Abstract
In this paper, a fiber optic sensor for adulteration detection in petrol and diesel by kerosene is proposed and demonstrated experimentally. The sensor consists of a multimode fiber with cladding substituted by a stimuli-responsive polydimethylsiloxane (PDMS) layer. The layer of PDMS swells/shrinks to a different extent in response to the level of adulteration, and as a result, the intensity of the output spectra changes. Unlike the existing fiber optic adulteration sensors that can only detect a change in refractive index (RI) due to the concentration change of adulterant, this sensor is responsive to hydrocarbon chain length and provides selective detection. The average sensitivities for petrol and diesel adulteration for PDMS coated fiber probe is 281 counts/% and 134 counts/% respectively, in the kerosene concentration range of 0–50%. This sensor can detect the presence of kerosene contamination up to 1% in petrol and diesel and thus furnish opportunities for the growth of portable fuel adulteration sensors.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Adulteration is an unlicensed or illegal introduction of a foreign substance into a superior quality product that degrades the original commodity’s quality. This foreign substance is known as an adulterant, and the process is called adulteration due to which item fails to hold the actual properties and constitution [1,2,3]. Adulterations in fluids such as fuel, milk, honey, edible oil, etc. are common, widespread malpractice from the past several years, leading to several hazards and immense threats to both environments as well as human health. Petrol and diesel are extensively used automobile fuels for electricity generation and transport globally, being a low boiling mixture of hydrocarbons. Petrol is a petroleum-derived commodity accommodating a composition of aliphatic hydrocarbons and liquid aromatic compounds lying between C4–C12 carbon atoms having a boiling range of 30–225 \(^\circ \mathrm{C}\). Although the hydrocarbons of diesel fuel are similar to petrol, the molecular weight and carbon numbers are higher, where the boiling range lies to 180–370 \(^\circ \mathrm{C}\) and carbon number within C8–C21 [4, 5]. An adulteration process involves the addition of organic solvents, including light aliphatic (C4–C8), heavy aliphatic (C13–C15), and aromatic hydrocarbons. Suppliers intentionally use cheap commodities for adulteration to raise their gains. The adulterant “kerosene (C6–C16)” is usually mixed into petrol and diesel due to its low cost and readily miscible nature. Also, this organic solvent is readily available in the market at a subsidized rate. Since kerosene has overlapping hydrocarbons with petrol and diesel, it has strong potential to be used as an adulterant and support a standard route to earn financial profits. The use of adulterated fuel cause deterioration of the engine parts, reduces the performance of automobiles, and pollutes the environment through the emission of toxic substances. The excessive emission of carbon monoxide, oxides of nitrogen, and hydrocarbons increase the greenhouse gasses in the environment [6,7,8].
As per the existing literature, different analytical techniques are available to monitor the adulteration of automobile fuels, such as chromatography, volumetry, spectroscopy, gravimetry, distillation, vapor pressure, kinematic viscosity, and elemental content [2, 3]. But all these sensing technologies involve old, heavy, complex, expensive, and tedious laboratory methods. Moreover, various approaches were also available in the market to evaluate the fuel qualities, which involve some parameter detections such as density, stability, flash point, odor based method, cetane number, cetane index, ultrasonic technique, polycyclic aromatics, hydrocarbon composition, etc. [4, 9]. The above-mentioned conventional methods have some limitations in terms of their equipment size, sensitivity, stability, analysis time, costs, level of accuracy, rigorousness, and sample amount requirement. Apart from these, some sensors based on optical waveguides were developed for optical sensing of the fuel adulteration level of unauthorized contamination, for example, Infra-red method, Image processing method, etc. [8]. They offer several benefits over the other conventional sensing methodology due to their high sensitivity, small size, easy fabrication process, rapid detection with reasonable accuracy, and safety from flammable liquids. An optical sensor has been designed to monitor the low concentration of kerosene in diesel [10] where laser light was reflected through a liquid fuel layer put on a glass surface, and the sensing principle is based on an RI mismatch between the glass plate and fuel samples. Another planner waveguide-based surface plasmon resonance (SPR) sensor was reported recently [5] to measure the kerosene concentration in automobile fuel by involving an angular interrogation mechanism with a metal-clad coating including sample flow cell; nevertheless, the experimental setup seems complex and costly.
