Abstract
Acetylene black is a special type of carbon black, obtained from the thermal decomposition of acetylene. Acetylene flames enable the investigation of the soot optical properties. A reliable knowledge of the optical properties of acetylene soot is necessary for interpreting the optical measurements in flames and the development of acetylene black applications in industry. However, a wide variation in the optical properties of soot is observed in different formation conditions. The main factors affecting soot formation in flames are fuel composition, equivalence ratio, and reaction time connected with the height above a burner. In this study, we measured the absolute value of the refractive index function of acetylene soot E(m,1064) at a wavelength of 1064 nm, as well as the ratio of the refractive index functions E(m,1064)/E(m,532) at laser wavelengths of 532 nm and 1064 nm in a premixed acetylene/air flame using laser-induced incandescence. The soot structure was studied using high-resolution electron microscopy. The obtained results showed that E(m,1064) increased from 0.19 to 0.53 and that the ratio of E(m,1064)/E(m,532) increased from 1.12 to 1.55 according to the height above a burner. These changes in the optical properties are attributed to differences in soot structure, such as different distances between graphene planes inside soot crystallites, which decreased from 0.43 to 0.36 nm as the height above the burner increased from 5 to 20 mm. It was concluded that the optical properties and structure of acetylene soot differ from those formed in other hydrocarbon flames.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Soot particles and carbon black constitute an important subject of combustion and material science, and their properties, to date, have attracted the attention of researchers. A large amount of data on the optical properties of carbonaceous particulates has been accumulated due to its importance in practical applications (see, for example, review papers [1,2,3]). Acetylene black is a special type of carbon black characterized by its purity of carbon, high degree of crystalline orientation and relatively high electrical conductivity compared to other sources of carbon black [4, 5]. These properties make it an ideal material in the production of dry cells, rubber reinforcements, cables, tires, belts, hoses, flooring, shoes, surface heating elements, black pigment, electronic components, etc. [6, 7]. Obviously, the specific properties of carbon black or soot depend on the type of fuel used for its formation. The difference between acetylene and other hydrocarbons lies in the special role that these molecules play during the formation of carbon particulates in pyrolysis and combustion. The common mechanism of soot formation is well described by Haynes and Wagner [8]. The pyrolysis of hydrocarbons produces smaller intermediates for the formation of the first aromatic ring, followed by the addition of other aromatic and alkyl species to produce polyaromatic hydrocarbons (PAHs). The formation of PAHs is believed to follow the HACA (H abstraction, C2H2 addition) mechanism [9]. The stacking of PAH results in the generation of soot precursors, and further soot formation proceeds by the agglomeration and surface growth of small particles with a size of the order of 1 nm [10]. In this work, we focus on the measurements of the optical properties of acetylene soot to find its differences from the soot produced using other hydrocarbons.
In early studies, the optical properties of soot were determined by the intensity of polarized light reflected from the surface of pressed soot pellets. Such measurements provide the direct values of the refractive index of soot [11,12,13]. However, in a review [1], the authors noted that the data obtained using pressed soot pellets may be in error due to cavities inside them, which could result in 20% smaller values of n and κ in spectral soot refractive index m = n – ik.
In situ determination of the soot refractive index in the visible and infrared spectral regions was carried out in numerous well-known studies [14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33] using the laser light absorption and scattering measurements. The interpretation of extinction/scattering measurements requires knowledge of the structure of soot aggregates, which is not pre-determined under the conditions of such measurements.
One of the promising methods for studying the properties of soot particles is the method of laser-induced incandescence (LII) [34,35,36,37,38,39,40]. When a laser pulse heats the particles to high temperatures, thermal radiation occurs. The intensity of this radiation depends on the optical properties of the condensed phase, which are determined by the refractive index of particle material. Thus, the laser-induced incandescence signals contain information about soot optical properties, which can be extracted by analyzing the recorded radiation. The advantage of the LII method for particle diagnostics is the use of volumetric laser energy absorption, which is independent of particle aggregation. From the other side, particle aggregation is essential for other methods, such as light extinction or light scattering. This is true in the frame of the Rayleigh–Debye–Gans (RDG) theory. The absorption cross section of soot aggregate predicted by the diffusion limited cluster aggregation (DLCA) can differ by 30% compared to that predicted by RDG [41].
Models of soot optical properties generally adopt approximations of uniform primary particle properties. However, the presence of atoms other than carbon (H, O), as well as annealing processes of soot at high temperatures, can affect optical properties such as the soot refractive index. Transition electron microscopy (TEM) studies show a different structure near the core and at the surface of soot primary particles, which can be a reason for the non-uniformity of their optical properties [42]. Charalampopoulos and Felske [10], who studied the refractive index of soot particles at various heights above a burner (HAB), noted an effect of flame conditions on the optical constants of soot. In addition, a wide variation in soot’s optical properties under different formation conditions was observed in [17].
Choi et al. [18] were the first to use gravimetric sampling as an independent measurement of the soot volume fraction to determine the dimensionless extinction coefficient with the light extinction technique. Using the gravimetrically determined soot volume fraction, they measured a mass-specific light extinction coefficient value of 8.6 in an acetylene/air premixed flame, which was a factor of two greater than the value of 4.9 predicted using a commonly accepted value for the refractive index of soot m = 1.57–0.56i [11, 21]. The later measurements of the mass-specific light extinction coefficient reported values between 8 and 10 for many flame conditions and fuels in the visible and near-infrared ranges of the spectrum [17, 18, 23,24,25,26, 28, 31].
The value of refractive index function E(m, 1064) for soot at a wavelength of 1064 nm was first directly measured in [43] by laser-induced incandescence in a diffusion ethylene flame.
The refractive index function E(m) is determined from the spectral refractive index:
Later, the value of E(m,532) at a wavelength of 532 nm was measured under the same conditions [44]. Bladh et al. [45] measured the value of E(m,1064) by LII in a premixed ethylene/air flame in dependence on height above a burner. A similar dependence of E(m,1064) on HAB was obtained in a methane/air premixed flame [46]. In another study [47], E(m,1064) for soot was investigated by LII during acetylene pyrolysis in a shock tube reactor in dependence on primary particle size. Finally, in a recent review [48], the authors concluded that the E(m) of soot aerosol in the visible and near-infrared should be greater than 0.32.
Two-wavelength excitation was proposed in [49] to determine the ratio of soot refractive index function E(m) at wavelengths of 532 and 1064 nm by laser-induced incandescence in laminar-diffusion ethylene and acetylene/air premixed flames. The ratio E(m,1064)/E(m,532) was also investigated for methane/oxygen and diesel/air flat premixed flames [50]. Table 1 presents the data of the refractive index function E(m,1064) and E(m,1064)/E(m,532) ratio, obtained in acetylene/air flames using different measurement methods.
The variety of optical properties of soot particles is obviously associated with the peculiarities of their internal structure [51]. In the last decade, various soot structures have been investigated by high-resolution transmission electron microscopy (HR TEM). This allows for the determination of the structural parameters of the soot “skeleton”, which consists of individual fringes (the edge of the graphene planes observable by high-resolution TEM). Such parameters include the fringe length, fringe tortuosity, and interlayer spacing of graphene planes in crystallite in soot primary particles [52]. The relationship between the regularities of the structure of young and mature soot particles and their UV–visible absorption coefficient has been investigated in [53]. The authors of [53] showed that the specific soot absorption increases with the soot graphitization process, which is characterized by a decrease in the H/C ratio and an increase in graphitic planar structures.
