Abstract
The laser-induced damage threshold (LIDT) of optical thin films is one of the major constraints in development of high repetition rate femtosecond laser systems. In this work, the optical anti-reflection HfO2 thin films are prepared by magnetron sputtering, and the optical properties of the films can be modulated by changing the sputtering power and O2/Ar gas flow ratio. The Urbach energy and the atomic ratio of O/Hf are calculated to analyze the point defects in the films, and the band structure of the film is obtained by photoluminescence spectra and density functional theory. To evaluate the major factors to the film failure, the laser-induced damage test is carried out for HfO2 films with different irradiation times using the high repetition rate femtosecond laser system. The results show that the damage threshold of the HfO2 thin films can reach 0.92 J/cm2 (500 k-on-1), and the laser-induced damage performance is limited by the point defects and fatigue effect. This work reveals the relationship between deposition parameters and optical properties of thin films, which provides reference for the design and manufacture of optical anti-reflection thin films with better damage resistance to high repetition rate femtosecond laser.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The high-power femtosecond laser renders the wide spectrum of application in science and industry, including compact laser accelerators [1], astrophysics [2] and material modifications [3]. Further increase of output power and repetition rate of the high-power femtosecond laser systems is the main trend in ultrafast laser science and technology. However, the progress of development is substantially hindered by low laser-induced damage threshold (LIDT) of critical laser components [3]. Especially, the LIDT of optical films is the major obstacle to improve the output power and repetition rate [4, 5]. The physical mechanism of the femtosecond laser damage in wide band gap materials has been studied extensively in the literatures. For femtosecond laser pulses, the irradiation damage is the result of a sequence of complex physical processes, including free carrier generation through multiphoton and avalanche ionization [5,6,7,8]. References [9, 10] studied the irradiation damage of oxide thin films using femtosecond laser, and the results showed that the LIDT was proportional to the optical band gap of the films. However, the research on the damage behavior of optical films under ultra-high repetition rate femtosecond laser irradiation is rare.
HfO2 thin films have diverse excellent properties such as thermal, chemical and mechanical stabilities, as well as low optical absorption and scattering, high refractive index, high transparency and wide optical band gap [11,12,13,14]. Therefore, the HfO2 thin films are widely used as anti-reflection and high-reflection coatings in the field of femtosecond laser. However, the performance of HfO2 thin films mainly depends on the preparation method and deposition condition of the films. Nowadays, HfO2 films are prepared by electron beam evaporation [15], atomic layer deposition [12, 16] and magnetron sputtering [13, 14], etc. The results indicate that the thin films prepared by electron beam evaporation have considerable laser scattering loss and poor thermal stability. Although atomic layer deposition technology can accurately control the composition and thickness of thin films, its slow deposition rate and expensive precursor limit the large-scale application of the technology in the field of photoelectric thin films. In contrast, magnetron sputtering has ability to produce uniform, good adhesion, high crystallinity, high surface quality and low laser scattering films. Moreover, the dense films produced by magnetron sputtering have high damage resistance in the femtosecond regime. Thus, the magnetron sputtering technique was selected to prepare the HfO2 thin films with high quality. At present, a large number of studies mainly focus on exploring the relationship between deposition parameters and microstructure, lacking of further characterization and exploration of laser-induced damage threshold under femtosecond irradiation [11, 14, 17, 18].
In this work, the influences of sputtering power and O2/Ar gas flow ratio on the microstructure, surface, band gap, defects and optical properties of HfO2 films are systematically investigated. After that, all HfO2 thin films are subjected to laser damage test under 515 nm, 250 fs and 500 kHz laser system, and the damage morphology is observed by the optical microscope. This work aims to investigate the correlation between magnetron sputtering deposition parameters and LIDT of HfO2 thin films.
2 Experimental
2.1 Preparation of films
The HfO2 thin films were prepared on fused silica substrates by radio frequency (RF) magnetron sputtering in a mixed O2/Ar atmosphere using a high purity hafnium target (Beijing Gaoke New Material Technology Co., 99.99% purity) with a diameter of 50 mm and a thickness of 6 mm. Fused silica (20 × 20 mm2, 99.99% purity) samples were purchased from ZhongNuo Advanced Material (Beijing) Technology Co., Ltd with similar surface qualities to ensure the comparison of test data. Before coating, all the substrates were firstly etched for 5 min in a buffered hydrofluoric acid solution (BHF, 1%HF + 15%NH4F + 84%H2O) in order to remove the surface contamination and polishing residuals. Then the substrates were cleaned immediately with acetone, ethanol, and deionized water and then dried at 55 °C. The magnetron sputtering device is designed and manufactured by our group and China Nuclear Tongchuang (Co., Ltd.) with 60 mm distance from the target to the substrate. The fused silica substrates were mounted on the substrate holder with a rotation speed of 10 rpm to obtain a uniform HfO2 thin films. Before sputtering, a base pressure of 2 × 10–3 Pa was achieved in chamber to ensure the purity of HfO2 thin films. The deposition of HfO2 thin films was conducted at the sputtering powers of 200 W, 300 W and 400 W with the O2/Ar gas flow ratios of 50/150, 66/132 and 100/100 (sccm). The deposition time was fixed at 120 min. Nine groups of HfO2 thin films were prepared for structural and morphological characterization, and the refractive index and deposition rate were calculated. Table 1 presents the sample labels of nine groups. Finally, the optical anti-reflection HfO2 films with an optical thickness of 3λ/4 (λ = 515 nm) were prepared for laser damage threshold test.