From the last couple of decades, optical fiber-based sensors have become one of the most demanding categories in the sensing market in favor of every chemical, physical, biological, environmental, and industrial sensing applications [11,12,13]. Fiber optic sensors possess numerous advantageous features of high sensitivity, lightweight, minimized cost, good resistance to chemical corrosion and high temperature, immune to electromagnetic (EM) interference, and the capability of remote sensing. Along with that, easy fabrication, fast detection, excellent accuracy, and the possibility of making devices portable and compact for real-time and in-situ measurements are the key factors to use [14,15,16,17]. There are a few reports in the literature for the identification of adulteration in automotive fuels using optical fiber sensors [7, 18, 19]. In 1999, Roy [20] developed a sensor based on an evanescent field-based etched clad fiber optic sensor by measuring the output power in reference to the kerosene amount present in petrol and diesel. Pathak et al. [21] proposed a sensor, using no-core fiber (NCF) as sensing head spliced between two multimode fibers (MMF) for detection of petrol adulteration. However, the bare optical fiber sensors are unsuitable for detecting the adulteration of diesel efficiently as the RI of diesel is close to the material of fiber. There is another report on SPR based fiber optic technique for kerosene detection in the fuel [22]. Although plasmonic-based sensors are highly sensitive due to their surface phenomena, these devices must contain at least a plasmon active materials (e.g., Au, Ag, Cu, etc.) and require a heavy coating machine. While the micron size silica fiber is not an easy task to coat by the thin film uniformly, and that makes it much more expensive and practically unaffordable sometimes. From the above discussion, it can be observed that the reported sensors primarily detect fuel adulteration by measuring the RI variation of the fuel due to addition of adulterants and hence are unable to perform specific or selective detection.
PDMS is an organic polymer belonging to the siloxane family and contains carbon and silicon. It is a thermally curable polymer and shows desirable, innovative intrinsic properties like the ease of fabrication, high dielectric coefficient, oxygen permeability, flexibility, biocompatibility, optical transparency, etc., which makes it attractive [23,24,25]. These outstanding characteristics of PDMS are due to the presence of a methyl group along the Si–O–Si backbone. The surface, structural and optical properties of PDMS have crucial importance which confers applications in chemical, physical, and biomedical fields. Several articles have been published in the literature using PDMS film for sensing various parameters such as chemicals, gasses, temperature, pressure, magnetic fields, load, strain, etc. [13, 26,27,28,29]. In this communication, a fiber optic sensor with its cladding modified with PDMS is proposed and experimentally demonstrated for selective detection of adulteration in fuels. Unlike the reported fiber optic sensors, the detection phenomena of this sensor do not simply depend on the RI variation of the contaminated fuel. Indeed, the sensor response depends on the reversible swelling/shrinkage of the coated PDMS layer in response to different hydrocarbon content. Additionally, due to the PDMS coating, the RI of the contaminated fuel has only a secondary effect on the transmitted light, so the proposed sensor can also detect the adulteration of diesel. The sensor works on an intensity modulation scheme, which is easier to implement and cost-effective. The details of the operating principle and experimentation on the sensor are demonstrated in the successive sections.
2 Working principle
2.1 Refractive loss
In an uncladded fiber optic probe, the light propagation is in direct contact with the external medium; the effective RI of the guided light is affected by the nature of the outer medium [30]. An increase in RI of the medium surrounding core exposed region decreases the local numerical aperture (NA), and hence the attenuation of EM energy occurs due to refraction loss. Therefore, depending on the interaction between light propagating through fiber and sensing medium, a variation in output power of guided light will be observed. There is also the possibility that a fraction of light entering the sensing region cannot follow the TIR condition to be guided but undergoes Fresnel reflections at the interface between glass and the outer medium [17]. The magnitude of such reflections will depend essentially on the RI difference between glass and the surrounding medium. In such a case, the magnitude will diminish with an increase of analyte concentration, and these reflections could also subscribe to the output power.