Alfe et al. [54] studied the influence of flame temperature on soot structure. The optical and electrical properties of carbonaceous particles formed in a premixed ethylene/air flame have been observed in [55], where it was found that the optical bandgap decreases with an increase in graphene planes and decreasing interlayer spacing between parallel layers in soot crystallite. The detailed evolution of the soot structure in premixed ethylene and benzene flames according to HAB has been presented in [56]. However, to date, a detailed investigation of the correlation between the structure and refractive index function of acetylene flame soot has not been presented.
The goal of the present study is to determine the values of E(m) at a wavelength of 1064 nm and the ratio of refractive index functions of acetylene soot at wavelengths of 1064 and 532 nm depending on HAB in a premixed acetylene/air flame, using the LII method. Another purpose of this work is to investigate the fine structure of acetylene soot particles by HR TEM to determine the correlation between the E(m) values and the soot structure at different HABs.
2 Experimental
2.1 Burner
Experiments were carried out in a premixed acetylene/air flame using a standard McKenna burner (Holthuis & Associates) with a bronze porous surface 62 mm in diameter and with a 5-mm-wide external bronze shroud ring. The stable and uniform parameters were achieved along the horizontal axis of the flame using a brass disk with a diameter of 60 mm and a thickness of 20 mm installed at a height of 23 mm above the burner. In the assumption of a one-dimensional structure of gas flow in the burner flame, the molar fractions of substances and the temperature in the flame change depending only on the height above a burner surface. In our experiments, the following molar composition of gases was used: C2H2: 13.28%; O2: 18.45%; N2: 68.27%. Gas consumption was controlled using RRG-10 mass-flow controllers (Eltochpribor). The fuel/oxidizer ratio was 1.8 times above the stoichiometric ratio (equivalence ratio). The C/O ratio was 0.72, the cold gas velocity was 6.8 cm/s, the acetylene consumption was 84 standard liters/hour, and the air consumption was 545 standard liters/hour (at standard temperature 293 K).
A Pt–Rh(6%)/Pt–Rh(30%) uncoated thermocouple was used to measure the temperature of the flame. The gas temperature was evaluated while considering conductive and radiative heat losses, according to [57]. The uncertainty of the temperature measurement taking into account this correction is evaluated to be ± 80 K.
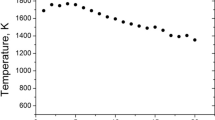
Under the conditions of our experiment, the soot was quickly deposited on the surface of the thermocouple junction even at low heights above a burner. Thus, measuring the temperature in extremely sooting flames is not an easy task. Due to the unknown emissivity of the thermocouple junction covered by soot at different HABs, we have supposed three cases of influences of soot deposition on thermocouple surface: the emissivity of the thermocouple junction is 0.95 (soot emissivity) at all HABs; the emissivity of the thermocouple junction gradually increases from the emissivity of a pure metal surface (ε = 0.11) to soot emissivity (ε = 0.95) with an increase in HAB; and the increase in the emissivity of the thermocouple junction due to soot deposition is ignored. In addition, the flame temperature was measured using the two-color LII method described below in Sect. 2.4.2 at HABs 5, 7, 10, 13, 15, 17, and 20 mm with accuracy ± 150 K. Figure 1 shows the centerline flame temperature measured by a thermocouple with three cases of corrections and temperature measurements by two-color LII. Note that after temperature measurement at a selected HAB, the thermocouple junction was thoroughly cleaned of soot. From Fig. 1, one can see that the temperature measurements using gradual junction emissivity correction agreed well with the LII flame temperature measurements. Hence, this correction is plausible. These data were used below for the evaluation of the optical properties of acetylene soot by LII.
Centerline acetylene/air flame temperature. 1—Measurements by thermocouple using gradual junction emissivity correction due to soot deposition; 2—measurements by two-color LII; 3—measurements by thermocouple using constant junction emissivity equal to soot emissivity; 4—measurements by thermocouple using correction of conductive heat loss and ignoring the increase of junction emissivity due to soot deposition
2.2 LII measurements
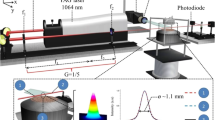
A pulsed Nd:Yag laser LQ 129 (Solar Laser Systems) was used to heat soot particles to temperatures higher than the temperature in the flame. The central part of the laser beam was cut using a diaphragm 1 mm in diameter. After that, the laser beam was directed through the center of the flame. The laser-induced incandescence from laser-heated soot particles was collected at a right angle to the laser beam using a homemade two-color LII device, presented in Fig. 2. LII registration was carried out using two photomultiplier modules, H6780-20. The modules were equipped with narrow-band filters with centers at wavelengths of 450 and 670 nm with 10 nm FWHM for two-color LII temperature measurements. The LII-detected wavelengths of 450 and 670 nm were chosen because this was the best compromise between sensitivity to temperature measurements, photomultiplier sensitivity, and avoidance of C2 emission. The signals were recorded using a LeCroy WavePro 7100 oscilloscope with a 1-GHz band pass. A separate series of experiments was carried out with laser irradiation of 532 or 1064 nm.
Figure 3 shows the normalized intensity of laser emission versus time. This time energy profile was measured using photomultiplier module H6780-20 (Hamamatsu) at laser wavelength 532 nm (rise time 0.78 ns). The temporal profiles of the 1064 nm and 532 nm pulses will not be identical, due to the non-linear nature of frequency doubling. However, usually, they are short enough for this difference to not be significant with regard to the eventual measurements.
The spatial energy profile of the laser beam was controlled using a laser profilometer WinCamD-UCD12 (DataRay). The diaphragm/flame distances 95 cm at 1064 nm and 47 cm at 532 nm were chosen so that the diameter of the laser beam in the flame center would be the same. The cross-sections of the laser spatial energy profiles measured at wavelengths 532 and 1064 nm are presented in Fig. 4. The laser energy spatial profile measured at 532 nm is noisier because of the lower sensitivity of a detector. The integral pulse energy was determined using a pyroelectric sensor ES145C (Thorlabs). The laser fluence was calculated by dividing the measured energy after the diaphragm by the cross-section area taken at 1/e2 of the peak of a Gaussian approximation of the spatial laser energy profile.
For measurement of the ratio of E(m,1064)/E(m,532) of soot, the amplitude of LII signals at a wavelength of 450 nm induced by laser wavelengths of 532 and 1064 nm was detected with a delay of 50 ns after the onset of the laser pulse. This avoided the contribution of fluorescent radiation from polycyclic aromatic hydrocarbons, induced by 532 nm laser excitation, which usually appears in the flames [49]. The amplitudes of LII signals were measured in the laser fluence range 0.05–0.3 J/cm2 and included the non-sublimation regime and regime above the soot sublimation threshold. The valuable measurements of E(m) and the ratio of E(m,1064)/E(m,532) were performed below the soot sublimation threshold due to the strong influence of sublimation on the amplitudes of LII signals. The soot sublimation process intensifies the cooling of the particles, which results in a flattening of the particle temperature, and the LII amplitudes do not increase with a rise in laser fluence. Besides that, the soot sublimation led to the interference of the incandescence with molecular emission like C2 [58]. This point will be discussed below.