2.2 Characterization
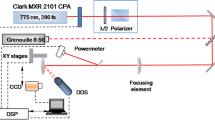
The crystal structure analysis was carried out by the Bruker AXS D8 ADVANCE X-ray diffractometer (XRD) with Cu Kα (λ = 1.5406 Å, 40 kV, 40 mA) radiation. The scanning speed and scanning range were 2°/min and 10°–70°, respectively. The surface and cross-section morphologies of HfO2 thin films were observed using a field-emission scanning electron microscope (FE-SEM, FEI Inspect F). In order to obtain the detailed surface morphology and roughness, an atomic force microscopy (AFM) was used to analyze the surface morphologies of the films and substrates and the average roughness for each sample was calculated with three randomly selected square areas of 1 × 1 µm2. The elemental composition and distribution of the films were analyzed with an energy-dispersive X-ray spectroscope (EDS). The atomic ratio of O and Hf was calculated from the results tested by using an X-ray photoelectron spectroscope (XPS, Quantum 2000 Scanning ESCA Microprobe instruments), and the chemical compositions and states were analyzed. The optical transmittance and absorption spectra of films were measured using UV–Vis-NIR spectrophotometer (Lambda 750S) in the range 200–800 nm by using dual beam mode to remove the influence of substrate. The photoluminescence (PL) spectra were measured by a fluorescence spectrophotometer (FLS1000). The laser damage tests were performed by a high repetition rate femtosecond laser operated at 515 nm with pulse duration of 250 fs and repetition rate of 500 kHz. The laser beam was focused on the film surface with a beam diameter of 3.6 μm. The damage morphologies of samples were observed by the optical microscope and SEM, and the S-on-1 test procedure [ISO Standard No. 21254-2] was employed for the LIDT measurements.
3 Results and discussion
3.1 Crystal structure
The XRD patterns of all samples under different sputtering powers and O2/Ar gas flow ratios are shown in Fig. 1a. All HfO2 thin films present crystalline structure of monoclinic phase (JCPDS-34-0104) and space group (P 21/a), and there are four dominant diffraction peaks of (− 111), (111), (020) and (002) planes [11]. The first-principles calculation results indicate that the monoclinic phase of HfO2 is the most stable phase at room temperature and atmospheric pressure [19]. Figure 1b shows the crystal structure of monoclinic HfO2. All the diffraction peaks gradually strengthen with the increasing sputtering power from 200 to 400 W, which indicates the improvement of crystal quality [14, 20]. The diffraction peaks of A1 sample are the weakest because the sputtering power and oxygen flow ratio are the lowest and it is difficult to form HfO2 thin films. In order to accurately investigate the effects of sputtering power and O2/Ar gas flow ratio on the microstructure of HfO2 thin films, we calculated the crystallinity, grain size, lattice constant corresponding to the (− 111) plane as well as the dislocation density and micro strain of all thin films since the (− 111) plane of HfO2 appeared in all the samples.
The crystallinity of HfO2 thin films is calculated by the area under the curve method [21], and the results are listed in Table 2. It is observed that the crystallinity significantly increases with the increasing sputtering power, because the high sputtering power will offer more energy for the mobility of particles on film surface to induce the crystallization of the films [20]. Under the condition of sputtering power of 400 W and O2/Ar gas flow ratio of 66/132, the film has the highest crystallinity.
The crystalline size is calculated using Scherrer’s formula [13, 18, 21]:
where K is known as a correction factor and taken the value 0.89; λ = 1.5406 Å is the wavelength of X-ray; θ is the diffraction angle corresponding to the (− 111) peak and β is the full width at half the maximum of the diffraction. The crystalline size of all HfO2 film particles ranges from 6.9 to 9 nm, as shown in Fig. 2a. In general, the crystalline size of the HfO2 thin films increases from 7.1 to 8.5 nm with the increase of sputtering power. The crystalline size of the HfO2 thin films also increases with the increase of O2/Ar gas flow ratio. This is because the higher mass and impact section of argon ions compared with oxygen. As the O2/Ar gas flow ratio increases, the average energy of the plasma bombarding Hf target decreases, thereby reducing the deposition rate. The previous study showed that the crystalline size is inversely proportional to the deposition rate [18]. The sufficient reaction energy and reaction gas are generally required to form thin films. However, it is difficult to form HfO2 crystalline at 200 W with an O2/Ar flow ratio of 50/150 so that only very weak diffraction peaks appear in the XRD curve of Sample A1. Therefore, the calculation of the crystalline size of Sample A1 using Scherrer's formula is not accurate, leading to the larger crystalline size of Sample A1 than B1 and C1 in Fig. 2a, consistent with the Ref. [21]. Due to the different volumes of O2 and Ar, the sputtering pressure is 1.8 Pa, 1.6 Pa and 1.4 Pa when the O2/Ar gas flow ratio is 50/150, 66/132 and 100/100, respectively. The mean free path (m) of the sputtering particle and sputtering pressure (P) has following relation [22]:
where δm is the particle diameter of the sputtering gas and Ts is the deposition temperature. The m decreases as the size of the gas cluster increases. This in turn increases the collision frequency, leading to agglomeration and growth of HfO2 particles before reaching the substrate.
The interplanar spacing values are calculated using the Bragg formula [17]:
where d is the interplanar spacing values; θ is the Bragg angle corresponding to (-111) peak and λ is the wavelength of X-ray. The value of d is found varying from 3.138 to 3.171 Å, consistent with the value of stress-free relaxed monoclinic HfO2 (d0 = 3.145 Å) [17]. As shown in Fig. 2b, the interplanar spacing values decreases with the increase of sputtering power. We also calculated the dislocation density and micro strain of HfO2 thin films using ρ = 1/D2 (D is the crystalline size calculated by Eq. (1)) [13] and ε = (d0 − d)/d [23], as shown in Fig. 2c and d, respectively. The changes of dislocation density and micro strain in thin films are consistent with internal stress. The stress of HfO2 thin film will reduce the cohesion between the film and the substrate, resulting in interfacial delamination and decohesion failure [24]. The poor interfacial adhesion and large film stress were regarded as adverse effects on LIDT [25].