The power attenuation mechanism due to refractive loss at the output end can be explained using ray optics, as shown in Fig. 1. When a medium of RI \({n}_{2}\) surrounds the uncladded portion, several rays (such as R1 and R2) are guided depending on the corresponding critical angle \({(\theta }_{c})\) (Fig. 1a). Whether the surrounding RI \({n}_{2}\) is replaced by a new medium of RI \({n}_{3}\) (\({n}_{3}>{n}_{2}\)), it led to an increase in critical angle \(({\phi }_{c})\). Consequently, the incidence angle for ray R1 is now smaller than the new critical angle, resulting in the ray R1 getting refracted and eventually lost (Fig. 1b). However, some percentage of the propagating light will be reflected into the fiber depending upon the Fresnel reflection coefficient and continue to propagate.
The general expression for refractive loss of a sensor can be given by [17, 20],
where, \({P}_{L}\) is the loss dB per unit length, \({\mathrm{NA}}_{a}\) and \({\mathrm{NA}}_{r}\) are the numerical apertures of sensing region in presence of sensing analyte and in the fact of reference medium, respectively. Such refractive loss is an expeditious response of the optical fiber due to bulk change in RI.
The cladding removed bare fiber core is coated with a stimuli-responsive PDMS layer in our proposed sensor which behaves as a substituted cladding. PDMS polymer undergoes significant swelling when exposed to saturated aliphatic hydrocarbons, and the degree of swelling is inversely proportional to the number of methylene units in the carbon chain [31]. Further, the swelling or shrinkage of the layer leads to RI variation of the PDMS layer which is used as the main detection criteria of our proposed sensor. Depending on the extent of swelling/shrinkage of the PDMS layer, the refraction loss at the core/PDMS interface will be different, leading to variation in the intensity count of output light.
3 Experimental
3.1 Probe fabrication
Cladding modification methodology was used to prepare the sensor probe, as shown in Fig. 2, where cladding is removed from the middle part of the fiber and cleaned. A step-index polymer clad silica (PCS) MMF having 0.39 NA and the core/cladding diameter of 200/225 µm is used, which was purchased from Thorlabs. This fiber is preferred due to easily removable cladding, robustness, and cost-effectiveness [14, 15]. Buffer jacket and the technology-enhanced clad silica (TECS) hard polymer cladding are removed mechanically with the help of a sharp razor blade; a 2 cm middle fiber portion was uncladded and used as a sensing head. Then unclad portion was kept in acetone for 10 min to remove the organic residues, then washed with deionized (DI) water and dried at room temperature.
The further step is to coat the uncladded region with prepared PDMS polymer. Since the PDMS is a thermally curable elastomeric polymer, it can be easily coated via dip and spin coating methods. The coating of the PDMS polymer layer was done by mixing the PDMS (Sylgard-184) pre-polymer (base) with a cross-linker (curing agent, i.e., hardener) in a 10:1 v/v ratio which was purchased from Sigma Aldrich. The dip-coating process is used to coat the sensing region because this process is protective and suitable for accurate deposition over the micro-scale optical fiber structures [32]. The step-by-step process is illustrated in Fig. 3. The pre-polymer and cross-linker were appropriately mixed for a few minutes, and then the core exposed region was dipped once and taken out followed by heat treatment for 5 h at 60 °C [13, 28, 33]. Now the probe is ready for sensing characterization with quite quick and straightforward methodology.
3.2 Experimental setup
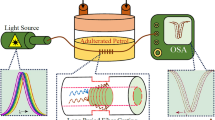
The experimental setup used for sensor characterization is depicted in Fig. 4. It consists of a broadband source (SLS201L/M – Thorlabs) of wavelength range 360–2600 nm, the prepared sensor probe with bare fiber connecting terminator (P/N: BFT1), and a portable spectrometer (ERA-2048*64) having 200–1100 nm wavelength detection range along with an electronic processing (personal laptop) to record the spectra. The sensing region was fixed in a prepared flow cell, and analytes of different concentrations were poured for the measurement. The adulterated fuel samples were prepared by mixing the different volume percent of kerosene in petrol and diesel which were purchased from the local Bharat petroleum outlet.
4 Results and discussions
4.1 FESEM analysis
The bare and PDMS coated sensing region of the probe was examined through field emission scanning electron microscopy (FESEM, Supra-55, Zeiss, Germany) characterization, and both are shown in Fig. 5. Since the PDMS polymer gel is sticky and heavy, hence we got bulk coating over fiber surface after polymerization by dip coating, which can be clearly observed in Fig. 5b.