2.3 Measurements of the E(m, 1064)/E(m,532) ratio
For measurement of the E(m, 1064)/E(m,532) ratio, the LII signals registered at 450 nm were chosen because the LII amplitude at this wavelength is higher than that at 670 nm for both excitation wavelengths. Figure 5 shows the amplitude of LII signals at 450 nm obtained with a delay of 50 ns after the beginning of the laser pulse at different laser fluences and heights above a burner. The 50 ns delay enables measurement of the LII amplitude without the influence of the fluorescence of polyaromatic hydrocarbon molecules at 532 nm laser excitation because the main fluorescence signal occurs during laser pulse [49].
At small fluences (less than 0.05 J/cm2), the amplitude of the LII signals did not exceed 0.02 a.u., which is below the sensitivity limit. At fluences above 0.05 J/cm2, the LII signals linearly increase until an energy density of 0.1–0.15 J/cm2 at 532 nm and 0.12–0.2 J/cm2 at 1064 nm. At larger fluences, the increase in LII signal amplitude slows down due to the influence of the sublimation process of soot particles. In addition to sublimation, the laser profile wings are responsible for slowing the rise in the LII signal. When the laser beam had a Gaussian-like profile, the wings effect led to inhomogeneous heating of carbon particles in the probe volume [58].
To find the ratio of E(m,1064)/E(m,532), it is necessary to determine the fluence values at which identical LII signals at both wavelengths are observed, as shown by the horizontal arrows in Fig. 5. Within the linear sections of fluence rise curves presented in Fig. 5, the same values of E(m, 1064)/E(m,532) can be extracted independently on the LII amplitude level. The method can be applied only at a low laser fluence (that is, without the sublimation of soot particles and the emission of C2 radicals). So, if the LII signals measured at both wavelengths are identical after successive irradiation with two laser pulses at different wavelengths and different laser fluences R0:
The following relation can be written according to Planck's law for thermal radiation at wavelength λi [49]:
where T(t) is the temperature reached by the particle at the moment of time t for each excitation wavelength 532 or 1064 nm.
Condition (9) is satisfied if T(t,532) = T(t,1064). As mentioned, this equality can be obtained by varying the laser fluence for each laser wavelength. Assuming the same spatial distribution of laser radiation and adjusting the laser fluence at 1064 and 532 nm, the ratio of the functions of the refractive indices can be expressed as [49]:
Thus, using the data obtained and the above method for determining laser fluences at 1064 nm and 532 nm, corresponding to equal LII signals, the dependences of the ratio E(m,1064)/E(m,532) on the flame height were found. These results are presented and discussed below.
2.4 Measurements of the values of E(m) at a wavelength of 1064 nm
2.4.1 LII model
For the two-color pyrometry of laser-heated soot, incandescence signals were measured through two narrow band-pass filters centered at wavelengths of 450 and 670 nm. The common LII model used for particle size evaluation considered the current particle temperature Tp during heating and cooling by solving the energy and mass balance Eqs. (11, 12):
Here, mp and cp are the mass and heat capacity of particles, t is the time, Jevap is the mass loss rate from the particle surface during the sublimation process, \(\dot{q}_{abs}\) is the laser energy per unit time absorbed by the particle, and \(\dot{q}_{evap}\), \(\dot{q}_{rad}\), and \(\dot{q}_{cond}\) are the energy loss rates from the particle in the processes of sublimation, thermal radiation, and conductive heat transfer with the molecules of bath gas, correspondingly.
During the laser heating of a soot particle, the heat capacity of the material is essentially changing. In the LII model, we used the heat capacity temperature dependence of solid graphite from [59] following [60]. The current particle mass in Eq. (11, 12) was converted to the current particle size using the particle density. Because the sizes of soot particles in our study were at least 12 nm, we suppose that this soot is close to mature. The common values for mature soot density were reported in the range of 1820–2050 kg/m3 [17]. We used the value of 1.86 g/cm3 reported in [40].
2.4.2 Refractive index function value determination
In the Rayleigh limit (dp < < λlaser), the energy absorption by a particle with a diameter dp can be expressed as [60]:
where g(t) is the time profile of laser intensity given by measured laser profile gm(t) (see Fig. 3) normalized and weighted by laser fluence R0 [J/m2]:
It should be noted that Eq. (13) is based on the RDG approximation and neglects the effect of aggregation on soot absorption. According to [41], the effect of soot aggregate absorption can be up to about 30%. In this study, only a 1064 nm laser incident light was used to determine E(m).
Neglecting the heat losses due to conductivity, sublimation, and thermal radiation, the maximum heat-up temperature of a particle at the end of laser pulse Tp0 can be obtained by the integration of Eq. (11) [61]:
Here, ρp is the particle density and Tg is the surrounding gas temperature.
On the other hand, two-color pyrometry can be applied for direct Tp0 measurements. The value of Tp0 can be determined from the ratio of two maximum amplitudes of LII signals obtained at two different wavelengths in the visible spectrum [62]:
where h, c, and kB are the Planck constant, light velocity in vacuum, and Boltzmann constant, respectively; λ1 and λ2 are the registered radiation wavelengths; I1 and I2 are the maximum amplitudes of the registered LII signals; ITL1, ITL2, and TTL are the radiation intensities and the brightness temperature of the calibration tungsten strip lamp defined by manufactured pyrometer and tungsten spectral emissivity εT(λ1), εT (λ2) on the wavelengths of λ1 and λ2; and E(m, λ1) and E(m, λ2) are the soot refractive index functions on wavelengths of λ1 and λ2.
Finally, the value of E(m) in (15) was adjusted until the temperature Tp0 was equal to that in Eq. (16). The spectral variation E(m, λ2)/E(m, λ1) of soot used in Eq. (16) was taken from data at a selected HAB, which is defined in Sect. 3.
2.4.3 Measured values of E(m) at a wavelength of 1064 nm in dependence on height above a burner
Figure 6 shows the E(m) values at a wavelength of 1064 nm determined by the equalizing of the measured peak temperature of laser-heated soot using two-color LII detection [see Eq. (16)] with the calculation of this temperature using the LII model (Eq. (15)), as was described above. These measurements were carried out only at the wavelength of 1064 nm because the laser irradiation of soot at the wavelength of 532 nm resulted in PAHs fluorescence and correspondingly affected the maximal amplitude of the LII signal [49].
