Abstract
The relationship between sample temperature and the plasma spectrum is of great significance to the water quality monitoring using the laser-induced breakdown spectroscopy (LIBS). In this work, we study the effect of temperature variation on the plasma spectrum in CaCl2 solution for on-line analysis using the LIBS. The effects of sample solution temperature from 25 to 80 °C on the emission spectral intensity, the signal-to-background ratio (SBR) and quantitative analysis of the laser-induced plasma are studied. When the temperature rises from 25 to 70 °C, the emission spectral intensity and the SBR of the laser plasma increase or get enhanced with the increase of temperature. However, when the temperature further increases from 70 to 80 °C, the spectral intensity and the SBR begin to decrease. The enhancement rate reaches the maximum at 70 °C. In quantitative analysis, the fitting curve and the root mean square errors (RMSE) increase with the increase of the sample temperature. The relevant parameters peak at 70 °C. At this optimal temperature, the correlation coefficients of Ca II 393.366 and Ca II 396.847 nm are found to be 0.9988 and 0.9953, respectively, with the RMSE decreased to 0.16 and 0.27%, respectively, and the lowest detection limits are 27.99 and 28.10 μg ml−1, respectively. This study demonstrates that the preheating to the samples can effectively improve the LIBS ability for on-line analysis and detection of water quality.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
With the aggravation of water quality calcification, the health problem of drinking water has become a priority issue. The main causes of water quality calcification are the infiltration of untreated industrial and domestic sewage and excessive exploitation of water sources, resulting in a series of hydrogeochemical effects. These factors promote the transfer of calcium-soluble salts into water sources and increase the content and hardness of calcium ions in water. Long-term consumption of high-hardness water can cause cardiovascular, neurological, urinary hematopoiesis and other systemic diseases [1, 2]. Therefore, detecting the water hardness has become a hot topic of research in the area of environmental protection.
At present, the determination methods of water hardness include the EDTA complex metric titration [3, 4], the chlorophosphonazo photometry and the 4-methylazo arsenazo III photometry [5]. However, these methods were tedious for the determination of calcium and magnesium ions in water samples and not suitable for quantitative analysis of water hardness. The laser-induced breakdown spectroscopy (LIBS) is a spectroscopic method that uses intense laser as a light source to induce plasma to analyze the element compositions and contents in the samples. It has many advantages, such as no need for pretreatment of samples, short analysis time, multi-element analysis, no secondary pollution and no restriction on sample morphology. It has been widely used in the detection of samples in solid [6,7,8,9,10,11,12,13,14], liquid [15] and gas states [16]. Therefore, more researchers are using the LIBS technique to monitor water quality. Katarína Skočovská [17] designed a special nozzle to control the liquid jet. The optimum signal-to-noise ratio (SNR) and the relative standard deviation (RSD) are obtained by optimizing the laser pulse energy, the lens-to-sample distance, the gate delay time and the gate width. The RSD obtained from the sample source controlled by a special nozzle decreases from 200 to 30% compared with the natural jet. Samek et al. [18] designed a liquid jet system to improve the limit of detection (LOD). The LOD for Li and U elements are lowered to 0.009 and 450 ppm, respectively. Yaroshchyk et al. [19] compared the sensitivities of LIBS analyzation of a static liquid surface with laminar liquid jets. The result shows that the LOD in liquid jets is four times lower in average. The LODs of different elements are estimated, which is 0.4 ppm for Mg and Ca, and 47 ppm for Ni. It has proved the ability of LIBS to measure trace elements in water, in which moving liquid measurement is better than static. H Sobral et al. [20] compared the spectral information between the aqueous solution state and the ice state under the same experimental conditions It is found that the average detection result of ice is 6 times lower than that of liquid. At the same time, many researchers who have ever worked on the LIBS water quality analysis are now studying other alternative water quality analysis and accuracy improvement methods [21,22,23,24]. However, the studies described above mainly focused on the improvement of liquid analysis method and the detection accuracy at room temperature, which did not involve the change of the spectral line caused by the temperature change at higher temperature and near the boiling point.