3.2 Surface morphology
Since the film morphology is the key factor to determine the optical performance, the surface and cross-sectional morphologies were studied by SEM. As shown in Fig. 3, the film surface is crack-free and it is composed of dense irregular particles. The HfO2 thin film has a dense and uniform surface morphology and the particles size is approximately 40 nm when the sputtering power is 300 W. In contrast, the surface morphology becomes rough and non-uniform when the sputtering power is 200 W and 400 W. This is because that low sputtering power can’t provide sufficient energy for migration and rearrangement of the sputtered particles. However, according to the research results in Refs. [14, 20], the large sputtering power will lead to the formation of void defects, which will also result in the rough surface of films.
The cross-sectional images of the films were obtained using SEM to study the deposition rate and interlayer growth mechanism, as shown in Fig. 4a. There is an obvious boundary between the HfO2 film and the substrate, indicating the high quality of the deposited films. The deposition rates of the HfO2 thin films are listed in Table 3, showing that the rate significantly increases with the increase of sputtering power. This is because the high sputtering power can improve the efficiencies of sputtering and molecular transport [14]. Moreover, the temperature of the substrate increases from 70 to 110 °C as the sputtering power increases. The higher temperature will transfer more energy to the particles on the substrate surface, thereby inducing the crystallization of the film and increasing deposition rate [26, 27]. At the sputtering power of 300 W and 400 W, the deposition rate of the HfO2 thin films decreases with the increase of O2/Ar gas flow ratio. The phenomenon agrees well with the results of XRD measurement. This is because the mass and impact section of oxygen ions are smaller, so the deposition rate decreases with an increase of the O2/Ar gas flow ratio [18]. However, at the sputtering power of 200 W, the deposition rate of the films increases with the increase of O2/Ar gas flow ratio. The reason is that the crystalline quality of the film and the adhesion of the particles are poor when the sputtering power at 200 W. The relationship between deposition rates and crystalline size are shown in Fig. 4b. It can be clearly observed that the crystalline size is inversely proportional to the deposition rate. At the high deposition rate, the HfO2 particles are covered by newly sputtered particles before they can grow up. On the contrary, the HfO2 particles can fully grow and form larger crystalline size when the deposition rate decreases [18].
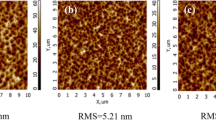
The surface morphologies of three representative substrates and three film samples were characterized by AFM before and after deposition, and the AFM images in a 1 × 1 µm2 square region and the root mean square (RMS) surface roughness data are shown in Fig. 5. The substrate surface with a low RMS roughness is uniform and free of scratch or dirt. As shown in Fig. 5d–f, the films are composed of dense grains, which is in agreement with the SEM results.
3.3 Chemical composition
The Samples A1 and C3 were selected for EDS test because of their very different deposition parameters. Figure 6 shows the EDS results, including elements in the films and distribution of Hf and O elements. The Si signal originates from the fused silica substrate, and the Au peak is due to the gold spraying treatment prior to SEM characterization. The EDS mapping images indicate the uniform distribution of Hf and O elements benefited from the mechanism of magnetron sputtering [28]. The SEM results indicate that the thickness of A1 and C3 samples is 239 nm and 455 nm (Fig. 4), respectively. The energy spectra of A1 and C3 samples contain a large amount of O element signals from the fused silica substrate because of characterization depth of EDS can reach 1 μm [29]. Therefore, the O/Hf ratio of A1 sample is much greater than 2. In contrast, the atomic ratio of C3 sample is closer to the stoichiometric ratio of HfO2 because of its larger thickness. Due to the fact that EDS can only perform semi-quantitative analysis of elements, it is necessary to conducted XPS analysis to accurately explore the elemental composition and valence states of the HfO2 thin films. Furthermore, in order to investigate the effects of sputtering power and O2/Ar gas flow ratio on elemental composition and valence states, the A1, C1 and C3 samples were selected for XPS test.
Figure 7a–f exhibit the XPS spectra of Hf 4f and O 1s of HfO2 thin films. The XPS full survey scan spectrum ( not given) mainly marks three elements: Hf, O and C, and the C signal with a binding energy of 284.8 eV was used to calibrate the position of each peaks [15]. As shown in Fig. 7a–c, the deconvolution peak of Hf 4f can be resolved into two components at 17.0 eV and 18.5 eV, corresponding to Hf 4f7/2 and Hf 4f5/2 of Hf4+, related to Hf–O bonding in the HfO2 thin films, respectively [15, 30, 31]. The spin–orbit splitting energies for A1, C1 and C3 samples are 1.563 eV, 1.542 eV and 1.557 eV, respectively, consistent with the reported value of Ref. [32]. As shown in Fig. 7d–f, a typical O 1s XPS spectrum of HfO2 thin films can be fitted into three Gaussian peaks located at about 530 eV (OC), 531.3 eV (OA) and 532.7 eV (OV), corresponding to crystal lattice oxygen (Hf–O), absorbed oxygen species on the surface and oxygen vacancy defects, respectively [30,31,32,33,34]. The binding energy represents the energy released when the atoms combine from a free state into composite particles, so the stability of the film is proportional to the binding energy. Therefore, with the increasing sputtering power and O2/Ar gas flow ratio, the peak of lattice oxygen shifts towards the higher energy (530.107 eV), indicating that the HfO2 thin films become more stable.
According to the XPS results, the atomic ratio of O/Hf can be calculated by the quantitative relative sensitively factors method [15]:
where IO and IHf are the areas of peaks corresponding to elements O and Hf, respectively, and S is the relative sensitivity factor. The calculated results are listed in Table 4. The ratio of O/Hf is sensitive to the O2/Ar gas flow ratio. It can be observed that the O/Hf ratio is lower than the stoichiometric ratio of HfO2 when the O2/Ar gas flow ratio is 50/150, indicating that the oxygen vacancy defects exist in the film because of the obvious sub-stoichiometry. The O/Hf atomic ratio of the film increases to 2.16 when the sputtering power is 400 W and the O2/Ar gas flow ratio is 100/100. The oxygen-enriched condition provides an excessive oxygen ions for the deposition, so a large number of unreacted oxygen ions enter into the film and are covered by newly generated HfO2 particles on the surface before moving so as to eventually form oxygen interstitial defects [30]. The O/Hf atomic ratio at the sputtering power of 400 W is greater than that of 200 W. The higher power provides more energy to the deposited particles to induce crystallization and reduce oxygen vacancy defects [20]. Moreover, the proportion of Hf 4f7/2/ Hf 4f5/2 also varies. As shown in Table 4, the ratios of Hf 4f7/2 to Hf 4f5/2 for Samples A1, C1 and C3 are 1.68, 1.70 and 1.89, respectively. The results show that the Hf 4f7/2 gradually dominates and the O/Hf ratio is proportional to Hf 4f7/2/ Hf 4f5/2. The change of Hf 4f7/2/ Hf 4f5/2 indicates that the change of electronic structure of the Hf–O bonds [35]. This may be due to the formation of more stable electronic structure of HfO2 thin films with the increasing sputtering power and O2/Ar gas flow ratio.