4.2 Fuel adulteration sensing experiment
To perform the fuel adulteration sensing experiments, samples with different levels of adulteration were put in the flow cell where the sensing region was fixed and the output spectra of the sensor were recorded for each sample. The whole experiment was carried out at room temperature and the output spectra were recorded after incubating the sensor for a fixed interval of 1 min in each of the sample solutions.
4.2.1 Petrol adulteration detection
When petrol samples with an increased level of adulteration were put successively in the sensing region, the output spectra of the sensor followed the downward trend, as shown in Fig. 6. As mentioned earlier, the polymer PDMS undergoes swelling when exposed to hydrocarbons and the extent of swelling is dependent on the chain length. This polymer swells more when the chain length is short and shrinks when the chain length increases. The petrol is composed of C4–C12 hydrocarbons while kerosene is composed of C6–C16 hydrocarbons. So, according to the hydrocarbon chain length, this polymer will be in a more swollen state when exposed to petrol and the degree of swelling will decrease when exposed to kerosene. Since the original fiber cladding of the sensor has been replaced by PDMS layer, light passing through the sensor directly interacts with this layer. When the kerosene content in the petrol sample increases, the PDMS layer will gradually shrink which eventually increases the RI of the PDMS layer. Hence, the light leakage through the sensing region will increase with the increase of RI of the PDMS layer, leading to a decrease in the transmitted light. Figure 6b shows the comparison between a bare fiber sensor and PDMS coated fiber sensor. The sensitivities observed for the bare and PDMS coated fiber sensors are 107 counts/% and 281 counts/%, respectively, and it can be clearly seen that the PDMS coating significantly enhances the sensor’s performance.
4.2.2 Diesel adulteration detection
Figure 7 depicts the response of the sensor when applied for diesel adulteration detection by kerosene. In contrary to the previous case, the output spectra follow an upward trend with the increase in adulteration level. The reason behind this is again related to the carbon chain length because kerosene is composed of C6–C16 hydrocarbons, while diesel contains hydrocarbons in the range of C8–C21. So, the chain length of diesel is more than kerosene. Accordingly, the sensing layer PDMS will be in a shrunken state in diesel and will successively swell when the kerosene content increases. The RI of the PDMS layer will also gradually decrease with an increase in the percentage of kerosene in diesel. This will lead to a decrease in light leakage from the sensor with augmented kerosene level and hence the intensity of output spectra increases. In this case also, PDMS coated sensor exhibits better performance as shown in Fig. 7b, and the sensitivities obtained for bare and PDMS coated sensors are 81 counts/% and 134 counts/%, respectively. The middle wavelength (750 nm) of the whole spectra was chosen for intensity counts determination and hence the sensitivity evaluation because near this wavelength, adequate and explicit intensity changes appear. For this purpose, a straight line parallel to the y-axis was drawn at this particular wavelength and recorded the change in intensity counts.
We can compare a few reported fiber optic automobile fuel adulteration sensors based on the intensity modulation sensing scheme. As the unit varies for the obtained results reported in the literature, we could not compare the performance of the sensors directly. So, we focused on structural simplicity and other advantages for comparison, as given in the Table 1. It is evident from the table that our reported sensor is fabrication feasible, selective, and suitable for both petrol and diesel adulteration detection compared to the sensors listed in the Table 1.
4.2.3 Response time
The response time of a sensor indicates how fast it can respond towards an external analyte change. In case of our fabricated sensor, the response time is recorded by incubating the sensor in pure diesel, kerosene and petrol samples successively, and the time for the output intensity of the sensor to get stabilized after transferring from one to another sample was recorded. As the hydrocarbon chain length of diesel is more than kerosene and petrol (hence RI is also high), we started the experiment with a pure diesel sample. When the diesel was replaced by pure kerosene, the output intensity increased due to swelling of the PDMS layer, and it took about 5 s to reach a stable output. So, the response time of the sensor in transferring from pure diesel to pure kerosene is 5 s. Thereafter, when the kerosene sample is replaced again by petrol sample, the PDMS layer swells more and output intensity again increases. In this case, the time taken to reach stable output was 9 s. It appears that the spectrum does not reach its original state when the liquid sample is removed from the sensing region. This happens because the recovery time of the sensor is longer than its response time. PDMS is in the most shrunken state when in diesel than another medium (kerosene, petrol, even air as can be seen in Fig. 8) and so, the recovery time is also longer in case of diesel than kerosene. The rate of recovery depends on the medium in which the sensor is kept. In our case, when we put the sensor out of diesel, it is in an air medium and the rate of recovery is w.r.t. air. If we consider response and recovery time in different combinations or in different orders like diesel/kerosene, diesel/petrol etc., the response and recovery time will also be different. Since here we are focusing on the quick response of the sensor, we consecutively transfer the analytes without giving it much time for their initial recovery. So, it can be concluded that the time response of the sensor in transferring from diesel to kerosene is faster than that of kerosene to petrol transfer.