In this study, we investigate the effect of sample temperature on the spectral intensity and detection sensitivity of LIBS by heating CaCl2 solution. The spectral intensity and the calibration curve fitting coefficient of the CaCl2 solution from 25 to 80 °C are compared comprehensively with the increase of temperature. Both the qualitative and quantitative analyses for the liquid elements at different temperatures are also performed, and the LODs are calculated.
2 Experimental setup and materials
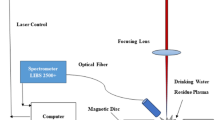
A schematic diagram of the experimental setup used in this work is shown in Fig. 1. The system is composed of a Nd: YAG laser (Surelite III 10, USA Continuum, pulse width 8 ns), a digital delay generator DG645, an Avantes fiber spectrometer (Avaspec-USB2), a fiber optic probe, a reflector, a Glan prism, a focusing lens, a peristaltic pump (Kamoer Lab UIP), a heating controller (TC200-EC), a separatory funnel, and hoses, nozzles, beakers and so on. The laser beam with a wavelength of 1064 nm was emitted through a reflector. The laser beam passes through an attenuation system consisting of a Glan prism and a half wave plate. The laser then focuses on a spot of 2 mm beneath the liquid surface by a 150-mm quartz lens. The plasma emission spectra were obtained using a focusing lens (with a focal length of 75 mm) placed at a 45° angle to the incident direction. The collected signal was coupled to the Avantes spectrometer via the fiber optic probe.
In this experiment, the energy of the laser pulse was adjusted by the attenuation system to control the induced plasma. The working sequence of each unit of the equipment, including the spectrometer, was controlled by the digital delay generator DG645 initially triggered by the Q-switch signal of the laser. The Nd: YAG laser was operated at 120 mJ of pulse energy and 10 Hz of repetition rate. The integration time of the spectrometer is 5 ms. The delay time of the spectral detection is 1.3 μs. The experiment was conducted in air environment under one atmospheric pressure and at a temperature of 22 °C and relative humidity of 40%.
The liquid jet device was powered by a peristaltic pump whose marching frequency was synchronized with the laser pulse. The CaCl2 solution was placed in a beaker. The liquid jet was refluxed into the dividing funnel through the hose and shot out by the nozzle (diameter of 1 mm). The velocity of the liquid jet was controlled at 65 ml/min. The aqueous solution analyzed in this work was Calcium (II) chloride, which was prepared from 99.7% (analytical pure AR) purity salts by the Beijing Chemical Plant. Eight different CaCl2 solutions (numbered as 1#, 2#, 3#, 4#, 5#, 6#, 7# and 8#) were used in the study. The concentrations of Ca (II) in the samples are shown in Table 1. The solution was heated by the thermistor HT10K and temperature controlled in real time by the TC200-EC heating controller via the temperature sensor inserted into the solution.
3 Results and discussion
3.1 Analytical lines selection
As shown in Fig. 2, the concentration of the CaCl2 solution in the beaker is 3000 μg ml−1, the solution was heated to 60 °C through the TC200-EC heating controller. Compared with that at 22 °C, the spectral intensity at 60 °C was significantly higher. However, the background noise at 60 °C was also higher than that at 22 °C. The difference of the element concentration at different temperatures could be reflected by the spectral intensity. Taking the spectral intensity and the nearby interference into account, the two analytical lines of Ca II 393.366 and Ca II 396.847 nm in the CaCl2 solution sample were screened out for measurement. The effect of the sample temperature on the two analytical lines in the CaCl2 solution were investigated.
The peak intensity reflects the content of the corresponding elements in the LIBS. The interference affects the peak intensity, thus influencing the ultimate quantitative analysis of the elements. As shown in Fig. 2, the collected LIBS spectral signal has large background noise interference, which seriously affects the accurate extraction of characteristic spectral lines and thus the accuracy of the detection results. Therefore, it is necessary to deduct the background spectrum to realize the baseline correction to improve the quantitative analysis results.