3.4 Optical properties
Figure 8 presents the transmittance and absorbance spectra of all HfO2 thin films at 200–800 nm. The average transmittance of the fused silica substrate at 400–800 nm is 92%, while the average transmittance of all HfO2 thin films in this wavelength range is higher than 92%. The sharp drop in transmittance below 240 nm is attributed to the substantial absorption of light associated with optical transitions in the band gap of HfO2 [36]. As shown in Fig. 8a, the peak transmittance of the film is close to 99%. Correspondingly, the absorbance of all HfO2 thin films samples is very low, especially in the wavelength range of 300–800 nm. As shown in Fig. 8b, it can be seen that the optical absorption edge appears near 220 nm. The enlarged image shows that the slope of the curve changes with the deposition parameters, which can be used to calculate the optical band gap of the HfO2 thin films. In addition, the oscillation in transmittance spectra from 80 to 99% is attributed to the interference effect of the HfO2 thin films resulted from the superposition of reflected beam from both sides of the film. There will be more interference formed in the transmittance spectrum for higher optical thickness and vice versa, and the optical thickness is proportional to the physical thickness. The number of peaks increases with the increase of sputtering power, indicating that the thickness of films has increased, which is consistent with the results by SEM. The peak intensity of transmittance is caused by defects in the film, especially oxygen vacancy defects that can lead to an increase of light absorption and decrease of transmittance [37]. The maximum transmittance increases with the increase of O2/Ar gas flow ratio because of the optimized stoichiometry of the HfO2 thin film. In addition, the good crystallinity and surface morphology reduce the scattering loss of the film [26].
The optical constants of thin films are crucial for the design and fabrication of optical and laser devices. According to classical electrodynamics, the refractive index and extinction coefficient of metal oxide films follow the Cauchy dispersion relationship [22, 38, 39]. Therefore, the refractive index (n) and extinction coefficient (k) can be calculated using the equations:
where Tmax and Tmin is the maximum and minimum transmission at the same wavelength, n1 and n0 represent the refractive indices of the substrate and air, respectively. The calculated results are shown in Fig. 9a and b. The refractive index and extinction coefficient decrease with the increase of wavelength. The refractive index of the HfO2 thin films at 515 nm varies from 1.79 to 2.05 with different sputtering powers and O2/Ar gas flow ratios. These results are well in agreement with the calculated values in the Refs. [13, 39, 40]. The extinction coefficient is nearly 0.005 in the wavelength range of 300–1200 nm for all the films. The refractive index of the HfO2 thin film is related to its mass density and roughness. The low refractive index corresponds to the high roughness and loose structure, while the high refractive index corresponds to the smooth surface and dense structure [13]. Optical thin film is simply a mixture of the pure dielectric material and the pores, and the porosity (P) and packing density (PD) are the indirect indicators of film densification. Consequently, the porosity and packing density were calculated using following formulas [14, 28, 41]:
where nf is the measured refractive index of HfO2 thin films; nb is the refractive index of the bulk material. The value of refractive index for bulk HfO2 with monoclinic phase is 2.12 [41]. As shown in Fig. 9c, the packing density increases with the increasing sputtering power, indicating that HfO2 thin films deposited with a highest sputtering power present the most closely packed structure [14]. On the contrary, the porosity decreases with the increasing sputtering power. Higher sputtering power can reduce structural defects in the thin film to obtain dense structure [14]. As the O2/Ar gas flow ratio increases, the unreacted oxygen leads to void defects in the film, which increases the porosity and reduces the refractive index. In addition, the packing density has an opposite trend to the interplanar spacing calculated by XRD, as shown in Fig. 9d. This result indicates that the thin films with smaller interplanar spacing have a denser structure.
It is observed from Fig. 8b that the absorption edge changes with different deposition parameters. In order to illustrate this difference more clearly, the optical band gap of HfO2 thin films was calculated using the following formula [21, 22, 42]:
where Eg is the optical band gap; B is a constant; α is the absorption coefficient; T and d is the transmittance and thickness of the film, respectively. The exponent n equals 2 because the HfO2 thin films follow the rule of indirect allowed transition [39], and it is related to the inter-band electronic transition from the O 2p to the Hf 5d between Γ and B points on the reciprocal lattice of monoclinic HfO2 [43, 44]. Figure 10 exhibits the variation of band gap with the deposition parameters. The optical band gap of all HfO2 thin films is between 5.17 and 5.46 eV. The optical band gap increases monotonically with the increase of sputtering power, while it first increases and then decreases with the increase of O2/Ar gas flow ratio. Many factors can influence the optical band gap, including crystalline size, packing density, defects density and stoichiometric ratio [43]. From the results of XRD and XPS, it is observed that sputtering power has a positive effect on the crystallinity and stoichiometric ratio of the HfO2 thin films, so the optical band gap increases with the increase of sputtering. As the number of oxygen vacancy defects decreases, the vacancy-related defect state becomes less delocalized and the valence band non-localization is reduced, resulting in a broadening of the band gap [45]. A large number of oxygen vacancy defects corresponds to smaller Eg and vice versa. Therefore, the increased Eg with O2/Ar gas flow ratio can be attributed to the decreased oxygen vacancy defects in deposited films [45]. However, the unreacted O2 will form oxygen interstitial defects in the film when the O2/Ar gas flow ratio is too large, which will instead reduce the optical band gap of the film, and the result is consistent with the XPS analysis. Therefore, the HfO2 thin films with the flow ratio of 66/132 present the largest optical band gap.