From Fig. 6, one can see that when the laser fluence increases above the onset of sublimation at around 0.12 J/cm2 [63,64,65,66], the apparent values of E(m) decrease. This could be attributed to the heat loss due to sublimation, neglected in the determination of the theoretical maximum of the particle heat-up temperature. Additionally, the influence of soot structure transformation due to the annealing process could be a reason for the apparent E(m) decrease with laser fluence rise. Therefore, the measurements of E(m) were performed below the sublimation threshold. To fulfill this condition, the found apparent values of E(m, 1064) were plotted as a function of the laser radiation energy density. At fluences of 0.05–0.12 J/cm2 (below the sublimation threshold), the extracted values of E(m) are stable (see the horizontal lines in Fig. 6). At fluences below 0.05 J/cm2, the LII signals are very noisy under the experimental conditions and do not allow for the extraction of reliable E(m) values. Thus, the true values of E(m, 1064) are located at a stationary level at a laser energy density between 0.05 and 0.12 J/cm2. The resulting dependencies of E(m, 1064) on HAB are given and discussed below.
The error of E(m) determination was evaluated as 15%, taking into account the ranges of the uncertainty of parameters: ρ=1860 kg/m3 ± 280 kg/m3, cp = 2000 kJ/kg ± 50 kJ/kg, Tp = 3500 K ± 200 K, Tg = 1800 K ± 150 K, and R0 = 0.1 J/cm2 ± 0.0025 J/cm2. However, the sensitivity of E(m) determination to the change of product ρ × cp due to the variation of soot maturity can be larger. Neglecting the essential variation of cp, the variation of soot density from nascent to mature soot can be in the range of 1300–1860 kg/m3 [40]. These changes can result in the 30% decrease in the measured E(m) values. As was mentioned above, we suppose that the soot in the investigated flame is the mature soot from 5 mm HAB because the size of the primary soot particles attained 12 nm. Nevertheless, an application of the nascent soot density value of 1300 kg/m3 to the measurements at 5 mm HAB will resulted to an E(m) value of 0.13, which is too low in comparison to the known flame data.
2.4.4 Transmission electron microscopy
HR TEM was used to analyze the structure of soot particles. Samples were collected by a thermophoresis method on copper grids 3 mm in diameter, covered with a continuous carbon layer. The grid was installed in a pneumatically driven holder for insertion into the flame at a certain height above a burner. The sum of the residence time and traveling time of the grid into the flat flame was 130 ms. Samples of particles were analyzed on an FEI Osiris transmission electron microscope with an accelerating voltage of 200 kV at the Federal Research Center for Crystallography and Photonics of the Russian Academy of Sciences. The low-resolution micrographs were used for the statistical treatment of primary particle size in soot aggregates (see Fig. 7). The example of soot particle size distribution measured at HAB of 10 mm is presented in Fig. 8. The count median diameter in this distribution was found to be 13.8 nm.
The algorithm for the HR TEM image analysis of the soot particle structure was previously reported [67,68,69]. Image processing was carried out using the free image analysis program “ImageJ” [70]. The processing procedure involved separate steps of allocating a characteristic section of the soot structure on the HR TEM image, fast Fourier transformation of the image, filtering, reverse Fourier transformation, binary image conversion, skeletonization, and quantitative analysis of the structure. Selected image regions were stored as 512 × 512-pixel 8-bit grayscale images. Raw digitized images were converted to the frequency domain by a fast Fourier transform and filtered using ring-shaped masks with dimensions of 0.335 nm (graphite interlayer spacing) and 0.7 nm (with which the Van der Waals forces between two layers can be considered negligible). After reverse transformation, the crystalline image features were accentuated and perfected, while features in the image with different spatial frequencies were weakened.
The filtered image was transformed into a two-phase image by setting a threshold brightness value for the phase boundary. The brightness threshold was set at a value that resulted in the maximum number of identified fringes. The binary image was further processed to create a “skeleton” structure of fringes by eroding dark areas until they were one pixel in width.
The further algorithm of image processing consisted of the separation of the aggregate structures, the disconnect of “X” and “Y” crossed structures, and the removal of small fringes. In “X” and “Y” structures, the two fringe branches that are most nearly 180° apart are kept together and the remaining branch is separated by the removal of a single pixel adjacent to the junction pixel. A fringe crossed by segments smaller than 0.142 nm (the C–C bond length) was considered as an individual rectilinear structure. Fringes smaller than 0.6 nm were eliminated and fringes consisting of more than three aromatic rings were considered. It is assumed that a plane less than 0.6 nm can consist of no more than 3 rings and 13 carbon atoms (benzene, naphthalene, and phenalene). Anthracene, phenanthrene, and pyrene are no longer included in this size range. Note that in the literature, it is believed that the basis of soot crystallites is formed by the structures of the coronene type 0.9–1.0 nm consisting of 24 carbon atoms [71]. In addition, to determine the process of graphitization of soot, the trend of changing parameters is important but not the parameters themselves. Note that the measurements of the exact values of the parameters of soot structure still represent a methodological problem because the user is free to choose this cut-off. Van der Waals forces were assumed to be negligible between graphene layers spaced more than 0.7 nm, so pairs of fringes with greater spacing or angles greater than 15° were considered as two single layers.
The statistical treatment of skeletonized fringes was analyzed automatically throughout the image using a custom Python script that allowed for the measurement of the fringe length, and fringe tortuosity (the ratio of the fringe length to the straight distance between end-points of the fringe) inside the structure of soot particles. The fringe separation distance was analyzed in the separate crystalline regions inside primary particles in manual mode using the program “ImageJ”.
Figure 9 shows an example of HR TEM image transformation into a carbon skeleton structure, throughout 512 × 512 pixel image selection, the binary image of selected part generation, and skeletonization of the binary image. The regions with staked parallel fringes were considered as the soot crystallites. An example of soot crystallite is marked by a circle inside a carbon skeleton structure in Fig. 9.
Processing of HR TEM image of acetylene soot extracted at 15 mm HAB. Sequentially shown: initial image, 512 × 512 pixel image of internal soot structure, filtered binary image of selected part, skeletonized image, and skeletonized image with deleted fringes shorter than 0.6 nm. The soot crystallite is marked by a circle
3 Results and discussion
Using data presented in Fig. 6, the dependence of E(m,1064) on height above a burner has been plotted in Fig. 10. These results demonstrate an increase in the absolute values of E(m,1064) from 0.19 to 0.53 in a premixed acetylene/air flame with an equivalence ratio of φ = 1.8 when HAB is increased from 5 to 20 mm. These data are compared to the results presented by Bladh et al. [45] E(m,1064) = 0.21–0.46 at HAB 7–17 mm for a premixed ethylene/air flame φ = 2.1, and data by Bejaoui et al. [46] for a methane/oxygen premixed flame with an equivalence ratio of φ = 2.15 E(m,1064) = 0.22–0.37 at HAB 7–15 mm.