4.2.4 Reversibility
The reversibility and hence the hysteresis behavior of our fabricated sensor was examined by conducting the petrol adulteration detection experiment in both increasing and decreasing levels of adulteration. The incubation time was nearly 1 min after putting the sample into the sensing region, which was the same for each measurement. The obtained results are depicted in Fig. 9a. So, it can be observed that the proposed sensor exhibits a small amount of hysteresis between ascending and descending orders of measurement. The percentage of hysteresis is calculated using the expression given below [21].
where \({Y}_{\mathrm{md}}\) is the middle point value of reversibility curve for descending order and \({Y}_{\mathrm{ma}}\) is for ascending order. \({Y}_{\mathrm{max}}\) and \({Y}_{\mathrm{min}}\) are the maximum and minimum value of intensity counts of the plot, respectively. So, the sensor exhibits a tolerable amount of hysteresis, i.e., 0.51%, and hence, the adulteration detection can be performed reversibly in either ascending or descending order of operation. The changes induced in PDMS due to the hydrocarbons is mechanically intriguing, but the impact is nearly reversible as per our observations and also as reported in the literature [31].
4.2.5 Repeatability
To check the repeatability of the sensor, we have picked two analytes, i.e., pure petrol with no contaminants and kerosene. So, we consider pure petrol as 0% and pure kerosene as 100% adulteration levels and then experimented. Several measurement cycles were performed between these two samples, and the obtained result is shown in Fig. 9b. From the plot, it is observed that the output intensity comes back to almost the same value after each measurement cycle. So, the results demonstrate that there is no significant drift between the measurement cycles, and hence response of the sensor is fairly repeatable. Moreover, the error or uncertainty in the sensor measurement obtained during experiments or taking different measurements can be calculated. If we compare the calibration curve and repeatability curve of petrol, the approximate value of uncertainty comes out to be 11%. This uncertainty between the two measurements may have arisen due to environmental perturbations (such as temperature, vibrations) or may be due to the volatile nature of petrol. We expect to minimize this by performing the entire experiment in a highly controlled environment and using a closed analyte chamber.
5 Conclusion
An optical fiber coated with PDMS as a stimuli-responsive layer for fuel adulteration detection is proposed and experimentally demonstrated in this paper. Swelling and shrinkage of the PDMS layer in response to different adulterated fuel sample is the essential detection criteria. The sensor exhibits different refraction losses concerning change in RI of the PDMS layer, and as a result, the output light intensity varies accordingly. The highest sensitivities of 281 counts/% and 134 counts/% are achieved in the range of 0–50% adulteration for petrol and diesel, respectively. In addition, the sensor shows fast response time, excellent repeatability, and reversibility with a meager amount of hysteresis. Further, the sensor is easy to fabricate, cost-effective, portable, and can provide selective detection. We anticipate that the proposed sensor can be efficiently utilized for in situ petrol and diesel adulteration detection with the above-mentioned advantageous features.