In addition, some researchers believe that the quantum size effect results in a change in Eg particularly if the crystalline size is less than 30 nm. As the nanoparticle size decreases, the electronic energy levels near the Fermi level change from quasi-continuous to discrete, leading to energy level splitting or band gap broadening [46]. The excitonic Bohr radius of HfO2 is between 13 and 2.2 nm [13]. Therefore, the quantum size effect can play a significantly role when the crystalline size of HfO2 is less than 30 nm [13, 18, 32, 47]. As shown in Table 5, the average optical band gap is inversely proportional to the average crystalline size, consistent with the quantum size effect. In Refs. [26, 48], HfO2 nanoparticles with a size of 15–37 nm were prepared with a corresponding optical band gap of 4.5–5.2 eV, which is consistent with this work.
3.5 Defects analysis
One of the major approaches to study crystal defects is to analyze the Urbach tail characteristics [49]. The change of Urbach energy is consistent with the change of defect density of the thin film, so the energy can reflect lattice defects [50]. In the exponential-edge region, the absorption coefficient is expressed by the so called Urbach equations [49, 51]:
where α is the absorption coefficient like Eq. (12); α0 is a constant and Eu is the Urbach energy which characterizes of the exponential-edge region. According to the equations, the plot of photon energy hν vs. ln α and Urbach energy Eu for deposition films are shown in Fig. 11a and b, respectively. As can be seen from the Fig. 11b, Eu decreases from 0.75 to 0.45 eV with the increasing sputtering power and O2/Ar gas flow ratio, indicating the decrease of defect size and density. The types of defects in HfO2 thin films include intrinsic defects and external defects [16, 52]. The intrinsic defects include point defects such as oxygen vacancy defects, hafnium vacancy and oxygen interstitial defects, and structural defects such as dislocations and interfacial overlaps. The external defects are mainly impurities. Therefore, we believe that oxygen-enriched conditions can reduce the oxygen vacancy defects in the HfO2 thin film, while higher sputtering power can reduce the structural defects of the film. Micro strain in thin films is caused by atoms difference, impurities, voids, and oxygen vacancy defects in thin films [16]. Figure 11c shows the relationship between Urbach energy and the absolute value of micro strain. The results indicate that the number of defects in the HfO2 thin film decreases under high sputtering power and large O2/Ar gas flow ratio, thereby reducing the micro strain and Urbach energy. Figure 11d exhibit the relationship between Urbach energy and optical band gap, showing that the change trend of the Urbach energy is opposite to that of optical band gap. It is obvious that the Urbach energy decreases but the band gap increases due to the disappear of defect energy levels of HfO2 thin films.
Photoluminescence spectra is another critical approach to study material defects [53]. The PL emission spectra of HfO2 thin films were studied with excitation wavelength of 355 nm, as shown in Fig. 12a. The results show that PL peaks occur at 442 nm (2.80 eV), 465 nm (2.67 eV) and 554 nm (2.23 eV), and there is no clear shift for the PL peak positions. It can be seen that the sputtering power and O2/Ar gas flow ratio can affect the intensity of PL peaks. The PL peaks intensity of the HfO2 thin film prepared at 200 W is higher than that of films prepared at 300 W and 400 W. We performed density functional theory (DFT) calculations using the unit cell parameters of cif document to obtain the band structure of defect free monoclinic HfO2, as shown Fig. 12b. The results indicate that PL excitation light (355 nm, 3.49 eV) cannot cause the thin film to produce photoluminescence spectra when there are no defect energy levels. The defects of HfO2 thin films are the main reasons for the enhancement of PL peaks [54]. Because the formation energy of hafnium vacancies is approximately twice that of the oxygen vacancies, the oxygen vacancies and oxygen interstitials are the most common defects [26, 31]. Combining with the stoichiometry results calculated by XPS, we believe that the oxygen vacancy and oxygen interstitial defects should be responsible for the PL spectra measured in the experiment.
Researchers have experimentally proved the presence of oxygen-related defects radiative defects states in HfO2 thin films [48, 55]. The electrons are captured in these oxygen vacancies and the ionized oxygen centers form. In the HfO2 with a monoclinic structure, there are two types of oxygen vacancies occupied by electrons, namely the three-fold (VO3) and four-fold (VO4), as each O atom is surrounded by 3 Hf atoms and 4 Hf atoms, respectively [56, 57]. The valence electron configurations are 5p65d36s1 for Hf and 2s22p4 for O, respectively [57]. Under excitation by 355 nm (3.49 eV), the electrons at the valence band are excited into the defect level. The PL peak will be generated when the electrons jump back to the valence band through the radiative recombination emission. The PL peaks located at 442 nm and 465 nm are attributed to the transitions from the energy levels of VO3 and VO4 to the valence band, while the peak at 554 nm is attributed to the transitions from oxygen interstitial defects to the valence band, respectively. [47]. Figure 13 presents the schematic diagram of PL peaks appeared in the HfO2 thin films. The overall low PL intensity in these samples may be due to the high compressive strain and high densification of the films prepared by magnetron sputtering [54].
3.6 LIDT
The surface damage morphologies of HfO2 thin films at different laser fluences are shown in Fig. 14 in a sequence that illustrates the evolution of femtosecond laser induced damage. Images (a) and (b) present the laser damage evolution with the irradiation time at 1 s and 5 s, respectively. In general, all films exhibit nano-bumps and damage craters [58]. Some inhomogeneous nano-bumps are formed on surface of the film in the initial stage, which are usually generated at the positions where the electric field is strongest in the film [4, 7]. Subsequently, the obvious black damage craters appeared on the surface of the film.