The values of E(m) at a wavelength of 1064 nm extracted using two-color LII according to the height above the burner. The symbols are the data obtained in this work, by Bladh et al. [42] for a premixed ethylene/air flame, and by Bejaoui et al. [46] for a methane/oxygen premixed flame. The horizontal lines are the data by Dalzel et al. [11], Köylü et al. [19], Krishnan et al. [28], and Zhu et al. [25] for acetylene soot collected in flames’ exhaust
Figure 10 shows that young soot that formed at low HAB (5–10 mm) has lower values of E(m,1064) than mature soot at higher HAB (15–20 mm). The observable difference of E(m) with increasing HAB can be as much as 1:2.8, which can be attributed to the different maturities of soot and must be accounted for in engineering calculations of soot absorption. It is seen that the E(m) values increase with HAB for the acetylene/air flame and the ethylene/air and methane/oxygen premixed flames. The absolute values of E(m) for acetylene soot are higher than that for ethylene and methane soot at about 1.2 times for low (5–10 mm) and high (15–20 mm) HABs. Note that the comparison of our values of E(m,1064) of acetylene soot and E(m,1064) data for soot collected in flames’ exhaust by Dalzel et al. [11], Köylü et al. [19], Krishnan et al. [28], and Zhu et al. [25] (presented in Fig. 10 by horizontal lines) is not correct due to strong E(m) dependence on HAB.
Of course, the E(m) determination is influenced by the uncertainty of the product ρ × cp (see Eq. (15)), and inaccurate knowledge of soot density and soot-specific heat restrains the accuracy of LII models and quantitative interpretation of LII signals. As was noted above, we supposed the constant value of the product ρ × cp with soot density 1860 kg/m3. The review by Ouf et al. [72] found a decrease in soot density from 1834 ± 187 kg/m3 to 1285 ± 217 kg/m3 with an increase in organic carbon content from 5 to 20%. However, in [72], the density of acetylene soot was found to be in the range of 1720–2050 kg/m3 due to low organic carbon content. From the other side, the reason for the non-monotonic data behavior in Fig. 10 can be a decrease of soot density with a decrease in HAB. Namely, at the lowest HABs of 5 and 7 mm the soot density can be lower in comparison to the density of mature soot, which can result in the decrease of E(m) values. However, the exact values of soot density in dependence on HAB for the acetylene soot are not well known.
The values of the ratio of E(m,1064)/E(m,532) for the acetylene soot extracted from LII amplitudes presented in Fig. 5 are summarized in Fig. 11 in dependence on HAB. One can see that the ratio E(m,1064)/E(m,532) is in the range of 1.12–1.55. The measured values of E(m,1064)/E(m,532) can be compared to other available data for the atmospheric flames. For comparison with our data, Fig. 11 presents data by Bejaoui et al. [50] for a methane/oxygen premixed flame, data by Eremin et al. [73] for an ethylene/air premixed flame, and data by Therssen et al. [49] for a premixed acetylene/air flame. Additionally, the values of E(m,1064)/E(m,532) for acetylene soot collected in flames’ exhaust regardless of HAB dependency by Dalzel et al. [11], Köylü et al. [19], Krishnan et al. [28], and Zhu et al. [25] are presented in Fig. 11 by horizontal lines. An uncertainty of E(m) ratio determination is defined by data point scattering and by the slope of lines drawn through experimental points before the sublimation threshold was evaluated to be ± 10%.
The ratio of E(m,1064)/E(m,532) versus HAB. Symbols are the data obtained in this work, by Therssen et al. [49] for an acetylene/air premixed flame, by Eremin et al. [73] for an ethylene/air premixed flame, and by Bejaoui et al. [50] for a methane/oxygen premixed flame. The horizontal lines are the data by Dalzel et al. [11], Köylü et al. [19], Krishnan et al. [28], and Zhu et al. [25] for acetylene soot collected in flames’ exhaust
From Fig. 11, one can see that the values of E(m,1064)/E(m,532) for acetylene soot are higher than that for ethylene and methane soot at corresponding HAB. The values of E(m,1064)/E(m,532) show a weak increase at HAB > 5 mm. Such behavior allows one to compare these data with results by Dalzel et al. [11], Köylü et al. [19], Krishnan et al. [28], and Zhu et al. [25] for acetylene soot collected in flames’ exhaust. One can see that the data by Bejaoui et al. [50] for a methane/oxygen premixed flame and the data by Eremin et al. [73] for an ethylene/air premixed flame are in good agreement with data by Dalzel et al. [11]. The data by Therssen et al. [49] for a premixed acetylene/air flame are in good agreement with data by Köylü et al. [19]. Our data from a premixed acetylene/air flame are closer to data by Zhu et al. [25]. The reason for the difference in our values of E(m,1064)/E(m,532) from the data by Therssen et al. [49] for premixed acetylene/air flames could be the essential distinction in soot size and structure because these flames were stabilized on a hybrid McKenna burner equipped with a central injector with inner diameter 4 mm. The results [49] were obtained in two premixed acetylene/air flames (equivalence ratio 2.9, cold gas velocity 4.2 m/s, and equivalence ratio 3.5, cold gas velocity 4.4 m/s). Thus, the flames’ temperature in [49] was not the same as that of the flame we used. Additionally, the difference in values of E(m,1064)/E(m,532) measured in acetylene/air flames from the data obtained in methane/oxygen and ethylene/air flames could be attributed to the differences in the kinetic mechanism of soot growth in the different fuels.
Finally, taking into account the data of the absolute values of E(m,1064) for soot (Fig. 10) and the ratio of E(m,1064)/E(m,532) (Fig. 11) according to HAB, one can find a value of E(m) (assuming linear dependence of E(m) on wavelength) at an arbitrary wavelength in the visible and near-infrared range of spectra.
In Fig. 12, the values of mean primary particle sizes measured using TEM images (see Fig. 7 and Fig. 8) are presented in dependence on HAB. The accuracy of TEM measurements of the mean particle size is evaluated as ± 9%. The primary particle sizes in a premixed acetylene flame increase from 11 nm up to 17 nm with a HAB increase from 5 to 20 mm. These data allow one to transform the dependences of E(m,1064) and E(m,1064)/E(m,532) on HAB to plots of these values versus soot particle size. These dependencies are presented in Figs. 13 and 14. The error bars in Figs. 13 and 14 correspond to the measurements presented in Figs. 10 and 11.
From Fig. 13, one can see that acetylene soot absorption essentially increases with the increase in the primary soot particle size. The rise of E(m,1064)/E(m,532) for acetylene soot (see Fig. 14) versus particle size is not as essential as the increase in the E(m,1064) value (see Fig. 13). The E(m,1064)/E(m,532) values increase only at a size rise of up to 13 nm. At the primary particle sizes of 13–18 nm, the value of E(m,1064)/E(m,532) is stable at about 1.4. The presented results mean that a change in the structure occurs with a change in the size of the soot nanoparticles. The data presented in Figs.12, 13, 14 allow for comparison with the results of other works in which changes in optical properties versus particle size are represented.
As noted in the introduction, the variation in soot’s optical properties is associated with a variation in the particle structure. Using HR TEM treatment of soot samples, we have analyzed the mean value of fringe length (Lf), fringe tortuosity (τ), and interlayer spacing (d) (when the parallel fringes were determined) in dependence on HAB. To determine the mean parameters, 30–35 images from each sample were analyzed. The data obtained are presented in Figs. 15, 16, 17.