References
G. Joel, L.N. Okoro, Recent advances in the use of sensors and markers for fuel adulteration detection : a review. Int. J. Res. Sci. Innov. VI(XI), 82–89 (2019)
S.K. Dilip, T.V.S. Pillai, Estimating fuel adulteration in automobiles using robust optical fiber sensors. Microprocess. Microsyst. 79(September), 103289 (2020). https://doi.org/10.1016/j.micpro.2020.103289
A. Datta, A. Saha, Investigation of an ultra-sensitive fiber-optic fuel adulteration sensor by propagating a higher-order Bessel-Gauss beam. Optik (Stuttg) 243(June), 167408 (2021). https://doi.org/10.1016/j.ijleo.2021.167408
B. Lv, M. Himanth, Review on the detection of fuel adulteration through sensor based techniques. Int. J. Sci. Res. Publ. 7(9), 447–451 (2017)
G.C. Yadav, S. Prakash, G. Sharma, S. Kumar, V. Singh, Detection of kerosene adulteration in automobile fuel with a novel metal clad planar waveguide. Opt. Laser Technol. 119(Feburary), 105589 (2019). https://doi.org/10.1016/j.optlastec.2019.105589
M. Kumar, D. Engles, S. Prashar, A. Singh, Etched FBG as chemical sensor for fuel adulteration. Int. J. Eng. Res. Technol. 1(4), 1–5 (2012)
M. Chauhan, A.K. Pathak, T. Khanikar, V.K. Singh, A side-polished macro bend fiber sensor for the efficient detection of petrol adulteration. Work. Recent Adv. Photonics 194, 1–3 (2019). https://doi.org/10.1109/WRAP47485.2019.9013694
V.J. Felix, P.A. Udaykiran, K. Ganesan, Fuel adulteration detection system. Indian J. Sci. Technol. 8(January), 90–95 (2015). https://doi.org/10.17485/ijst/2015/v8iS2/59076
A.P. Gawande, J.P. Kaware, Fuel adulteration consequences in India : a review. Sci. Rev. Chem. Commun. 3(3), 161–171 (2013)
B. Kanyathare, K. Kuivalainen, J. Räty, P. Silfsten, P. Bawuah, A prototype of an optical sensor for the identification of diesel oil adulterated by kerosene. J. Eur. Opt. Soc. Publ. (2018). https://doi.org/10.1186/s41476-018-0071-2
Z. Tang et al., A U-shape fibre-optic pH sensor based on hydrogen bonding of ethyl cellulose with a sol-gel matrix. J. Light. Technol. (2020). https://doi.org/10.1109/JLT.2020.3034563
Y. Zhao, M. Lei, S. Liu, Q. Zhao, Smart hydrogel-based optical fiber SPR sensor for pH measurements. Sens. Actuators B Chem. 261, 226–232 (2018). https://doi.org/10.1016/j.snb.2018.01.120
R. Yang et al., PDMS-coated S-tapered fiber for highly sensitive measurements of transverse load and temperature. IEEE Sens. J. 15(6), 3429–3435 (2015). https://doi.org/10.1109/JSEN.2015.2388490
T. Khanikar, V.K. Singh, PANI-PVA composite film coated optical fiber probe as a stable and highly sensitive pH sensor. Opt. Mater. (Amst) 88(Septumber 2018), 244–251 (2019). https://doi.org/10.1016/j.optmat.2018.11.044
M. Chauhan, V.K. Singh, Fiber optic pH sensor using TiO2-SiO2 composite layer with a temperature cross-sensitivity feature. Opt. –Int. J. Light Electron Opt. 212, 164709 (2020). https://doi.org/10.1016/j.ijleo.2020.164709
M. Chauhan, V.K. Singh, Review on recent experimental SPR/LSPR based fiber optic analyte sensors. Opt. Fiber Technol. 64, 102580 (2021). https://doi.org/10.1016/j.yofte.2021.102580
N. Punjabi, J. Satija, S. Mukherji, Evanescent wave absorption based fiber-optic sensor—cascading of bend and tapered geometry for enhanced sensitivity. Smart Sensors, Meas. Instrum. (2015). https://doi.org/10.1007/978-3-319-10948-0
R. Biswas, M. Pradhan, A comparative analysis of all fiber optic sensors for detection of adulteration in fossil fuels. Opt. Quantum Electron. 62, 1–10 (2020). https://doi.org/10.1007/s11082-019-2179-z
S. Kher, S. Chaubey, J. Kishore, S.M. Oak, Detection of fuel adulteration with high sensitivity using turnaround point long period fiber gratings in B/GE doped fibers. IEEE Sens. J. 13(11), 4482–4486 (2013). https://doi.org/10.1109/JSEN.2013.2270312