To further investigate the detailed morphologies of the damage craters, three typical samples (A1, A3, and C1) with representative damage craters were selected for SEM analysis, and the results are shown in Fig. 15. It can be seen that all damage craters exhibit similar morphology, i.e., ablation and melting. After the laser energy is deposited in the film, the film material will be ablated and peeled off to leave a damage crater once the critical laser energy value is reached. In addition, the damage crater diameter increases rapidly with the increasing laser fluence, from smaller than to larger than the laser spot diameter (3.6 μm). This is because when the laser fluence is low, only the laser energy near the center of the laser beam can damage the film since the laser beam is near-Gaussian distribution. However, when the laser fluence is high enough, the entire laser beam can damage the film and transfer energy to the surrounding film material leading to larger damage craters [59]. According to the avalanche ionization theory, the femtosecond laser pulses can deliver energy to the laser-generated free carries by inverse bremsstrahlung (electron-photon-phonon interaction) that results in rapid thermalization of the free electrons and promotes them to the higher energy band. The high energy free electrons become capable of transferring enough energy to the valence band electrons by electron–electron collisions to promote the valence electrons to the conduction band [3, 60].
The LIDT is defined as the average value of the highest fluence that no damage detected and the critical fluence that damage begins to occur by the optical microscope. Figure 16 shows the LIDTs of the HfO2 thin films, and the values are from 0.6 to 0.9 J/cm2. Because the S-on-1 test at a higher number of laser pulses can bring out the fatigue effects, the LIDT decreases with the increasing irradiation time [6]. From Fig. 16a and b, it is obvious that the LIDT increases with the increasing sputtering power and O2/Ar gas flow ratio. Some studies have shown that under femtosecond irradiation, the LIDT is majorly governed by the electronic excitation and electric field intensity in films while the defects play a negligible role [6, 61]. However, our experiment results have shown a strong correlation between LIDT and the point defects. We calculated the relationship between LIDT and Urbach energy, as shown in Fig. 16c. The results indicate that the LIDT of HfO2 film is inversely proportional to the Urbach energy. This indicates that the higher point defect density will be responsible for the low LIDT under high repetition rate femtosecond laser irradiation. This is because the point defects such as oxygen vacancies and oxygen interstitials can increase the energy absorption of laser by the film during the femtosecond laser irradiation to accelerate the rate of avalanche ionization [3, 4, 9]. The diameters of damage crater ranges from 9 to 21 μm (Fluence is 0.96 J/cm2, irradiation time = 1 s), as shown in Fig. 16d. Because higher sputtering power can reduce the size and number of point defects, the damage size decreases with the increasing sputtering power. In contrast, the influence of O2/Ar gas flow ratio on damage size is relatively small.
4 Conclusion
In this work, all HfO2 thin films are deposited by magnetron sputtering, and the effects of deposition parameters on microstructure, chemical composition, optical properties and LIDTs of HfO2 thin films are investigated. As the sputtering power increases, the crystallinity, deposition rate, refractive index, optical band gap and LIDTs of films increase monotonically while the Urbach energy and PL intensity decrease monotonically. Therefore, we believe that the large sputtering power has a positive effect on improvement of the optical properties of thin films. In addition, the refractive index and Urbach energy decreases with the increasing O2/Ar gas flow ratio, whereas the optical band gap first increases and then decreases with the increasing O2/Ar gas flow ratio. The different O2/Ar gas flow ratios lead to the formation of different point defects, i.e., oxygen vacancies or oxygen interstitials. Therefore, the proper O2/Ar gas flow ratio is important for the preparation of high quality optical thin films. The irradiation damage results of the HfO2 thin films at high repetition rate femtosecond laser indicate that the LIDT is limited by the point defects and fatigue effect. In summary, the optimized deposition parameters are crucial to reduce the point defects in optical thin films to further improve their optical properties.
Data availability
Data underlying the results presented in this paper is not publicly available at this time but may be obtained from the authors upon reasonable request.
References
V. Malka, S. Fritzler, E. Lefebvre et al., Electron acceleration by a wake field forced by an intense ultrashort laser pulse. Science 298(5598), 1596–1600 (2002)
B.A. Remington, D. Arnett, R.P. Drake et al., Experimental astrophysics—modeling astrophysical phenomena in the laboratory with intense lasers. Science 284(5419), 1488–1493 (1999)
S.M. Zhang, C. Menoni, V. Gruzdev et al., Ultrafast laser material damage simulation—a new look at an old problem. Nanomaterials 12(8), 1259 (2022)
P.K. Velpula, M. Durak, D. Kramer et al., Evolution of femtosecond laser damage in a hafnia-silica multi-layer dielectric coating. Opt. Lett. 44(21), 5342–5345 (2019)
D.A. Alessi, H.T. Nguyen, J.A. Britten et al., Low-dispersion low-loss dielectric gratings for efficient ultrafast laser pulse compression at high average powers. Opt. Laser Technol. 117, 239–243 (2019)