According to our analysis, the mean fringe length and mean fringe tortuosity (see Figs. 15 and 16) do not differ significantly with HAB. The measured mean fringe length depends strongly on the lower critical size below which this value was not considered (0.6 nm, in our case). In studies by Alfe et al. [53, 54], the mean fringe length of young and mature methane, ethylene, benzene, and cyclohexane soot was defined using the “Analyze plan” method as Lf >0.25 nm and Lf >0.49 nm. The difference between these two values attains 1.5 times for the same soot. The typical values Lf of flame soot in [53, 54] were found to be 0.45–0.6 nm if Lf >0.25 nm and 0.7–0.87 nm if Lf >0.49 nm, which are essentially smaller than our values of 1.17–1.22 nm defined at Lf >0.6 nm. On the other hand, the value of Lf for ethylene and benzene soot obtained using the “Morphological analysis” method was found to be 0.94–1.27 nm [56], which is closer to our data (see Fig. 15). Additionally, the mean fringe length, evaluated for the same soot samples by Raman spectroscopy, was found to be 1–1.25 nm in [56]. Thus, the different methods resulted in the different values of mean fringe length. However, for investigation of soot structure transformation during their growth, it is more essential to define the tendency of change of Lf with HAB, because the Lf increase leads to soot graphitization. From Fig. 15, it is seen that the Lf value essentially changes at HABs lower than 7 mm for premixed flames.
The data on mean fringe tortuosity obtained in various works are presented in Fig. 16. The set of data shown in Fig. 16 demonstrates a weak tendency toward decreasing the values of fringe tortuosity on HAB. Our data on fringe tortuosity, τ = 1.27–1.32, are in good agreement with the data by Apicella et al. [56, 74], τ = 1.302–1.391, for ethylene and benzene flames and with the data by Zhang et al. [75], τ = 1.25, for diesel engine soot primary particles. The smaller values of τ = 1.16–1.19 were measured in [76] in an ethylene-nitrogen/air co-flow diffusion flame.
In Fig. 17, the mean graphene interlayer distance in soot crystallite d is plotted versus HAB. According to our analysis, the distance between graphene planes in acetylene soot decreases from 0.43 nm down to 0.36 nm with a HAB increase from 5 mm up to 20 mm. Based on qualitative analysis of skeletonized images, one can conclude that the amount of soot crystallites in primary particles increases with an increase in HAB. This means that the number of parallel stacked layers increases.
The interlayer distance d between two or more parallel stacked graphene planes is the key parameter of soot graphitization [51, 56, 74]. The values of d closer to graphite value 0.335 nm testifies to the higher graphitization of soot. However, the values of d for methane/oxygen, ethylene/air, benzene/air, and cyclohexane/air flame soot were found independently on HAB to be 0.38–0.4 nm in [53, 54, 56] and 0.44–0.445 nm for ethylene-nitrogen/air flame soot [76] (see Fig. 17).
Thus, the difference between acetylene soot and ethylene or benzene flame soot is the more graphitic structure at the large HABs caused by the smaller interlayer distance. Additionally, we registered an increase in the number of crystalline regions and the number of stacked graphene layers in the acetylene soot internal structure with a HAB increase using HR TEM images.
The observed changes in mean fringe length and mean graphene interlayer distance, and the increase in soot crystallite, resulted in soot graphitization, which consequently leads to a decrease in the optical bandgap and an increase in laser light absorption by soot particles [55]. With a decrease in the bandgap, longer wavelengths of radiation are absorbed. This resulted in increased absorption of electromagnetic radiation at a wavelength of 1064 nm as compared to the absorption at a wavelength of 532 nm with an increase in HAB. Thus, one can conclude that the graphitization of soot in acetylene/air flame with an increase in HAB is likely more intensive than in ethylene/air or benzene/air flames. This leads to the higher value of the ratio of E(m,1064)/E(m,532) (see Fig. 11) in contrast to methane and ethylene atmospheric flame soot.
4 Conclusion
The optical properties of soot particles from premixed acetylene/air premixed flame were investigated. The absolute values of E(m) at a wavelength of 1064 nm were measured using the LII method in dependence on HAB. These values of E(m) were found to increase from 0.19 to 0.53 as HAB increased from 5 to 20 mm. The ratio of the refractive index function at wavelengths of 532 and 1064 nm was determined by recording the amplitude of LII signals and measuring the laser fluence. With an increase in HAB from 5 to 20 mm, which corresponSded to different stages of soot particle growth, the E(m,1064)/E(m,532) ratio increased from 1.12 to 1.55. Variations in the E(m) and E(m) ratio values with HAB correspond with an increase in particle size from 11 to 18 nm.
The changes in the optical properties of soot were attributed to the process of soot particle growth and their structure transformation that occurred under flame conditions. The changes in the optical properties can be associated with a decrease in the distance between graphene planes in soot crystalline from 0.43 nm to 0.36 nm with HAB increasing from 5 mm to 20 mm. These changes resulted in soot graphitization, optical bandgap decreases and an increase in the laser light absorption by soot particles at longer a wavelength.
A comparison of the obtained results with those results in flat premixed ethylene/air and benzene/air flames led to the conclusion that acetylene soot is more graphitized at the same HABs.
References
S. Bard, P.J. Pagni, Carbon particulate in small pool fire flames. J. Heat Transf. 103, 357–362 (1981)
C.M. Sorensen, Light scattering by fractal aggregates: a review. Aerosol Sci. Technol. 35, 648–687 (2001)
T.C. Bond, R.W. Bergstrom, Light absorption by carbonaceous particles: an investigative review. Aerosol Sci. Technol. 40, 27–67 (2006)
T. Mendiara, M.U. Alzueta, A. Millera, R. Bilbao, A comparison of acetylene soot and two different carbon blacks: reactivity to oxygen and NO. Int. J. Chem. React. Engineer. 5, A100 (2007)
M. Singh, R.L. Vander Wal, Nanostructure quantification of carbon blacks. C J Carbon Ress 5(1), 2 (2019)
X. Bourrat, Electrically conductive grades of carbon black: Structure and properties. Carbon 31, 287–302 (1993)
M. Singh, R.L. Vander Waal, Nanostructure quantification of carbon blacks, C-Journal of Carbon Research, 5 (2019) 2.
B.S. Haynes, H.G. Wagner, Soot formation. Prog. Energy Combust. Sci. 7, 229–273 (1981)
M. Frenklach, Reaction mechanism of soot formation in flames. Phys. Chem. Chem. Phys. 4, 2028–2237 (2002)
H. Wang, Formation of nascent soot and other condensed-phase materials in flames. Proc. Combust. Inst. 33, 41–67 (2011)
W.H. Dalzell, A.F. Sarofim, Optical constants of soot and their application to heat-flux calculations. J. Heat Transf. 91, 100–104 (1969)
J.D. Felske, T.T. Charalampopoulos, H. Hura, Determination of the refractive indices of soot particles from the reflectivities of compressed soot pellets. Combust. Sci. Technol. 37, 263–284 (1984)
B.J. Stagg, T.T. Charalampopoulos, Refractive indices of pyrolytic graphite, amorphous carbon and flame soot in the temperature range 25° to 600 °C. Combust. Flame 94, 381–396 (1993)
S.C. Lee, C.L. Tien, Optical constants of soot in hydrocarbon flames. Proc. Combust. Inst. 18, 1159–1166 (1981)
T.T. Charalampopoulos, J.D. Felske, Refractive indices of soot particles deduced from in-situ laser light scattering measurements. Combust. Flame 68, 283–294 (1987)
H. Chang, T.T. Charalampopoulos, Determination of the wavelength dependence of refractive indices of flame soot. Proc. R. Soc. London A 430, 577–591 (1990)
R.A. Dobbins, G.W. Mulholland, N.P. Bryner, Comparison of a fractal smoke optics model with light extinction measurements. Atmos. Environ. 28, 889–897 (1994)
M.Y. Choi, G.W. Mulholland, A. Hamins, T. Kashiwagi, Comparisons of the soot volume fraction using gravimetric and light extinction technique. Combust. Flame 102, 161–169 (1995)
Ü.Ö. Köylü, G.M. Faeth, Spectral extinction coefficients of soot aggregates from turbulent diffusion flames. J. Heat Transf. 118, 415–421 (1996)
Ü.Ö. Köylü, Quantitative analysis of in situ optical diagnostics for inferring particle/aggregate parameters in flames: implications for soot surface growth and total emissivity. Combust. Flame 109, 488–500 (1996)
K.C. Smyth, C.R. Shaddix, The elusive history of m=1.57–0.56i for the refractive index of soot, Combust. Flame. 107 (1996) 314–320.