S. Roy, Fiber optic sensor for determining adulteration of petrol and diesel by kerosene. Sens. Actuators B. Chem. 55, 212–216 (1999). https://doi.org/10.1016/S0925-4005(99)00171-9
A.K. Pathak, R.K. Gangwar, P. Priyadarshini, V.K. Singh, A robust optical fiber sensor for the detection of petrol adulteration. Opt. - Int J. Light Electron Opt. 149, 43–48 (2017). https://doi.org/10.1016/j.ijleo.2017.09.036
R.K. Verma, P. Suwalka, J. Yadav, Detection of adulteration in diesel and petrol by kerosene using SPR based fiber optic technique. Opt. Fiber Technol. 43(April), 95–100 (2018). https://doi.org/10.1016/j.yofte.2018.04.011
A. Lamberti, A. Virga, P. Rivolo, A. Angelini, F. Giorgis, Easy tuning of surface and optical properties of PDMS decorated by Ag nanoparticles. J. Phys. Chem. B 119, 8194–8200 (2015). https://doi.org/10.1021/acs.jpcb.5b02581
M. Ionescu et al., Enhanced biocompatibility of PDMS (polydimethylsiloxane) polymer films by ion irradiation. Nucl. Inst. Methods Phys. Res. B 273, 161–163 (2012). https://doi.org/10.1016/j.nimb.2011.07.065
J.S. Velazquez-Gonzalez, Martınez-Pinon, D.M.-H. Fernando, D.A.M.-A.I. Hernandez-Romano, Surface plasmon resonance-based optical fiber embedded in PDMS for temperature sensing. IEEE J. Sel. Top. Quantum Electron. (2017). https://doi.org/10.1109/JSTQE.2016.2628022
Y.O.N.G. Wang, Q.I.N.G. Huang, W.E. Zhu, M.I. Yang, Elfed, and lewis, “novel optical fiber SPR temperature sensor based on MMF-PCF-MMF structure and gold- PDMS film.” Opt. Express 26(2), 1910–1917 (2018). https://doi.org/10.1364/OE.26.001910
Y. Chen et al., A flexible gastric gas sensor based on functionalized optical fiber. IEEE Sens. J. 16(13), 5243–5248 (2016). https://doi.org/10.1109/JSEN.2016.2544701
Q. Zhang et al., A flexible magnetic field sensor based on AgNWs & MNs-PDMS. Nonoscale Res. Lett. 14(27), 1–9 (2019). https://doi.org/10.1186/s11671-018-2826-5
C. Luo et al., An optimized PDMS thin film immersed fabry-perot fiber optic pressure sensor for sensitivity enhancement. Coatings 9(290), 1–10 (2019). https://doi.org/10.3390/coatings9050290
P. Wang, G. Brambilla, M. Ding, Y. Semenova, Q. Wu, G. Farrell, High-sensitivity, evanescent field refractometric sensor based on a tapered, multimode fiber interference. Opt. Lett. 36(12), 2233 (2011). https://doi.org/10.1364/ol.36.002233
D. Park, J. Hong, I.S. Park, C.W. Lee, J. Kim, A colorimetric hydrocarbon sensor employing a swelling- induced mechanochromic polydiacetylene. Adv. Funct. Mater. 24, 5186–5193 (2014). https://doi.org/10.1002/adfm.201400779
S. Noimark et al., Carbon-nanotube—PDMS composite coatings on optical fibers for all-optical ultrasound imaging. Adv. Funct. Mater. 26, 8390–8396 (2016). https://doi.org/10.1002/adfm.201601337
X. Ning, J. Yang, C.L. Zhao, C.C. Chan, PDMS-coated fiber volatile organic compounds sensors. Appl. Opt. 55(December), 3543–3548 (2018). https://doi.org/10.1364/AO.55.003543
Acknowledgements
Authors are thankful to DST-SERB for providing the research grant with vide project no: DST (SERB) (222)/2018/635/AP and IIT(ISM) Dhanbad, India, for providing the experimental facilities.
Author information
Authors and Affiliations
Contributions
MC: experimentation and manuscript writing, TK: idea and manuscript writing, VKS: supervision, overall organization, and fund acquisition.
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Chauhan, M., Khanikar, T. & Singh, V.K. PDMS coated fiber optic sensor for efficient detection of fuel adulteration. Appl. Phys. B 128, 89 (2022). https://doi.org/10.1007/s00340-022-07809-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00340-022-07809-8