3.2 Algorithm description
There are a large number of mixed local minima in the actually measured spectra, and not all the minima are useful as background information, such as the noise signals. Therefore, a minimum baseline automatic background calibration algorithm is proposed in this paper. The LIBS spectrum can be defined as,
where the parameter \({\text{f}}\left( {\text{x}} \right)\) represents the spectrum collected by the spectrometer, the parameter \({\text{g}}\left( {\text{x}} \right)\) stands for the spectrum baseline and background values, and the parameter \({\text{Y}}\left( {\text{x}} \right)\) represents the series of characteristic spectral peaks; the series of spectral characteristic peaks are obtained by subtracting the baseline from the spectrum. In this experiment, the baseline deduction algorithm is used to effectively remove the influence of the background noise on the spectral data analysis and improve the accuracy of the LIBS quantitative analysis. The corrected results are shown in Fig. 3, and it can be seen that the algorithm has effectively removed the background noise.
3.3 Relationship between spectral line intensity and sample temperature
In the experiment, the sample was heated to different temperatures from 25 to 80 °C, and eight sets of data were collected at each sampling temperature. The spectral data with the background noise removed were obtained, and each spectral data was obtained by averaging the eight sets of the collected data to obtain the confidence intervals.
where \(\mu\) represents the sample mean, \(\sigma\) the overall standard deviation, n the number of samples, and \(z_{{\frac{\alpha }{2}}}\) is called the Z value (which can be obtained by referring to the standard normal distribution). As shown in Fig. 4, a blue confidence interval with a confidence of 95% is obtained. The spectral intensity data varying with temperature are nonlinearly fitted to obtain the fitted curve (the red fitting curve). The spectra varying with temperature are shown in Fig. 4. Almost all the data processed by the minimum baseline automatic calibration background algorithm are within the blue confidence interval.
At the same time, the plasma temperature and electron density at different sample temperatures of 25, 50, 70 and 80 °C are calculated through the characteristic spectral lines of Ca element to explore the reason of the spectral enhancement effect. We selected five Ca elements to determine the electron temperature. Table 2 lists the spectral line parameters of these five Ca ions. When the laser pulse energy is 120 mJ, the spectrometer integration time is 5 ms and the delay time of spectral detection is 1.3 μs, the plasma temperatures at the solution temperatures of 20, 50, 70 °C and 80 °C were calculated by Boltzmann plane method. The corresponding electron number density is calculated by Stark broadening method through HI 656.2 nm spectral line.
In Fig. 4, it can be found that both the spectral intensities of the Ca element increase with the increase of the sample temperature. When the temperature of the CaCl2 solution increases from 25 to 60 °C, the spectral intensity increases rapidly. The main reason is that the sample surface reflectance decreases as the sample temperature increases [25]. The coupling energy with the laser pulse energy causes more pulse energy to ablate the sample to form the plasma, and higher ablated material evaporation shows stronger emission spectral intensity in the LIBS, resulting in the higher spectral intensity. When the temperature of the CaCl2 solution increases from 60 to 70 °C, the spectral intensity increases slowly. The ablation quality of the CaCl2 solution tends to be saturated. In Table 3, it can be found that the plasma temperature rises from 6627 K at 25 °C to 7986 K at 70 °C. In this process, the electron density is also increased, as well as the corresponding spectral intensity. The plasma temperature and electron density peak at 70 °C, which are 7986 K and 2.0 \(\times\) 1018 cm−3, respectively. When the temperature further increases from 70 to 80 °C, the spectral intensity begins to decrease. The main reason is that the CaCl2 solution starts to generate a large amount of water vapor at this temperature when it flows out through the liquid jet system. And extra laser energy is absorbed when passing through the water vapor. Simultaneously, in the larger plasma volume, the trailing edge of the laser pulse energy is consumed by the water vapor reflection and scattering [26], which results in a decrease in the spectral intensity.
To quantitatively describe the relationship between the spectral intensity and the sample temperature, eight sets of data were collected and averaged for one data point at each sampling temperature. After subtracting the background noise, the emission intensity of the laser plasma is measured and plotted as a function of the sample temperature and the histogram of the signal-to-background ratio (SBR). The variation trend is shown in Fig. 5.
It can be seen from Fig. 5 that both the emission spectral intensity of the laser plasma and the SBR increase with the increase of the sample temperature from 25 to 70 °C, and then tend to decrease from 70 to 80 °C. The enhancement ratios for the spectral lines of Ca II 393.366 and Ca II 396.847 nm are shown in Table 4. The spectral data and the SBR at 25, 50, 70 and 80 °C are listed for comparison.