P.K. Velpula, D. Kramer, B. Rus, Femtosecond laser-induced damage characterization of multilayer dielectric coatings. Coatings 10(6), 603 (2020)
B. Ma, J.Q. Han, J. Li et al., Damage characteristics of dual-band high reflectors affected by nodule defects in the femtosecond regime. Chin. Opt. Lett. 19(8), 081403 (2021)
Q.Y. Feng, X.T. Zu, B.Y. Wang et al., First-principles study of metallic impurities induced 355 nm UV laser absorption in fused silica. J. Mate. Res. Technol. JMR&T 21, 2906–2914 (2022)
B. Mangote, L. Gallais, M. Commandre et al., Femtosecond laser damage resistance of oxide and mixture oxide optical coatings. Opt. Lett. 37(9), 1478–1480 (2012)
D.B. Douti, T. Begou, F. Lemarchand et al., Analysis of laser energy deposition leading to damage and ablation of HfO2 and Nb2O5 single layers submitted to 500 fs pulses at 1030 and 343 nm. Appl. Phys. A Mater. Sci. Process. 122(7), 653 (2016)
J.X. Gu, H. Wei, F.F. Ren et al., Fabrication and performances of double-sided HfO2 anti-reflection films with ultra-high infrared transmittance. J. Alloy. Compd. 858, 158337 (2021)
C. Yin, M. Zhu, T. Zeng et al., HfO2/SiO2 anti-reflection films for UV lasers via plasma-enhanced atomic layer deposition. J. Alloy. Compd. 859, 157875 (2021)
A. Zahoor, C. Xu, T. Shahid et al., Effects of O2 flux on structure, optical properties and hydrophobicity of highly emissive antireflective HfO2 thin films by magnetron sputtering. Vacuum 197, 110824 (2022)
M. Mazur, T. Howind, D. Gibson et al., Modification of various properties of HfO2 thin films obtained by changing magnetron sputtering conditions. Surf. Coat. Technol. 320, 426–431 (2017)
J. Liu, X.L. Ling, X.F. Liu, Mechanism of annealing effect on damage threshold enhancement of HfO2 films in vacuum. Vacuum 189, 110266 (2021)
V. Beladiya, T. Faraz, P. Schmitt et al., Plasma-Enhanced atomic layer deposition of HfO2 with substrate biasing: thin films for high-reflective mirrors. ACS Appl. Mater. Interfaces 14(12), 14677–14692 (2022)
S. Jena, R.B. Tokas, J.S. Misal et al., Effect of O2/Ar gas flow ratio on the optical properties and mechanical stress of sputtered HfO2 thin films. Thin Solid Films 592, 135–142 (2015)
W.T. Liu, Z.T. Liu, F. Yan et al., Influence of O2/Ar flow ratio on the structure and optical properties of sputtered hafnium dioxide thin films. Surf. Coat. Technol. 205(7), 2120–2125 (2010)
X.H. Luo, W. Zhou, S.V. Ushakov et al., Monoclinic to tetragonal transformations in hafnia and zirconia: a combined calorimetric and density functional study. Phys. Rev. B 80(13), 134119 (2009)
B. Deng, G. He, J.G. Lv et al., Modulation of the structural and optical properties of sputtering-derived HfO2 films by deposition power. Opt. Mater. 37, 245–250 (2014)
W. Wu, Y.L. Tang, B. Li et al., Microstructure and optical properties of PbSe nanocrystalline films prepared by magnetron sputtering. Opt. Mater. 118, 111233 (2021)
V. Dave, P. Dubey, H.O. Gupta et al., Effect of sputtering gas on structural, optical and hydrophobic properties of DC-sputtered hafnium oxide thin films. Surf. Coat. Technol. 232, 425–431 (2013)
P.B. Nair, V.B. Justinvictor, G.P. Daniel et al., Effect of RF power and sputtering pressure on the structural and optical properties of TiO2 thin films prepared by RF magnetron sputtering. Appl. Surf. Sci. 257(24), 10869–10875 (2011)
D.Q. Zhao, F. Xu, G.G. Wang et al., SiO2/HfO2 laser film with enhanced protection and antireflection for sapphire infrared windows at high temperatures. ACS Appl. Electron. Mater. 3(10), 4611–4617 (2021)
M. Falmbigl, K. Godin, J. George et al., Effect of annealing on properties and performance of HfO2/SiO2 optical coatings for UV-applications. Opt. Express 30(8), 12326–12336 (2022)
Q.Q. Du, W.J. Wang, S.H. Li et al., Effects of substrate temperature on the structural, optical and resistive switching properties of HfO2 films. Thin Solid Films 608, 21–25 (2016)
B. Aguirre, R.S. Vemuri, D. Zubia et al., Growth, microstructure and electrical properties of sputter-deposited hafnium oxide (HfO2) thin films grown using a HfO2 ceramic target. Appl. Surf. Sci. 257(6), 2197–2202 (2011)
M. Mazur, A. Poniedzialek, D. Kaczmarek et al., Investigation of various properties of HfO2–TiO2 thin film composites deposited by multi-magnetron sputtering system. Appl. Surf. Sci. 421, 170–178 (2017)
A.S. Gorzalski, C. Donley, O. Coronell, Elemental composition of membrane foulant layers using EDS, XPS, and RBS. J. Membr. Sci. 522, 31–44 (2017)
B. Choi, H.U. Kim, N. Jeon, Uniformity of HfO2 thin films prepared on trench structures via plasma-enhanced atomic layer deposition. Nanomaterials 13(1), 161 (2023)
S.P. Swathi, S. Angappane, Enhanced resistive switching performance of hafnium oxide-based devices: effects of growth and annealing temperatures. J. Alloy. Compd. 913, 165251 (2022)
Y.J. Wang, Z.L. Lin, X.L. Cheng et al., Study of HfO2 thin films prepared by electron beam evaporation. Appl. Surf. Sci. 228(1–4), 93–99 (2004)
X.J. Li, Y.W. Li, G. Sun et al., Synthesis of a flower-like g-C3N4/ZnO hierarchical structure with improved CH4 sensing properties. Nanomaterials 9(5), 724 (2019)
K.S. Pasupuleti, M. Reddeppa, S.S. Chougule et al., High performance langasite based SAW NO2 gas sensor using 2D g-C3N4@TiO2 hybrid nanocomposite. J. Hazard. Mater. 427, 128174 (2022)
S.J. Kang, Y. Yi, C.Y. Kim et al., Chemical reaction at the interface between pentacene and HfO2. Phys. Rev. B 72(20), 205328 (2005)