J.-S. Wu, S.S. Krishnan, G.M. Faeth, Refractive indices at visible wavelengths of soot emitted from buoyant turbulent diffusion flames. J. Heat Transf. 119, 230–237 (1997)
G.W. Mulholland, M.Y. Choi, Measurement of the mass specific extinction coefficient for acetylene and ethylene smoke using the large agglomerate optics facility. Proc. Combust. Inst. 27, 1515–1522 (1998)
S.S. Krishnan, K.C. Lin, G.M. Faeth, Optical properties in the visible of overfire soot in large buoyant turbulent diffusion flames. J. Heat Transf. 122, 517–524 (2000)
J. Zhu, M.Y. Choi, G.W. Mulholland, L.A. Gritzo, Measurement of soot optical properties in the near-infrared spectrum. Int. J. Heat Mass Transf. 43, 3299–3303 (2000)
J. Zhu, M.Y. Choi, G.W. Mulholland, L.A. Gritzo, Soot scattering measurements in the visible and near-infrared spectrum. Proc. Combust. Inst. 28, 439–446 (2000)
N.A. Marley, J.S. Gaffney, JCh. Baird, C.A. Blazer, P.J. Drayton, J.E. Frederick, An empirical method for the determination of the complex refractive index of size-fractionated atmospheric aerosols for radiative transfer calculations. Aerosol Sci. Tech. 34, 535–549 (2001)
S.S. Krishnan, K.-C. Lin, G.M. Faeth, Extinction and scattering properties of soot emitted from buoyant turbulent diffusion flames. J. Heat Transf. 123, 331–339 (2001)
P. Van-Hulle, M. Talbaut, M. Weill, A. Coppalle, Inversion method and experiment to determine the soot refractive index: application to turbulent diffusion flames. Meas. Sci. Technol. 13, 375–382 (2002)
M. Schnaiter, H. Horvath, O. Mohler, K.-H. Naumann, H. Saathoff, O.W. Schock, UV-VIS-NIR spectral optical properties of soot and soot-containing aerosols. J. Aerosol Sci. 34, 1421–1444 (2003)
J. Zhu, A. Irrera, M.Y. Choi, G.W. Mulholland, J. Suo-Anttila, L.A. Gritzo, Measurement of light extinction constant of JP-8 soot in the visible and near-infrared spectrum. Int. J. Heat Mass Transf. 47, 3643–3648 (2004)
S.S. Iyer, T.A. Litzinger, S.-Y. Lee, R.J. Santoro, Determination of soot scattering coefficient from extinction and three-angle scattering in a laminar diffusion flame. Combust. Flame 149, 206–216 (2008)
F. Migliorini, S. De Iuliis, R. Dondè, M. Commodo, P. Minutolo, A. D’Anna, Nanosecond laser irradiation of soot particles: insights on structure and optical properties. Exper. Thermal and Fluid Science 114, 110064 (2020)
E. Gurentsov, A review on determining the refractive index function, thermal accommodation coefficient and evaporation temperature of light absorbing nanoparticles suspended in gas the phase using the laser-induced incandescence. Nanotechnol. Rev. 7, 583–604 (2018)
H.A. Michelsen, P.E. Schrader, F. Goulay, Wavelength and temperature dependences of the absorption and scattering cross sections of soot. Carbon 48, 2175–2191 (2010)
X. López-Yglesias, P.E. Schrader, H.A. Michelsen, Soot maturity and absorption cross sections. J. Aerosol Sci. 75, 43–64 (2014)
J. Yon, R. Lemaire, E. Therssen, P. Desgroux, A. Coppalle, K.F. Ren, Examination of wavelength dependent soot optical properties of diesel and diesel/rapeseed methyl ester mixture by extinction spectra analysis and LII measurements. Appl. Phys. B. 104, 253–271 (2011)
G. Cléon, T. Amodeo, A. Faccinetto, P. Desgroux, Laser induced incandescence determination of the ratio of the soot absorption functions at 532 nm and 1064 nm in the nucleation zone of a low pressure premixed sooting flame. Appl. Phys. B. 104, 297–305 (2011)
F. Migliorini, S. DeIuliis, S. Maffi, G. Zizak, Saturation curves of two-color laser-induced incandescence measurements for the investigation of soot optical properties. Appl. Phys. B. 120, 417–427 (2015)
C. Betrancourt, X. Mercier, F. Liu, P. Desgroux, Quantitative measurement of volume fraction profiles of soot of different maturities in premixed flames by extinction-calibrated laser-induced incandescence. Appl. Phys. B 125, 16 (2019)
C.M. Sorensen, J. Yon, F. Liu, J. Maughan, W.R. Heinson, M.J. Berg, Light scattering and absorption by fractal aggregates including soot. J. Quantit Spectrosc Radiat Transfer. 217, 459–473 (2018)
J. Lahaye, G. Prado, Morphology and internal structure of soot and carbon blacks, in Particulate carbon. ed. by D.C. Siegla, G.W. Smith (Springer, Boston, 1981), pp. 33–55
D.R. Snelling, F. Liu, G.J. Smallwood, Determination of the soot absorption function and thermal accommodation coefficient using low-fluence LII in a laminar coflow ethylene diffusion flame, Combust. Flame 136, 180–190 (2004)
D. Snelling, K. Thomson, F. Liu, G. Smallwood, Comparison of LII derived soot temperature measurements with LII model predictions for soot in a laminar diffusion flame. Appl. Phys. B 96, 657–669 (2009)
H. Bladh, J. Johnsson, N.-E. Olofsson, A. Bohlin, P.-E. Bengtsson, Optical soot characterization using two-color laser-induced incandescence (2C-LII) in the soot growth region of a premixed flat flame. Proc. Combust. Inst. 33, 641–648 (2011)
S. Bejaoui, S. Batut, F. Therssen, N. Lamoureux, P. Desgroux, F. Liu, Measurements and modeling of laser-induced incandescence of soot at different heights in a flat premixed flame. Appl. Phys. B 118, 449–469 (2015)
A.V. Eremin, E.V. Gurentsov, E. Popova, K. Priemchenko, Size dependence of complex refractive index function of growing nanoparticles. Appl. Phys. B 104, 285–295 (2011)
F. Liu, J. Yon, A. Fuentes, P. Lobo, G.J. Smallwood, J.C. Corbin, Review of recent literature on the light absorption properties of black carbon: refractive index, mass absorption cross section, and absorption function. Aerosol Sci. Tech. 54, 33–51 (2020)
