated Sr8ZnSc(PO4)7: a novel near-ultraviolet converting yellow-emitting phosphor for white light-emitting diodes. ACS Appl. Mater. Interfaces 4, 6661–6668 (2012)
R.T. Maske, A.N. Yerpude, R.S. Wandhare, A. Nande, S.J. Dhoble, Combustion synthesized novel SrAlBO4:Eu3+ phosphor: structural, luminescence, and Judd-Ofelt analysis. Opt. Mater. 141, 113893 (2023)
J. Zhang, Y. Dai, B. Liu, B. Han, Luminescence quenching of Er3+/Sm3+ in color-tunable NaLaMgWO6:Er3+, Sm3+ phosphor. Optik 172, 1129–1133 (2018)
Y. Cheng, Y. Gao, H. Lin, F. Huang, Y. Wang, Strategy design for ratiometric luminescence thermometry: circumventing the limitation of thermally coupled levels. J. Mater. Chem. C 6, 7462–7478 (2018)
D. Chen, S. Liu, Y. Zhou, Z. Wan, P. Huang, Z. Ji, Dual-activator luminescence of RE/TM:Y3Al5O12(RE = Eu3+, Tb3+, Dy3+; TM = Mn4+, Cr3+) phosphors for self-referencing optical thermometry. J. Mate. Chem. C 4, 9044–9051 (2016)
W. Piotrowski, K. Trejgis, K. Maciejewska, K. Ledwa, B. Fond, L. Marciniak, Thermochromic Luminescent Nanomaterials Based on Mn4+/Tb3+ codoping for temperature imaging with digital cameras. ACS Appl. Mater. Interfaces 12, 44039–44048 (2020)
C.W. Struck, W.H. Fonger, Thermal Quenching of Tb+3, Tm+3, Pr+3, and Dy+3 4fn emitting states in La2O2S. J. Appl. Phys. 42, 4515–4516 (1971)
M.-H. Yu, D. Zhao, R.-J. Zhang, Q.-X. Yao, L. Jia, Dual luminescent peaks, energy transfer and temperature sensor properties of a new Eu2+/Mn2+ co-doped phosphor. J. Lumin. 257, 119760 (2023)
Q. Wang, M. Liao, Q. Lin, M. Xiong, Z. Mu, F. Wu, A review on fluorescence intensity ratio thermometer based on rare-earth and transition metal ions doped inorganic luminescent materials. J. Alloys Compd. 850, 156744 (2021)
F. Huang, D. Chen, Synthesis of Mn2+:Zn2SiO4–Eu3+:Gd2O3 nanocomposites for highly sensitive optical thermometry through the synergistic luminescence from lanthanide-transition metal ions. J. Mater. Chem. C 5, 5176–5182 (2017)
Y. Gao, F. Huang, H. Lin, J. Zhou, J. Xu, Y. Wang, A novel optical thermometry strategy based on diverse thermal response from two intervalence charge transfer states. Adv. Func. Mater. 26, 3139–3145 (2016)
D. Ananias, F.A. Paz, D.S. Yufit, L.D. Carlos, J. Rocha, Photoluminescent thermometer based on a phase-transition lanthanide silicate with unusual structural disorder. J. Am. Chem. Soc. 137, 3051–3058 (2015)
X. Zhang, Y. Huang, M. Gong, Dual-emitting Ce3+, Tb3+ co-doped LaOBr phosphor: Luminescence, energy transfer and ratiometric temperature sensing. Chem. Eng. J. 307, 291–299 (2017)
L. Peng, Q. Meng, W. Sun, Size dependent optical temperature sensing properties of Y2O3: Tb3+, Eu3+ nanophosphors. RSC Adv. 9, 2581–2590 (2019)
S. Li, Q. Meng, S. Lü, W. Sun, Optical properties of Sm3+ and Tb3+ co-doped CaMoO4 phosphor for temperature sensing. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 214, 537–543 (2019)
G. Bao, K.L. Wong, D. Jin, P.A. Tanner, A stoichiometric terbium-europium dyad molecular thermometer: energy transfer properties. Light Sci Appl 7, 96 (2018)
X. Zhang, Z. Zhu, Z. Guo, Z. Sun, Y. Chen, A ratiometric optical thermometer with high sensitivity and superior signal discriminability based on Na3Sc2P3O12: Eu2+, Mn2+ thermochromic phosphor. Chem. Eng. J. 356, 413–422 (2019)
P. Wang, J. Mao, L. Zhao, B. Jiang, C. Xie, Y. Lin, F. Chi, M. Yin, Y. Chen, Double perovskite A2LaNbO6:Mn4+, Eu3+ (A = Ba, Ca) phosphors: potential applications in optical temperature sensing. Dalton Trans. 48, 10062–10069 (2019)
H. Zhou, N. Guo, M. Zhu, J. Li, Y. Miao, B. Shao, Photoluminescence and ratiometric optical thermometry in Mn4+/Eu3+ dual-doped phosphor via site-favorable occupation. J. Lumin. 224, 11731 (2020)
Acknowledgements
This work was supported by the Natural Science Foundation of Hunan Province, China (No. 2020JJ4589).
Funding
Natural Science Foundation of Hunan Province,China, 2020JJ4589.
Author information
Authors and Affiliations
Contributions
HuiRao Liu: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Validation, Writing - original draft. Xiaoliang Yang: Conceptualization; Funding acquisition; Methodology; Project administration; Resources; Supervision; Writing - review & editing. Siguo Xiao: Conceptualization, Funding acquisition, Methodology, Project administration, Resources, Writing - review & editing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Liu, H., Yang, X. & Xiao, S. Color tunability of NaLaMgWO6:Mn4+, Tb3+ phosphor and its potential application in temperature sensor and wavelength detector. Appl. Phys. B 129, 186 (2023). https://doi.org/10.1007/s00340-023-08133-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00340-023-08133-5