F. Rubio, Optical properties of reactively sputtered Ta2O5 films. Jvacscitechnol 21(4), 1043–1045 (1982)
Z.B. He, W.D. Wu, H. Xu et al., The effects of O2/Ar ratio on the structure and properties of hafnium dioxide (HfO2) films. Vacuum 81(3), 211–214 (2006)
J.C. Manifacier, J. Gasiot, J.P. Fillard, A simple method for the determination of the optical constants n, k and the thickness of a weakly absorbing thin film. J. Phys. E Sci. Instrum. 9, 1002–1004 (1976)
M. Vargas, N.R. Murphy, C.V. Ramana, Structure and optical properties of nanocrystalline hafnium oxide thin films. Opt. Mater. 37, 621–628 (2014)
M.F. Al-Kuhaili, S.M.A. Durrani, I.A. Bakhtiari et al., Influence of hydrogen annealing on the properties of hafnium oxide thin films. Mater. Chem. Phys. 126(3), 515–523 (2011)
S. Kermadi, N. Agoudjil, S. Sali et al., Microstructure and optical dispersion characterization of nanocomposite sol-gel TiO2-SiO2 thin films with different compositions. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 145, 145–154 (2015)
S. Pokhriyal, S. Biswas, R. Prajapati, Structural, optical, and electrical properties of e-beam deposited metamaterials of granular CdSe thin films on glass substrates with a thin buffer layer of HfO2 dielectric. Mater. Chem. Phys. 294, 126950 (2023)
M.C. Cisneros-Morales, C.R. Aita, The effect of nanocrystallite size in monoclinic HfO2 films on lattice expansion and near-edge optical absorption. Appl. Phys. Lett. 96(19), 191904 (2010)
J.W. Park, D.K. Lee, D. Lim et al., Optical properties of thermally annealed hafnium oxide and their correlation with structural change. J. Appl. Phys. 104(3), 033521 (2008)
J. Gao, G. He, B. Deng et al., Microstructure, wettability, optical and electrical properties of HfO2 thin films: effect of oxygen partial pressure. J. Alloy. Compd. 662, 339–347 (2016)
A. Campos, N. Troc, E. Cottancin et al., Plasmonic quantum size effects in silver nanoparticles are dominated by interfaces and local environments. Nat. Phys. 15(3), 275–280 (2019)
Y. Sebti, T. Chauveau, M. Chalal et al., Assessment of the morphological, optical, and photoluminescence properties of HfO2 nanoparticles synthesized by a sol–gel method assisted by microwave irradiation. Inorg. Chem. 61(17), 6508–6518 (2022)
R. Kumar, V. Chauhan, N. Koratkar et al., Influence of high energy ion irradiation on structural, morphological and optical properties of high-k dielectric hafnium oxide (HfO2) thin films by atomic layer deposition. J. Alloy. Compd. 831, 154698 (2020)
F. Urbach, The long-wavelength edge of photographic sensitivity and of the electronic absorption of solids. Phys. Rev. 92(5), 1324–1324 (1953)
J. Xie, B. Li, Y.J. Li et al., Study of ZnS thin films prepared by RF magnetron sputtering technique. Acta Phys. Sin. 59(8), 5749–5754 (2010)
A. Hakeem, M. Ramzan, E. Ahmed et al., Effects of vacuum annealing on surface and optical constants of hafnium oxide thin films. Mater. Sci. Semicond. Process. 30, 98–103 (2015)
Y. Zhao, W. Gao, J. Shao et al., Roles of absorbing defects and structural defects in multilayer under single-shot and multi-shot laser radiation. Appl. Surf. Sci. 227, 275–281 (2004)
X. Xiang, X.T. Zu, S. Zhu et al., Optical properties of metallic nanoparticles in Ni-ion-implanted alpha-Al2O3 single crystals. Appl. Phys. Lett. 84(1), 52–54 (2004)
S. Papernov, M.D. Brunsman, J.B. Oliver et al., Optical properties of oxygen vacancies in HfO2 thin films studied by absorption and luminescence spectroscopy. Opt. Express 26(13), 17608–17623 (2018)
T.V. Perevalov, V.S. Aliev, V.A. Gritsenko et al., Electronic structure of oxygen vacancies in hafnium oxide. Microelectron. Eng. 109, 21–23 (2013)
A.S. Foster, F.L. Gejo, A.L. Shluger et al., Vacancy and interstitial defects in hafnia. Phys. Rev. B 65(17), 174117 (2002)
L.G. Wang, H.L. Tu, Y.H. Xiong et al., Formation of the dopant-oxygen vacancy complexes and its influence on the photoluminescence emissions in Gd-doped HfO2. J. Appl. Phys. 116(12), 123505 (2014)
T. Noah, A. Abdallah, T. Michael et al., Comparison of damage and ablation dynamics of multilayer dielectric films initiated by few-cycle pulses versus longer femtosecond pulses. Opt. Lett.. Lett. 45(9), 2672–2675 (2020)
X.B. Cheng, Z.X. Shen, H.F. Jiao et al., Laser damage study of nodules in electron-beam-evaporated HfO2/SiO2 high reflectors. Appl. Opt. 50(9), C357 (2011)
H.X. Deng, X.T. Zu, X. Xiang et al., Quantum theory for cold avalanche ionization in solids. Phys. Rev. Lett. 105(11), 113603 (2010)
J. Jasapara, A.V.V. Nampoothiri, W. Rudolph et al., Femtosecond laser pulse induced breakdown in dielectric thin films. Phys. Rev. B 63(4), 045117 (2001)
Acknowledgements
This work was supported by the Key Project of National Natural Science Foundation of China-China Academy of Engineering Physics joint Foundation (NSAF, Grant No. U1830204) and the National Natural Science Foundation of China (Grant No. 12105037).
Author information
Authors and Affiliations
Contributions
YW: Samples preparation and characterization, analysis, writing original draft. XX: Conceptualization, methodology, discussion, English, superivision. JiY: Discussion. LY: Experiment, discussion. WW: Experiment, discussion. XW: Experiment. MT: Experiment. BL: Funding acquisition. XZ: Funding acquisition.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wu, Y., Yu, J., Yang, L. et al. Modulation of microstructure and optical properties of anti-reflection HfO2 films by sputtering power and O2/Ar flow ratio. Appl. Phys. B 129, 168 (2023). https://doi.org/10.1007/s00340-023-08118-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00340-023-08118-4