E. Therssen, Y. Bouvier, C. Schoemaecker-Moreau, X. Mercier, P. Desgroux, M. Ziskind, C. Focsa, Determination of the ratio of soot refractive index function E(m) at the two wavelengths 532 and 1064 nm by laser induced incandescence. Appl. Phys. B 89, 417–427 (2007)
S. Bejaoui, R. Lemaire, P. Desgroux, F. Therssen, Experimental study of the E(m, k)/E(m, 1064) ratio as a function of wavelength, fuel type, height above the burner and temperature. Appl. Phys. B 116, 313–323 (2014)
C. Jager, Th. Henning, R. Schlogl, O. Spillecke, Spectral properties of carbon black. J. Non-Crystalline Solids 258, 161–179 (1999)
V. Fernandez-Alos, J.K. Watson, R. vander Wal, J.P. Mathews, , Soot and char molecular representations generated directly from HRTEM lattice fringe images using Fringe, Combust. Flame 158, 1807–1813 (2011)
M. Alfe, B. Apicella, R. Barbella, J.N. Rouzaud, A. Tregrossi, A. Ciajolo, Structure–property relationship in nanostructures of young and mature soot in premixed flames. Proc. Combust. Inst. 32, 697–704 (2009)
M. Alfe, B. Apicella, J.N. Rouzaud, A. Tregrossi, A. Ciajolo, The effect of temperature on soot properties in premixed methane flames. Combust. Flame 157, 1959–1965 (2010)
G. De Falco, M. Commodo, C. Bonavolonta, G.P. Pepe, P. Minutolo, A. D’Anna, Optical and electrical characterization of carbon nanoparticles produced in laminar premixed flames. Combust. Flame 161, 3201–3210 (2014)
B. Apicella, P. Pre, M. Alfe, A. Ciajolo, V. Gargiulo, C. Russo, A. Tregrossi, D. Deldique, J.N. Rouzaud, Soot nanostructure evolution in premixed flames by High Resolution Electron Transmission Microscopy (HRTEM) Proc. Combust. Inst. 35, 1895–1902 (2015)
D. Bradley, K.J. Matthews, Measurement of high gas temperatures with fine wire thermocouples. J. Mech. Eng. Sci. 10, 299–305 (1968)
C. Schulz, B.F. Kock, M. Hofmann, H.A. Michelsen, S. Will, B. Bougie, R. Suntz, G. Smallwood, Laser-induced incandescence: recent trends and current questions. Appl. Phys. B 83, 333–354 (2006)
M.W. Chase, C.A. Davies, J.R. Downey et al., JANAF Thermochemical Tables, Third Edition. J. Phys. Chem. Ref. Data 14, 667 (1985)
H.A. Michelsen, Understanding and predicting the temporal response of laser-induced incandescence from carbonaceous particles. J. Chem. Phys. 118, 7012–7045 (2003)
P. Roth, A.V. Filippov, In situ ultrafine particle sizing by a combination of pulsed laser heatup and particle thermal emission. J. Aerosol Sci. 27, 95–104 (1996)
B. Kock, C. Kayan, J. Knipping, H.R. Ortner, P. Roth, Comparison of LII and TEM sizing during synthesis of iron particle chains. Proc. Combust. Inst. 30, 1689–1697 (2004)
G.D. Yoder, P.K. Diwaker, D.W. Hahn, Assessment of soot particle vaporization effects during laser-induced incandescence with time-resolved light scattering. Appl. Opt. 44, 4211–4219 (2005)
S. De Iuliis, F. Migliorini, F. Cignoli, G. Zizak, Peak soot temperature in laser induced incandescence measurements. Appl. Phys. B 83, 397–402 (2006)
F. Goulay, P.E. Schrader, L. Nemes, M.A. Dansson, H.A. Michelsen, Photochemical interferences for laser-induced incandescence of flame-generated soot. Proc. Combust. Inst. 32, 963–970 (2009)
F. Goulay, P.E. Schrader, X. Lopez-Yglesias, H.A. Michelsen, A data set for validation of models of laser-induced incandescence from soot: temporal profiles of LII signal and particle temperature. Appl. Phys. B 112, 287–306 (2013)
H.-S. Shim, R.H. Hurt, N.Y.C. Yang, A methodology for analysis of 002 lattice fringe images and its application to combustion-derived carbons. Carbon 38, 29–45 (2000)
N. Galvez, C. Herlin-Boime, C. Reynaud, Clinard, J-N Rouzaund, carbon nanoparticles from laser pyrolysis. Carbon 40, 2775–2789 (2002)
S.A. Pfau, A. La Rocca, M.W. Fay, Quantifying soot nanostructures: importance of image processing parameters for lattice fringe analysis, Combust. Flame 211, 430–444 (2020)
C.A. Schneider, W.S. Rasband, K.W. Eliceiri, NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 9, 671–675 (2012)
V. Fernandez-Alos, J.K. Watson, R. Vander Wal, J.P. Mathews, Soot and char molecular representations generated directly from HRTEM lattice fringe images using Fringe, Combust. Flame 158, 1807–1813 (2011)
F.-X. Ouf, S. Bourrous, S. Fauvel, A. Kort, L. Lintis, J. Nuvoli, J. Yon, True density of combustion emitted particles: a comparison of results highlighting the influence of the organic contents. J. Aerosol Sci. 134, 1–13 (2019)
A.V. Eremin, E.V. Gurentsov, R.N. Kolotushkin, The change of soot refractive index function along the height of premixed ethylene/air flame and its correlation with soot structure. Appl. Phys. B 126, 125 (2020)
B. Apicella, P. Pré, J.N. Rouzaud, J. Abrahamson, R.L. Vander Wal, A. Ciajolo, A. Tregrossi, C. Russo, Laser-induced structural modifications of differently aged soot investigated by HR TEM, Combust. Flame 204, 13–22 (2019)
Y. Zhang, R. Zhang, L. Rao, S. Kook, A Comparison between in-flame and exhaust soot nanostructures in a light-duty diesel Engine. SAE Technical Paper (2017). https://doi.org/10.4271/2017-01-0710
M.L. Botero, Y. Sheng, J. Akroyd, J. Martin, J.A.H. Dreyer, W. Yang, M. Kraft, Internal structure of soot particles in a diffusion flame. Carbon 141, 635–642 (2019)
Acknowledgments
The reported study was funded by Russian Science Foundation, project 19-79-10204.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Drakon, A.V., Eremin, A.V., Gurentsov, E.V. et al. Optical properties and structure of acetylene flame soot. Appl. Phys. B 127, 81 (2021). https://doi.org/10.1007/s00340-021-07623-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00340-021-07623-8