Combined with Fig. 5 and Table 4, it is found that when the temperature is from 25 to 70 °C, the emission spectral intensity and the SBR of the laser plasma increase and the substrate noise decreases with the increase of temperature. The enhancement ratio reaches the maximum at 70 °C.
3.4 Quantitative analysis
The internal standard method can overcome the effect of spectral fluctuations caused by the experimental conditions to a certain extent. The TC200-EC was used to heat the eight different concentration of samples, as shown in Table 1. Twenty (20) pairs of spectrograms were collected for each sample. The calibration curve for trace elements in the calcium chloride solution is established by an internal standard method as shown in Fig. 6. The X-axis represents the element concentration, and the Y-axis the intensity ratio of the characteristic line and the reference line. The selected spectra for the calibration analysis are Ca II 393.366 and Ca II 396.847 nm. The relevant parameters can be found in Table 5.
According to the calibration curve in Table 5, the curve fitting degree and the root mean square error (RMSE) increase with the increase of temperature. When the temperature reaches the boiling point of 70 °C, the relevant parameters begin to decrease. The RMSE and the detection limit (LOD) decrease with the increase of temperature. When the temperature reaches the boiling point of 70 °C, the relevant parameters begin to rise. When the temperature is higher than the boiling point (70 °C), the related parameters begin to decline. The correlation coefficients of Ca II 393.366 nm and Ca II 396.847 nm increase from 0.9788 and 0.9769 at 25 °C to 0.9988 and 0.9953 at 70 °C, respectively. The RMSE of Ca II 393.366 nm and Ca II 396.847 nm decrease from 0.85 and 1.24% at 25 °C to 0.16 and 0.27% at 70 °C, respectively. The minimum LOD of Ca II 393.366 and Ca II 396.847 nm can reach 27.99 μg ml−1 and 28.10 μg ml−1 at 70 °C, respectively. At 80 °C, the minimum relative standard deviation (RSD) of Ca II 393.366 nm is 0.5880. Comparing the RSD values at different temperatures, it is found that the data are not stable, such problems will be explored in the next step. The RSD of Ca II 396.847 nm decreases from 67.02% at 25 °C to 60.84% at 70 °C and reaches the lowest point. The plasma spectrum collected at 70 °C can thus be applied to the qualitative and quantitative analysis of trace elements in the solution quality monitoring process.
4 Conclusions
In this work, the effect of the temperature of the CaCl2 solution samples on the plasma emission was studied for the purpose of exploring the LIBS ability for water quality quantitative analysis and detection. First, the minimum baseline automatic background correction algorithm was used to deduct the background noise and obtain the spectral data with the background noise removed. Second, the effects of temperature variation of the CaCl2 solution from 25 to 80 °C on the spectral intensity and the SBR were studied. The results show that when the sample temperature increases from 25 to 70 °C, the spectral intensities of Ca II 393.366 and Ca II 396.847 nm increase by 3.07 times and 2.43 times, respectively, and the SBRs increase by 3.05 times and 3.4 times, respectively. And third, the effects of the sample temperatures of 25, 50, 70 and 80 °C on the quantitative analysis of Ca concentration in the sample solution were also studied. The results show that both the fitting curve and the RMSE increase with the increase of the sample temperature. The relevant parameters peak at 70 °C. The correlation coefficients of Ca II 393.366 and Ca II 396.847 nm are 0.9988 and 0.9953, respectively. The RMSEs decrease to 0.16 and 0.27%, respectively. The lowest detection limits of Ca II 393.366 and Ca II 396.847 nm are 27.99 μg ml−1 and 28.10 μg ml−1, respectively. This study shows that it is practical to quantitatively apply the LIBS in the environmental detection and water quality investigation. And when the output power of the pulsed laser in the LIBS is limited, preheating of the sample to a proper temperature just below the boiling point can effectively improve the detection ability.
References
R. Chandrajith, C.B. Dissanayake, T. Ariyarathna, H.M. Herath, J.P. Padmasiri, Sci. Total. Environ. 409(4), 671–675 (2011)
H.M.S. Wasana, D. Aluthpatabendi, W.M.T.D. Kularatne, P. Wijekoon, R. Weerasooriya, J. Bandara, Environ. Geochem. Health. 38(1), 157–168 (2016)
M.C. Yappert, D.B. Donald, J. Chem. Educ. 74(12), 1422 (1997)
M.E. Mahmoud, G.M. El Zokm, A.E.M. Farag, M.S. Abdelwahab, Environ. Sci. Pollut. Res. 24(22), 18218–18228 (2017)
H. Zhang, W. Wu, Anal. Sci. 34(3), 305–310 (2018)
D.A. Cremers, Appl. Spectrosc. 41(4), 572–579 (1987)
M.L. Shah, A.K. Pulhani, G.P. Gupta, B.M. Suri, Appl. Opt. 51(20), 4612–4621 (2012)
A.K. Knight, N.L. Scherbarth, D.A. Cremers, M.J. Ferris, Appl. Spectrosc. 54(3), 331–340 (2000)
B. Sallé, D.A. Cremers, S. Maurice, Spectrochim. Acta, Part B. 60(4), 479–490 (2005)
G. Cristoforetti, S. Legnaioli, V. Palleschi, A. Salvetti, E. Tognoni, Spectrochim. Acta, Part B. 59(12), 1907–1917 (2004)
Z.Q. Hao, C.M. Li, M. Shen, X.Y. Yang, K.H. Li, L.B. Guo, X.Y. Li, Y.F. Lu, X.Y. Zeng, Opt. Express. 23(6), 7795–7801 (2015)
T. Zhang, L. Liang, K. Wang et al., J. Anal. At. Spectrom. 29, 2323–2329 (2014)
F.C. DeLucia, J.L. Gottfried, C.A. Munson et al., Appl. Opt. 47(31), G112–G121 (2008)
A.W. Miziolek, V. Palleschi, I. Schechter. Cambridge University Press, Cambridge (2006). https://doi.org/10.1007/s00216-011-5073-5
A. Kumar, F.Y. Yueh, J.P. Singh, Appl. Opt. 42(30), 6047 (2003)
L.M. Wang, Y.J. Zhang, Y. He, K. You, J.G. Liu, W.Q. Liu, Spectrom. Spectr. Anal. 32(4), 910–914 (2012)
K. Skočovská, J. Novotný, D. Prochazka, P. Pořízka, K. Novotný, J. Kaiser, Rev. Sci. Instrum. 87(4), 233 (2016)
O. Samek, D.C.S. Beddows, J. Kaiser, S.V. Kukhlevsky, M. Liska, H.H. Telle, A.J. Whitehouse, Opt. Eng. 39(8), 2248–2262 (2000)
P. Yaroshchyk, R.J.S. Morrison, D. Body, B.L. Chadwick, Spectrochim. Acta, Part B 60, 986 (2005)
H. Sobral, R. Sanginés, A. Trujillo-Vázquez, Spectrochim. Acta, Part B 78, 62–66 (2012)
A. Pichahchy, D. Cremers, M. Ferris, Spectrochim. Acta, Part B. 52, 25 (1997)
B. Charfi, M.A. Harith, Spectrochim. Acta, Part. B. 57, 1141 (2002)
A. Casavola, A. De Giacomo, M. Dell’Aglio, F. Taccogna, G. Colonna, O. De Pascale, S. Longo, Spectrochim. Acta, Part B. 60, 975 (2005)
R. Knopp, F.J. Scherbaum, J.J. Kim, Fresenius. J. Anal. Chem. 355, 16–20 (1996)
S. Kumar, J. Park, S.H. Nam et al., Plasma Sci. Technol. 22(7), 074009 (2020)
L. Flamigni, J. Koch, D. Gunther, J. Anal. At. Spectrom. 29(2), 280–286 (2014)
Acknowledgements
This paper was supported by National Natural Science Foundation of China (NSFC, No. 51374040), Department of Science and Technology of Jilin Province of China (Grant No. 20200403008SF) and Education Department of Jilin Province of China (Grant No. JJKH20210737KJ).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
There are no conflict to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Lin, J., Yang, J., Gao, X. et al. The effect of solution temperature on the quantitative analysis of laser-induced breakdown spectroscopy. Appl. Phys. B 128, 127 (2022). https://doi.org/10.1007/s00340-022-07843-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00340-022-07843-6